We have updated recommendations on 12 controversial topics that were published in the 2013 National Consensus on the diagnosis, risk stratification and treatment of patients with pulmonary embolism (PE). A comprehensive review of the literature was performed for each topic, and each recommendation was evaluated in two teleconferences. For diagnosis, we recommend against using the Pulmonary Embolism Rule Out Criteria (PERC) rule as the only test to rule out PE, and we recommend using a d-dimer cutoff adjusted to age to rule out PE. We suggest using computed tomography pulmonary angiogram as the imaging test of choice for the majority of patients with suspected PE. We recommend using direct oral anticoagulants (over vitamin K antagonists) for the vast majority of patients with acute PE, and we suggest using anticoagulation for patients with isolated subsegmental PE. We recommend against inserting an inferior cava filter for the majority of patients with PE, and we recommend using full-dose systemic thrombolytic therapy for PE patients requiring reperfusion. The decision to stop anticoagulants at 3 months or to treat indefinitely mainly depends on the presence (or absence) and type of risk factor for venous thromboembolism, and we recommend against thrombophilia testing to decide duration of anticoagulation. Finally, we suggest against extensive screening for occult cancer in patients with PE.
El objetivo del presente documento es actualizar el consenso previo publicado en 2013, en relación con 12 áreas controvertidas en el manejo de la tromboembolia de pulmón (TEP). Para cada área se realizó una exhaustiva revisión bibliográfica y una propuesta de recomendación, sometida a un proceso de debate interno en dos teleconferencias sucesivas. En relación con el diagnóstico, recomendamos no utilizar la escala Pulmonary Embolism Rule Out Criteria (PERC) de forma aislada para descartar la TEP y, cuando haya indicación de dímero D, recomendamos emplear un punto de corte ajustado a la edad. Sugerimos utilizar la angiotomografía computerizada de tórax como prueba de imagen para el diagnóstico de la mayoría de los pacientes con sospecha de la enfermedad. Se recomienda utilizar anticoagulantes de acción directa (en vez de antagonistas de la vitamina K) para el tratamiento de la mayoría de los pacientes con TEP, y se sugiere utilizar anticoagulación para la mayoría de los pacientes con TEP subsegmentaria. Se recomienda no colocar un filtro de vena cava inferior en la mayoría de los pacientes. Si se indica tratamiento de reperfusión, el panel recomienda utilizar fibrinolisis sistémica a dosis completas. La duración de la anticoagulación está condicionada principalmente por la presencia (o ausencia) y el tipo de factor de riesgo para enfermedad tromboembólica venosa, y recomendamos no realizar estudios de trombofilia para decidir la duración de la anticoagulación a la mayoría de los pacientes con TEP. Finalmente, sugerimos no realizar cribado extendido de cáncer oculto en pacientes con TEP.
This consensus for the management of pulmonary embolism (PE) was developed with the collaboration of experts from the following scientific societies: the Spanish Society of Cardiology (SEC), the Spanish Society of Emergency Medicine (SEMS), the Spanish Society of Internal Medicine (SEMI), the Spanish Society of Radiology (SRAM), the Spanish Society of Thrombosis and Hemostasis (SETH), and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).
To produce this consensus, the coordinators identified 12 critical questions, each of which was assigned to 2 panel members. Each pair performed a systematic review and proposed a recommendation that was submitted for internal discussion in 2 successive teleconferences. The recommendations were then put to an anonymous vote: a consensus of more than 80% was required for final approval. The coordinators then combined the various proposals in a single draft document that was submitted for a final critical review by all panel members. The strength of the recommendation was graded in accordance with the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system, based not only on the quality of the evidence, but also on other factors such as the risk-benefit ratio, patient and practitioner values, preferences, cost, and resource consumption.
1. Is it is safe to use only the PERC scale (without any additional testing) to rule out PE in the emergency department?The Pulmonary Embolism Rule-out Criteria (PERC) should not be used alone (without any additional diagnostic tests) in the emergency department to rule out PE.
The PERC rule (Table 1) was developed to reduce the number of additional tests in the diagnosis of patients with suspected symptomatic acute PE. Patients with a negative score would not require any additional tests (not even d-dimer) to rule out the diagnosis of the disease.
