The increasing prevalence of highly specialized units and the growing number of lung cancer screening programs have not only reduced lung cancer mortality by at least 20%,1 but have also led to a rise in the detection of subcentimeter and/or subsolid pulmonary nodules (PN). These small masses can be challenging to manage and require a multidisciplinary approach.
The diagnostic yield of transthoracic needle biopsy in peripheral nodules measuring <20mm is 70%, and the procedure is associated with a 9.6% incidence of pneumothorax,2 so surgical resection may be necessary, either for diagnosis or for treatment. Intraoperative detection of small nodules by digital palpation can be difficult during minimally invasive surgery, and conversion to open surgery is sometimes required for a better examination of the parenchyma. Algorithms, such as that developed by Tamura,3 have been designed to identify cases that would benefit from pre-operative marking. The indication should be determined by the surgical team on the basis of their experience and setting. Various pre-surgical techniques can be used for locating PNs, including hook wires, coils, the injection of different substances such as lipiol, barium, cyanocrylate or methylene blue, and ultrasound.
Given the growing demand, new techniques for locating PNs have been developed and existing methods have been refined. Given the wide range of techniques available, we present a review of the latest developments.
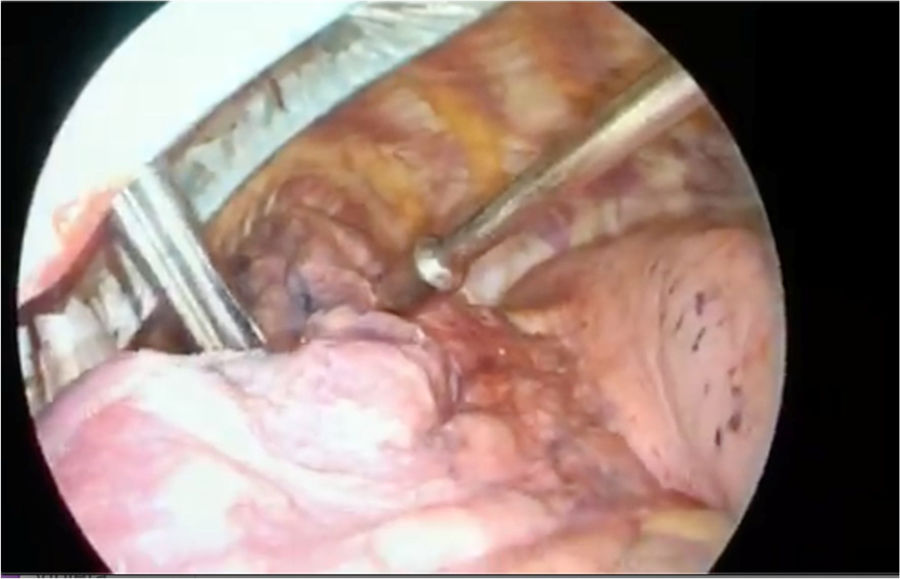
Radio-guided surgery: technetium 99m-labeled macroaggregated albumin (Tc 99m MAA) using a hand-held gamma probeIn 2000, Chella et al.4 described the detection of PNs by injecting Tc 99m-labeled albumin microspheres that were subsequently located intraoperatively using a gamma-probe. They reported an efficacy of 100%. Daniel et al.5 studied various radiotracers and found that Tc 99m MAA was more persistent and showed lower intraparenchymal diffusion due to its greater molecular size. In 2019, Galetta et al.6 reported on their 10 years of experience. The complications they described were asymptomatic pneumothorax (13.4%), intraparenchymal hemorrhages (13.7%), failure to locate the PN in 3 patients, and allergic reactions in 2 (Fig. 1).
Vollmer et al.7 adapted the Galetta technique and incorporated methods developed for parathyroid adenoma surgery, breast cancer, and sentinel node biopsy. They used a portable gamma-camera (PGC) in the operating room to confirm resection margins, although the specimen also required pathology analysis. With this technique, they were able to locate 100% of the 38 marked nodules. The use of a PGC for assessing margins is associated with a high negative predictive value.
Radiotracer marking is a safe and reliable technique. It has been shown to be superior to other methods, such as hook wires or ultrasound,8 and the failure rate is low – Galetta et al. describe only 3 failures in their cohort of 262 cases. However, Vollmer's innovative PCG guarantees the success of the test whenever the marker is located within or caudal to the nodule.