PERC rule.
| Age <50 years |
| Heart rate <100 |
| SaO2 >94% in room air |
| No previous history of DVT or PE |
| No recent trauma or surgery |
| No hemoptysis |
| No estrogen use |
| No unilateral inflammation of a leg |
| The score is negative if the patient meets ALL of the above criteria |
DVT: deep vein thrombosis; PE: pulmonary embolism; SaO2: arterial oxygen saturation.
A recent systematic review reported an overall sensitivity of 95% and a negative probability ratio of 0.211. The quality of evidence was very low to low: 1) in most of the studies, the score was calculated retrospectively; 2) several studies had a high risk of bias; 3) the baseline diagnosis of PE was different in each publication; and 4) patients with a low suspicion of PE were included.
In a clinical trial that included 1916 patients, the PERC rule reduced the need for computed tomography angiography (CT angiogram) by 10%, mean emergency room stay by 36 min, and the need for hospital admission (13% vs. 16%), with no significant differences in diagnostic error (0.1% vs. 0%) or mortality in the first three months of follow-up (0.3% vs. 0.2%)2. The quality of evidence provided by this trial is very low to low: 1) the risk of inclusion bias was high; 2) baseline characteristics of patients in the group assigned to the PERC rule were different from those in the control group; 3) the risk of inaccuracy was high; and 4) the prevalence of PE in the control group was very low (3%).
Based on this evidence, and given that the prevalence of PE among patients attending emergency services for suspected acute symptomatic PE is higher in Spain3, the panel recommends the use of well-validated clinical scales (Wells scale [Appendix A Table S1]) that also require d-dimer and/or imaging tests.
2. Should the cut-off point used in the diagnostic algorithm for PE be fixed or adjusted (for age or clinical signs)?The use of D-dimer with an age-adjusted cutoff is recommended in patients with a low or moderate clinical probability of PE or with clinically unlikely PE.
D-dimer with a cut-off point adjusted for clinical signs (the YEARS algorithm) should be used in patients (including pregnant women) with suspected PE.
The use of D-dimer with a cut-off adjusted for clinical probability (Wells criteria) is recommended in patients with a low or moderate clinical likelihood of PE or with clinically unlikely PE.
The use of D-dimer with a cut-off point adjusted for age or clinical signs is not recommended in patients with cancer or kidney failure.
In recent years, new strategies for the diagnosis of PE based on the use of adjusted d-dimer cut-off points have been evaluated. The first is the use of an age-adjusted cut-off point (age multiplied by 10) in patients over 50 years of age4. The second (the YEARS algorithm) uses a 1000 ng/mL d-dimer cut-off point in patients who do not meet any of the following criteria: 1) symptoms or signs of deep vein thrombosis (DVT), 2) hemoptysis, and 3) PE as the most likely diagnosis; and a cut-off point of 500 ng/mL if one or more of the above criteria are met5. The third involves the use of a d-dimer cut-off point of 1000 ng/mL in patients with a low clinical probability of PE (according to the Wells criteria); and 500 ng/mL in patients with a moderate clinical probability6.
Several studies have analyzed the safety (i.e., failure rate defined as the incidence of thromboembolic events at 3 months among patients who were not anticoagulated due to failure of the strategy used) and efficacy (i.e., absolute difference in CT angiograms performed) for the overall population and for specific subgroups of patients with suspected PE (Table 2). In the overall population, the failure rates of the new approaches are similar to or lower than conventional strategies (e.g., the fixed d-dimer cut-off), but more effective. A recent study has demonstrated the safety and efficiency of the YEARS algorithm in a subgroup of pregnant patients7. However, in patients with cancer or renal impairment, these strategies still require external validation before they can be recommended for routine use.
Safety and efficiency of the use of fixed or adjusted d-dimer cut-off points4–6.
| Fixed d-dimer | Age-adjusted d-dimer | Symptom-adjusted d-dimer (YEARS) | d-dimer adjusted for clinical probability | |
|---|---|---|---|---|
| Global population | ||||
| Error rate | 0.65 (0.30–1.11) | 0.94 (0.58–1.5) | 0.61 (0.36–0.96) | 0 (0.00–0.29) |
| Efficacy | – | 4.6 (4.3–4.8) | 13 (10–15) | 17.6 (15.9–19.2) |
| Pregnant women | ||||
| Error rate | – | – | 0.21 (0.04–1.2) | – |
| Efficacy | – | – | – | – |
| Cancer | ||||
| Error rate | – | 3.8 (3.1–4.4) | 7.4 (5.0–11) | – |
| Efficacy | 2.6 (0.57−11) | 1.4 (0.15–12.6) | 2.6 (1.3–5.2) | – |
| Renal failure | ||||
| Error rate | – | – | – | – |
| Efficacy | – | – | – | – |
The use of ventilation/perfusion (V/Q) scintigraphy instead of chest CT angiogram is not recommended as an imaging test for the diagnosis of most patients with suspected symptomatic acute PE.
A V/Q scan is recommended for imaging in the diagnosis of hemodynamically stable patients with suspected symptomatic acute PE and allergy to iodinated contrast agents or severe renal impairment (i.e., creatinine clearance <30 mL/min).
The use of V/Q scans has been replaced by chest multidetector CT angiogram as the imaging test of choice for the diagnosis of most patients with suspected symptomatic acute PE8. Unlike V/Q scans, chest CT angiogram is available in most hospitals, requires less time to conduct and interpret, is highly sensitive and specific, offers a lower percentage of inconclusive results, provides prognostic information, and can give an alternative diagnosis to PE in up to 50% of cases9,10.
Currently, V/Q scans are mainly reserved for patients with known allergy to iodinated contrast agents and for patients with severe renal impairment who are not in a dialysis program8. In pregnant patients with suspected PE, both scintigraphy and CT angiogram expose the mother and fetus to a radiation dose that is well below the danger threshold (100 mGy)11. If scintigraphy is indicated in pregnant patients with suspected PE, chest X-ray must be normal, and only a Q scan should be performed (without the V component). Although the yield of compression ultrasound of the lower extremities is less in pregnant patients than in others with suspected PE12, it seems reasonable to indicate it as the first test in the diagnostic algorithm.
Appendix A Figs. S1 and S2 show diagnostic algorithms for patients with suspected PE and for pregnant patients with suspected PE, respectively.
4. Is it safe to treat selected patients with symptomatic acute PE as outpatients? How can these patients be identified?Outpatient treatment (in the first 24 h after diagnosis) is recommended in low-risk patients with symptomatic acute PE.
The use of either the simplified Pulmonary Embolism Severity Index (sPESI) or the Hestia criteria for identifying low-risk patients who are candidates for outpatient treatment is recommended.
Home treatment (discharge within 24 h after diagnosis) in a subgroup of low-risk patients with symptomatic acute PE reduces complications associated with hospital admission, improves quality of life, and reduces related healthcare costs. A meta-analysis that included 11 studies with 1258 patients with PE treated on an outpatient basis showed a rate of recurrent venous thromboembolism (VTE) of 1.47% during the first 3 months of follow-up13. The rate of fatal PE was 0.47%, while major and fatal intracranial bleeds occurred at rates of 0.81% and 0.29%, respectively.
The growing use of direct-acting oral anticoagulants and the validation of various tools for identifying patients with a low short-term risk of complications has fostered early discharge and even home care in a subgroup of patients with symptomatic acute PE. The clinical practice guidelines of the European Society of Cardiology8 recommend the use of prognostic scales (such as sPESI and Hestia criteria) [Appendix A Table S2]) to identify patients with low-risk PE who might benefit from home treatment. The HOME-PE clinical trial showed that both scales can be used interchangeably to identify these patients14. While interobserver agreement in sPESI is greater than in the Hestia criteria (making it more useful for doctors with limited experience in the management of PE)15, additional criteria (active bleeding or high risk of bleeding, heparin-induced thrombocytopenia, severe renal impairment, lack of adherence to the prescribed anticoagulant treatment, inadequate home setting, etc.) must be taken into consideration to ensure that outpatient treatment of PE is safe (Appendix A Fig. S3).
5. Are DAOCs the anticoagulant treatment of choice in PE? What are the exceptions?The use of direct-acting oral anticoagulant (DAOCs) instead of vitamin K antagonists (VKA) is recommended for the anticoagulant treatment of most patients with symptomatic acute PE.
DAOCs are not recommended for the anticoagulant treatment of patients with a known diagnosis of triple positive antiphospholipid syndrome, pregnant and breastfeeding patients, and patients with severe renal impairment.