Use of fluorescence and indocyanine green imaging technologyThe injection of dyes for the detection of PN is a widely used technique with a large body of evidence, but is hampered by diffusion and intraoperative visualization issues.
Fluorescence imaging technology is based on the use of fluorescence-emitting contrasts in combination with an imaging system. The fluorescent dye accumulates selectively in the tissues and can be detected with a specific imaging system.
Indocyanine green (ICG) is a molecule that binds to plasma proteins and emits a wavelength of 805–830nm. It is a safe substance with a very low incidence of toxicity and allergic reactions (0.01%).9
It can be located in a variety of ways, including intravenous injection, CT-guided transthoracic localization, or electromagnetic navigational bronchoscopy (ENB).
In 2015, Anayama et al. demonstrated the feasibility of ENB-guided ICG labeling in a porcine model.10 The procedure is based on locating the lesion using CT software prior to or on the day of surgery. Although there is currently no consensus as to when CT should be performed for reconstruction, it is clear that the closer it is to surgery, the more reliable the reconstruction. The patient is placed in a prone position on an electromagnetic table. A real-time virtual ENB is performed that is reconstructed by the system. The coordinates of the PN are entered and the bronchoscope is guided there to mark it. ICG 0.5ml is injected followed by sterile serum 0.5ml to flush the working channel, and the nodule can then be located using a camera with near-infrared detection.11
Okusanya et al. 12 labeled PNs with ICG that were then located using a CT-guided transthoracic needle and resected, demonstrating advantages such as speed of labeling, high sensitivity, and safety. However, Yang et al.13 reported a higher rate of procedure-related complications, such as pneumothorax (0 vs. 16.7%, p=0.029) and focal intrapulmonary hemorrhage (3.3 vs. 26.7%, p=0.008) when they compared the EBN and transthoracic methods in 2 propensity score-matched cohorts.
EBN is also used to guide transthoracic puncture. This novel approach uses an intraoperative electromagnetic tracking sensor for percutaneous localization. Electromagnetic navigation enables real-time tracking of the procedure by detecting the electromagnetic tip of the needle stylet. A preoperative chest CT is performed, obtaining slices in inspiration and expiration and the entry point is selected using planning software. The needle is placed at the entry point and the path to the nodule is guided by the navigation system. Once it is located in the nodule, the intended material, be it a hook wire, a seed, or a dye, is injected. Hung14 found no differences compared with CT-guided transthoracic localization in terms of effectiveness or complications, although CT-guided localization was faster.
ICG injection is a useful technique for the localization of pulmonary nodules. One of the drawbacks is that manipulation of the lung during surgery may cause parenchymal diffusion. The injection technique used will depend on the facilities available in each center, although it appears that the use of EBN is associated with a lower rate of complications. The use of electromagnetic guidance for puncture may be an option for radiologists with limited experience in transthoracic localization, since it has not so far been shown to offer advantages over the conventional technique.
Virtual-assisted lung mapping (VAL-MAP)VAL-MAP is a lung-labeling technique that uses different lung markings placed by ENB. It indicates the lung area to be resected by marking the pleural surface with dyes or by inserting microcoils using 3-dimensional CT. This information not only facilitates node resection, but also provides a wide oncologically accurate margin.15
Sato et al.16 analyzed the results of VAL-MAP and found it to be safe. In their cohort, 0.8% presented important complications, and 3.6% presented pneumothorax, 1.2% pneumomediastinum, and 1.2% alveolar hemorrhage. Resection was successful in 99% of cases. The authors remarked that one of the limitations of the technique is to obtain safe margins in deep nodules.
Ueda et al.17 performed a small variant of the technique using VAL-MAP to place microcoils instead of dyes, so that deep nodules could be resected more safely while maintaining optimal margins. The results of the VAL-MAP 2.0 study are pending publication.
ConclusionsThere are a great number of minimally invasive techniques that facilitate localization and resection of PNs. Advances in these techniques, such as the introduction of the PGC in radioisotope labeling or the use of EBN for the injection of substances such as ICG, are driving the creation of new, less invasive, safer, and more reliable methods, often incorporating 3-dimensional reconstruction technology such as VAP-MAP.