Four DOACs are currently approved for the treatment of patients with DVT and/or PE: 3 factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban), and 1 direct thrombin inhibitor (dabigatran). Several clinical trials have tested the efficacy and safety of DAOCs compared with VKA in the treatment of patients with VTE. A meta-analysis of 6 clinical trials that included 27,023 patients with VTE demonstrated the non-inferiority of DOACs compared to VKAs in terms of efficacy (fatal or non-fatal recurrent VTE) (relative risk [RR], 0.90; 95% confidence interval [95% CI], 0.77–1.06)16. Compared with standard anticoagulant treatment, DOACs significantly reduced the risk of major bleeds (RR, 0.61: 95% CI, 0.45–0.83), intracranial bleeds (RR, 0.37; 95% CI 0.21–0.68), fatal bleeds (RR, 0.36; 95% CI, 0.15–0,84), and clinically relevant non-major bleeds (RR, 0.73; 95% CI, 0.58–0.93). Unlike VKAs, DOACs have predictable bioavailability and pharmacokinetics, so monitoring is unnecessary and they are easier to use. Appendix A Fig. S4 shows DOAC dosing for the treatment of patients with VTE.
DOACs are contraindicated in some clinical situations: triple positive antiphospholipid syndrome, renal impairment with creatinine clearance <15 mL/min (apixaban, edoxaban, and rivaroxaban) or <30 mL/min (dabigatran), pregnancy and breastfeeding. They should be used with caution if there is a risk of drug interactions (P-glyoprotein [P-gp] and cytochrome P450 3A4 [CYP3A4] inhibitors/inducers) or malabsorption, and scant clinical experience is available on their use in patients with thrombosis in unusual sites (upper extremity DVT, catheter-related thrombosis, cerebral venous sinus thrombosis, or splanchnic vein thrombosis). Table 3 provides guidelines on the choice of oral anticoagulant treatment in different clinical situations.
Oral anticoagulants for the treatment of pulmonary embolism.
| Factor | VKA | Apixaban | Dabigatran | Edoxaban | Rivaroxaban |
|---|---|---|---|---|---|
| Need for reperfusion treatmenta | X | X | X | ||
| No need for parenteral anticoagulation | X | X | |||
| Administration once daily | X | X | X | ||
| GERD or use of PPI | X | X | X | X | |
| Poor therapeutic compliance | X | ||||
| Possibility of administration without food | X | X | X | X | |
| Malnutrition, vitamin K deficiency | X | X | X | X | |
| Administration dissolved in liquid | X | X | X |
GERD: gastroesophageal reflux disease; PPI: proton pump inhibitors; VKA: vitamin K antagonists.
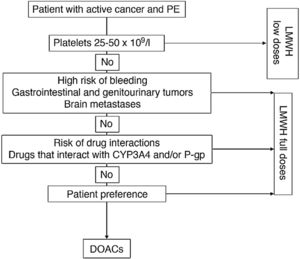
The use of low-weight molecular heparins (LWMH), apixaban, edoxaban, and rivaroxaban instead of VKA is recommended for the anticoagulant treatment of patients with PE caused by cancer.
LWMHs in monotherapy have been the conventional treatment of choice for PE associated with cancer. Compared with VKA, they have shown a statistically significant reduction in recurrent VTE (RR, 0.58), with no significant differences in major or minor bleeds or mortality17.
Four clinical trials have been published to date comparing dalteparin with DAOCs (apixaban [2], edoxaban, and rivaroxaban). In contrast to DAOCs, dalteparin is associated with a significant increase in recurrent VTE (RR, 1.55, 95% CI, 1.19–2.03) and a significant reduction in clinically relevant non-major bleeds (RR, 0.68; 95% CI, 0.54–0.86), mainly associated with gastrointestinal tumors18. No statistically significant differences were observed in major bleeds (RR, 0.74; 95% CI, 0.52–1.06).
Fig. 1 shows an algorithm for selecting anticoagulant treatment in patients with PE associated with cancer.
Anticoagulation in patients with PE associated with cancer.
CYP3A4: cytochrome P450 3A4; DOACs: direct-acting oral anticoagulants; LMWH: low molecular weight heparin; PE: pulmonary embolism; P-gp: P-glycoprotein.
For patients with platelet counts <25 × 109/L, retrievable inferior vena cava filter placement is recommended over platelet transfusion and anticoagulant therapy.
The use of anticoagulant treatment is recommended for most patients with subsegmental PE.
NB: In patients with subsegmental PE and a high risk of bleeding (but no formal contraindication for anticoagulation), the possibility of avoiding anticoagulant therapy can be considered, provided the patient meets all the criteria: involvement of a single subsegmental artery, no concomitant DVT on imaging tests, adequate cardiopulmonary reserve, and no permanent risk factor for VTE.
Since chest multidetector CT angiogram allows better visualization of the peripheral pulmonary arteries, the generalized use of this technique has led to an increase in the detection of subsegmental PEs (with no involvement of the more proximal arteries) that now account for approximately 10%–15% of all diagnosed PEs19.
However, it is still unclear whether subsegmental PEs are false positives (radiological artefacts), and their clinical significance (if they represent more benign forms of the disease) has not been fully determined. In a meta-analysis of 14 studies, the rate of bleeds among patients receiving anticoagulation for subsegmental PE was 8.1% (no data are available on the group of patients who were not anticoagulated). The frequency of VTE recurrence at 90 days among anticoagulated patients was 5.3% vs. 3.9% among those who were not anticoagulated, with respective mortality rates of 2.1% vs. 3.0%, respectively20. In contrast, an analysis of two prospective studies that included a total of 116 patients with subsegmental PE (16% of all PEs) reported recurrent VTE in 3.6% of patients in the subsegmental PE group vs. 2.5% in patients with more proximal PE21.
Given the lack of robust evidence on the best management of subsegmental PE, the panel took into consideration in particular the low risk of associated major bleeds within 3 months of starting anticoagulation (particularly with DOACs [recommendation 5]). However, this is one of the scenarios that requires an individualized assessment of the risk of recurrence, the risk of bleeding, and patient preferences. For this reason, the use of anticoagulant treatment may be ruled out, particularly in patients whose symptoms suggest a greater risk of bleeding than usual (RIETE score [Appendix A Table S3]) if the risk of thrombosis is low (i.e., involvement of a single subsegmental artery, no concomitant DVT on imaging tests, adequate cardiopulmonary reserve and no permanent risk factor for VTE).
8. What are the indications for placing an inferior vena cava filter?Placement of a retrievable vena cava filter is not recommended in most patients with symptomatic acute PE.
A retrievable vena cava filter is recommended in patients with symptomatic acute PE and absolute contraindication for anticoagulation.
The results of clinical trials suggest that placing an inferior vena cava filter in patients with VTE reduces the risk of recurrence presenting as PE, increases the risk of recurrence presenting as DVT, and does not change mortality22,23. Based on these findings and on data from observational studies24, the panel recommends filter placement in patients with absolute contraindication for anticoagulation. Whenever possible, a retrievable filter should be placed and removed as soon as anticoagulation can be started.
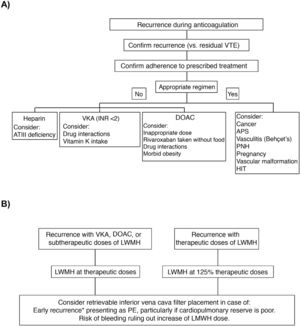
In a clinical trial that included 399 patients with symptomatic acute PE, concomitant DVT, and poor cardiopulmonary reserve, placement of an inferior vena cava filter did not significantly reduce VTE recurrence in the first 3 months of follow-up (RR, 2.0; 95% CI, 0.5–7.9), so routine use in this scenario is not recommended25. Clinical practice guidelines vary with respect to recommendations on the placement of an inferior vena cava filter in patients with VTE recurrences despite adequate anticoagulation. Fig. 2 shows an algorithm for the management of patients with VTE recurrences during anticoagulant therapy.
(A) Research and (B) management of patients with VTE recurrences during anticoagulant therapy.
*In the first 14 days after the index event.
APS: antiphospholipid syndrome; ATIII: antithrombin III; DAOC: direct acting oral anticoagulant; HIT: heparin-induced thrombocytopenia; INR: international normalized ratio; LMWH: low molecular weight heparin; PE: pulmonary embolism; PNH: paroxysmal nocturnal hemoglobinuria; VKA: vitamin K antagonists; VTE: venous thromboembolism.
Full-dose systemic fibrinolysis is recommended in most patients with symptomatic acute PE and indication for reperfusion treatment.
The use of percutaneous catheter-directed therapy (percutaneous thrombectomy, local fibrinolysis, or both) or reduced-dose systemic fibrinolysis is recommended in patients with symptomatic acute PE, indication for reperfusion treatment, and relative contraindications for the use of systemic fibrinolysis at full doses.
Surgical embolectomy or percutaneous catheter-directed treatment (percutaneous thrombectomy) is recommended in patients with symptomatic acute PE, indication for reperfusion treatment, and absolute contraindication for the use of full-dose systemic fibrinolysis.
Reperfusion treatment is indicated in patients with high-risk symptomatic acute PE (hemodynamically unstable) and patients with intermediate-to-high risk who deteriorate hemodynamically after starting anticoagulant therapy8 (Appendix A Table S4). Currently available reperfusion treatments are: systemic therapy (full-dose or reduced-dose systemic fibrinolysis), local catheter-directed therapy (percutaneous thrombectomy, local fibrinolysis, or both), and surgery (surgical embolectomy).
For patients with symptomatic acute PE and indication for reperfusion treatment, the panel recommends the use of full-dose systemic fibrinolysis. This recommendation is based on: 1) the results of a clinical trial in 34 patients with massive PE in which local administration of recombinant tissue plasminogen activator (rtPA) did not offer any significant benefit over systemic therapy26, and 2) the accumulation of robust evidence on the clinical benefits of full-dose systemic fibrinolysis27 and the limited (weak) evidence on the clinical benefits of reduced-dose systemic fibrinolysis, percutaneous catheter-directed therapies, or surgical embolectomy28,29.
However, between one third and one half of patients with acute PE have some contraindication for full-dose systemic fibrinolysis, and in up to 10% of cases it is not effective. For patients with absolute contraindications for fibrinolysis (Table 4), the therapeutic options are surgical embolectomy or percutaneous thrombectomy (without local fibrinolysis), depending on local experience and availability. For patients with no absolute contraindication for full-dose systemic fibrinolysis, but with a high perceived risk of bleeding, a network meta-analysis suggested that systemic fibrinolysis at reduced doses and catheter-directed local treatment were associated with the best risk/benefit balance30. Again, local experience and availability determine the choice of procedure. The BACS scale has been designed and validated to identify patients with acute PE with an increased risk of bleeding on fibrinolytic therapy31 (Appendix A Table S5).
Thrombolytic dosing and contraindications for use.
| Drug | Regimen | Contraindications |
|---|---|---|
| rtPA | Full dose: 100 mg in 2 h | Absolute |
| Reduced dose: 0.6 mg/kg (maximum 50 mg) | History of hemorrhagic or cryptogenic stroke | |
| Ischemic stroke in previous 6 months | ||
| CNS cancer | ||
| Major trauma, surgery, or TBI in the previous 3 weeks | ||
| Bleeding diathesis | ||
| Active bleeding | ||
| Relative | ||
| TIA in previous 6 months | ||
| Oral anticoagulation | ||
| Pregnancy or puerperium | ||
| Non-compressible vascular punctures | ||
| Traumatic CPR | ||
| Refractory hypertension (SBP > 180 mmHg) | ||
| Advanced liver disease | ||
| Infectious endocarditis | ||
| Active peptic ulcer | ||
| Streptokinase | Loading dose of 250,000 IU in 30 min, followed by 100,000 IU/h in 12–24 h | |
| Accelerated regimen: 1.5 million IU in 2 h | ||
| Urokinase | Loading dose of 4400 IU/kg in 10 min, followed by 4400 IU/h in 12–24 h | |
| Accelerated regimen: 3 million IU in 2 h | ||
CNS: central nervous system; CPR: cardiopulmonary resuscitation; rtPA: recombinant tissue plasminogen activator; SBP: systolic blood pressure; TIA: transient ischemic attack; TBI: traumatic brain injury.
Anticoagulation should be discontinued within 3 months of the first episode of PE secondary to a transient major risk factor that has been resolved.
Indefinite anticoagulation is recommended in patients with a persistent major risk factor (e.g., active cancer, antiphospholipid syndrome, history of 2 or more idiopathic thromboembolic events).
Indefinite anticoagulant therapy is recommended in men with idiopathic PE.
The use of additional tools (e.g. clinical characteristics, d-dimer, predictive scales, thrombophilia studies) is recommended to determine the duration of anticoagulant treatment in a) women with idiopathic PE, b) patients with PE due to a transient minor risk factor that has been resolved, c) patients wishing to discontinue anticoagulation (regardless of their risk of recurrence), and d) patients in whom the risk/benefit of indefinite anticoagulant therapy is unclear (e.g., men with idiopathic PE and high risk of bleeding).
After an episode of symptomatic acute PE, patients require a minimum of 3 months of anticoagulant treatment32. At that time, the decision on the duration of therapy should combine the risk of recurrence, the risk of bleeding, and the patient’s preferences. Although anticoagulation reduces the risk of recurrence by about 90%, its use increases the risk of major bleeding 2 to 6-fold. Moreover, anticoagulants only “protect” patients while they are using them8, so the decision consists not of establishing a defined treatment period, but of determining whether to discontinue anticoagulation or indicate it indefinitely.
The presence (or absence) and type of VTE risk factor is the most important variable in determining the duration of anticoagulation (Table 5). Patients diagnosed with PE due to a transient, major risk factor that has been resolved have a very low risk of recurrence when anticoagulation is discontinued33, and the panel recommends that it be discontinued after 3 months of treatment. At the other end of the spectrum are patients with a persistent major risk factor (e.g., active cancer, antiphospholipid syndrome, history of 2 or more idiopathic thromboembolic events). These patients have a very high risk of recurrence when anticoagulation is discontinued, and should receive treatment indefinitely, or until the risk factor has been resolved. Men with idiopathic PE have an intermediate risk of recurrence that lies between the 2 groups described above, and is 2.2 times higher than women, so we recommend that they received indefinite anticoagulation34.
Risk factors for PE.
| Risk factor |
|---|
| Transient |
| Major |
| Major surgery (general anesthesia >30 min) |
| Confined to hospital bed for medical cause ≥3 days |
| Trauma with fractures |
| Cesarean section |
| Minor |
| Minor surgery (general anesthesia <30 min) |
| Confined to bed at home for medical cause ≥3 days |
| Confined to hospital bed for medical cause <3 days |
| Use of estrogen/contraception |
| Pregnancy or puerperium |
| Long trips |
| Leg injury (without fracture) associated with reduced mobility for ≥3 days |
| Permanenta |
| Major |
| Active cancer |
| Antiphospholipid syndrome |
| History of 2 or more idiopathic thromboembolic episodes |
| Minor |
| Inflammatory bowel disease |
| Active autoimmune disease |
Source: adapted from Konstantinides et al.8.
Certain subgroups of patients may benefit from additional information (clinical characteristics, d-dimer, HERDOO2 rule, and thrombophilia studies) for a better assessment of the risk of recurrence when anticoagulation is discontinued (Appendix A Table S6):
- 1.
Women with idiopathic PE.
- 2.
Patients with PE due to a transient, minor risk factor that has been resolved (e.g., estrogen use, prolonged travel, immobilization, and minor surgery) (Table 5).
- 3.
Patients who wish to stop anticoagulation (regardless of their risk of recurrence).
- 4.
Patients with an unclear risk/benefit ratio for the use of indefinite anticoagulant therapy (e.g., men with idiopathic PE and high risk of bleeding).
In most patients with PE, thrombophilia studies to decide on the duration of anticoagulation are not recommended.
Antiphospholipid antibody determination before starting anticoagulation with DOACs for an episode of symptomatic acute PE is not recommended.
No clinical trials have been published that have evaluated the benefit of thrombophilia studies when deciding on the duration of anticoagulation in patients with PE35, and epidemiological data provide inconclusive results on the risk of recurrence associated with a positive hereditary thrombophilia study36.
For patients with PE due to a transient, major risk factor that has been resolved, the duration of anticoagulant treatment is 3 months (recommendation 10), and a positive thrombophilia study should not change this duration37. Patients with a permanent major risk factor (for example, cancer or a history of 2 or more idiopathic thromboembolic events) should receive anticoagulation indefinitely or until the risk factor has been resolved; thrombophilia studies are not indicated38.
In some patients, the risk/benefit ratio for the indefinite use of anticoagulant therapy is unclear, so the clinician can use certain prognostic tools to support their decision on the duration of anticoagulation (Appendix A Table S6). Of these tools, thrombophilia studies are of the least value when used in isolation.
Although the use of DOACs in patients with triple positive antiphospholipid syndrome (lupus anticoagulant, anticardiolipin antibodies, and anti-beta-2 microglobulin) is not recommended39, pre-screening was not performed in any of the clinical trials that evaluated the efficacy and safety of DOACs in the treatment of VTE. The panel does not recommend determining anti-phospholipid antibodies prior to initiating anticoagulation with DOACs for an episode of symptomatic acute PE, although it can be considered in young patients with idiopathic PE if underlying connective tissue disease is suspected.
Appendix A Table S7 provides information on hereditary and acquired thrombophilia studies.
12. Is screening for occult cancer indicated in patients with PE?Extensive screening (beyond medical history, physical examination, basic laboratory tests, and chest imaging tests performed to diagnose the PE episode) for occult cancer is not recommended in patients with provoked PE.
Extensive screening for occult cancer is not recommended in patients with idiopathic PE.
Approximately 5% of patients with idiopathic VTE will be diagnosed with occult cancer (i.e., not apparent at the time of diagnosis of VTE) in the year following the thromboembolic episode40. Screening for occult cancer could theoretically detect early-stage tumors and reduce cancer-related mortality. However, studies published to date have shown no clinical benefit for extensive screening compared to limited screening (medical history, physical examination, basic laboratory tests [complete blood count, biochemistry with liver enzymes and calcium, hemostasis and urine analysis], and chest imaging tests performed to diagnose the PE episode) in patients with VTE.
A meta-analysis of 10 studies with individual data from 2316 patients with idiopathic VTE followed for a minimum of 12 months after the thromboembolic episode40 reported that the prevalence of occult cancer was initially twice as high (95% CI, 1.2–3.4) in patients who underwent extensive screening (usually with computed tomography [CT] or positron emission tomography [PET]) compared with the group receiving limited screening, although there were no significant variations at 12 months (OR, 1.4; 95% CI, 0.9–2.1). Furthermore, there were no statistically significant differences in the proportion of patients with early-stage cancer or in cancer-related mortality, although most studies did not provide data on long-term mortality.
Depending on the evidence available, patients with VTE should only undergo limited screening that is appropriate for their age and gender (breast, colon, prostate, and uterus).
FundingThis consensus document has not received funding.
Conflict of interestsJosé Luis Lobo, Sergio Alonso, Juan Arenas, Pere Domènech, Pilar Escribano, Sonia Jiménez, María Lázaro and Manuel Monreal declare that they have no conflict of interest.
Carmen Fernández-Capitán has received speaking fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Leo-Pharma, Pfizer, Rovi and Sanofi and consultancy fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Leo-Pharma, Pfizer, Rovi, and Sanofi.
Luis Jara-Palomares has received speaking fees from Actelion Pharmaceuticals, Bayer, Leo Pharma, Menarini, Pfizer, and Rovi, and consultancy fees from Actelion Pharmaceuticals, Bayer, Leo Pharma, Menarini, Pfizer, and Rovi.
Ramón Lecumberri has received speaking fees from Boehringer Ingelheim, Daiichi-Sankyo, Leo-Pharma, Rovi, and Sanofi, and consultancy fees from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Leo-Pharma and Sanofi, and research support from Rovi.
Pedro Ruiz-Artacho has received speaking fees from Bristol-Myers Squibb, Daiichi-Sankyo, Leo-Pharma, Pfizer, and Rovi, and consultancy fees from Bristol-Myers Squibb, Daiichi-Sankyo, Leo-Pharma, Pfizer, and Rovi.
David Jiménez has received speaking fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Leo-Pharma, Pfizer, Rovi, and Sanofi, consultancy fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Leo-Pharma, Pfizer, Rovi, and Sanofi, and research support from GSK, Daiichi-Sankyo, Rovi, and Sanofi.
Sergio Alonso, Albert Antolín-Santaliestra, Juan Arenas, Antonio Castro-Fernández, Carlos Israel Chamorro-Fernández, Pere Domènech, Pilar Escribano, Carmen Fernández-Capitán, María Fernández-Velilla, Alberto García-Ortega, Olga Gavín, José Ramón González-Porras, Luis Jara-Palomares, David Jiménez, Sonia Jiménez, María Lázaro, Ramón Lecumberri, José Luis Lobo, Alicia Lorenzo-Hernández, Manuel Monreal, Ana Moretó, Raquel Morillo, Remedios Otero, Jorge Pedraza-García, Pascual Piñera-Salmerón, Juan Plasencia-Martínez, Ramón Puchades-Rincón-de Arellano, Pedro Ruiz-Artacho, Giorgina Salgueiro-Origlia, Teresa Sancho-Bueso, Carmen Trinidad-López, Yale Tung-Chen, and María Teresa Velázquez-Martín.