In addition to idiopathic pulmonary fibrosis (IPF), other diffuse interstitial lung diseases (ILD) are also associated with pulmonary fibrosis and occur in a variable proportion of patients, depending on the entity. The name given to this fibrotic component, that may progress despite treatment, is progressive pulmonary fibrosis (PPF). In this context, PPF is not an entity per se but a common clinical condition or behavior that may occur in association with different types of fibrosing diffuse ILDs, compromising patient prognosis. PPF is identified from worsening clinical, physiological, and/or radiological criteria during patient follow-up. Randomized clinical trials in patients with IPF or progressive non-IPF ILD have shown that treatment with antifibrotic drugs, either nintedanib or pirfenidone, slows progression. We are seeing the start of a new era in the clinical management of this subgroup of patients, offering the perfect opportunity for exploring still uncharted territories.
Además de la fibrosis pulmonar idiopática (FPI), otras enfermedades pulmonares intersticiales difusas (EPID) desarrollan fibrosis pulmonar, lo cual ocurre en una proporción variable en función de la entidad. Este componente fibrótico puede progresar a pesar de las medidas terapéuticas adoptadas, lo que se conoce como fibrosis pulmonar progresiva (FPP). En este contexto, la FPP no es una entidad per se sino una condición clínica o comportamiento común que pueden desarrollar diferentes EPID fibrosantes, y compromete el pronóstico del paciente. La FPP se identifica por criterios de empeoramiento clínico, fisiológico y/o radiológico durante el seguimiento del paciente. Ensayos clínicos aleatorizados en pacientes con FPI o EPID no-FPI progresiva han demostrado que el tratamiento con medicamentos anti-fibróticos, sea nintedanib o pirfenidona, enlentece su progresión. Se abre una nueva era en el manejo clínico de este subgrupo de pacientes y una ventana de oportunidad para investigar incógnitas aún existentes.
Pulmonary fibrosis arises from the uncontrolled accumulation of extracellular matrix in the lung parenchyma that hinders gas exchange.1–6 As this alteration progressively affects more alveolar-interstitial units, the lung loses its structure and function, leading to respiratory failure and death.1 Fibrosis is always present in idiopathic pulmonary fibrosis (IPF), a disease caused by an aberrant epithelial response, but appears to a greater or lesser extent in other diffuse interstitial lung diseases (ILD) as a result of an uncontrolled inflammatory response during disease development.7 Fibrosis may be suspected when certain clinical and functional characteristics and specific HRCT and/or histological signs are identified5–12 (Table 1). In addition to IPF, the main diagnostic options in a patient with fibrotic ILD are: (1) fibrotic hypersensitivity pneumonitis (fHP), (2) fibrotic ILD associated with systemic autoimmune disease (SAD), mainly rheumatoid arthritis and systemic sclerosis; (3) ILD associated with exposure to inorganic dust (pneumoconiosis), such as asbestosis; (4) fibrotic non-specific interstitial pneumonia (fNSIP); (5) unclassifiable ILD (uILD).5–12 Other ILDs may also present pulmonary fibrosis or progress to fibrosis (Fig. 1), though this is a rare occurrence. The key to obtaining a definitive diagnosis and optimizing the initial treatment of any fibrotic ILD is multidisciplinary evaluation, which involves analysis of HRCT (± histological) patterns together with a detailed history and comprehensive serological tests to assess causes or associations.1 If, despite this, diagnostic probability or confidence is less than 50% for a specific entity, or if the patient's clinical status makes it impossible to perform the studies required to achieve a provisional diagnosis, the case should be classified as fibrotic uILD, in other words, diffuse fibrotic interstitial lung disease that cannot be classified into any specific entity using the diagnostic tools currently available.13
Signs associated with pulmonary fibrosis.
| Symptoms | Bilateral Velcro cracklesFamilial aggregation |
| LFTs | Decline in FVC and/or TLCFEV1/FVC ratio>0.8 (in the absence of obesity, thoracic deformities, or neuromuscular disease) |
| Chest HRCT | Reticular pattern or septal thickening (non-nodular)Traction bronchiectasisHoneycombingReduction in lung volume (associated with any of the foregoing signs) |
| Lung biopsy | Collagen depositHoneycombingFibroblastic foci |
The clinical signs suggestive of pulmonary fibrosis or fibrosing ILD in a patient with progressive dyspnea on exertion and an interstitial pattern on chest X-ray are: family members with pulmonary fibrosis (familial aggregation), Velcro crackles on auscultation, decline in FVC, TLC or both, and FEV1/FVC ratio>0.8 (in the absence of other causes that could alter these variables, such as obesity, thoracic deformities, pleural thickening or neuromuscular disease). Signs of septal thickening, traction bronchiectasis, traction bronchiectasis, bronchiectasis or honeycombing, which are usually associated with reduced lung volume, on chest high-resolution computed tomography (HRCT). Of these, honeycombing has been shown to be irreversible. Although chest HRCT images are highly sensitive, septal thickening and traction bronchiectasis are also seen in inflammatory processes. Whenever lung biopsy is indicated and feasible, the observation of parenchymal destruction with collagen deposits confirms the existence of fibrosis. Microcystic honeycombing and fibroblastic foci are also observed in some cases. Histology confirms the suspicion of pulmonary fibrosis.
FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HRCT: high resolution computed tomography; LFTs: lung function tests; TLC: total lung capacity.
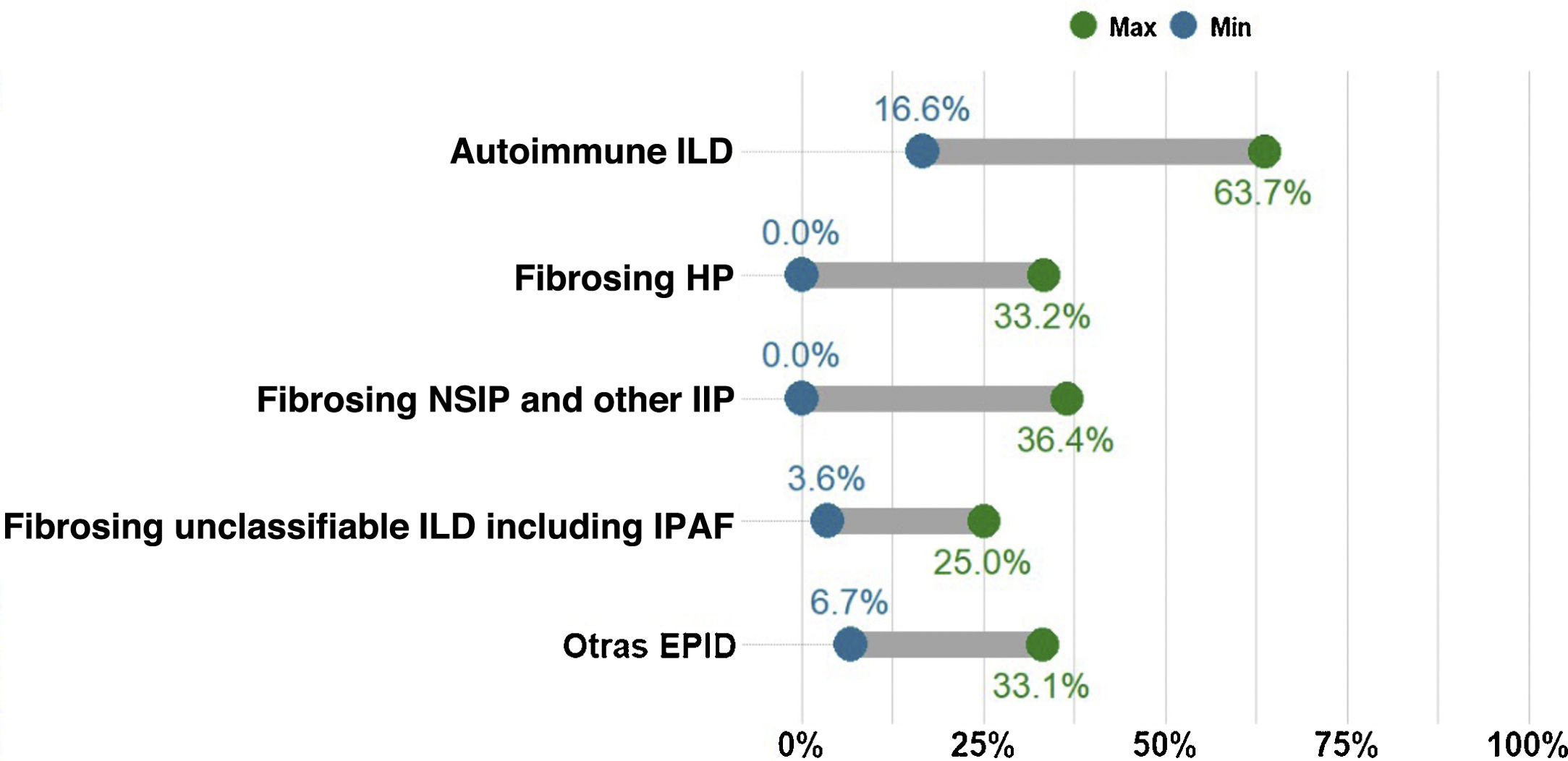
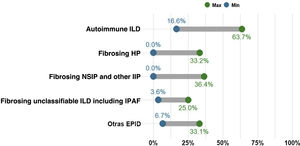
Percentage of cases with non-IPF ILD presenting with progressive pulmonary fibrosis. The proportion of patients with fibrotic ILD in a multidisciplinary ILD unit varies considerably. However, the most common entity is idiopathic pulmonary fibrosis (IPF), which is also always progressive. The chart shows the current percentage range of patients with different types of non-IPF progressing fibrotic ILDs, including fibrosing or chronic hypersensitivity pneumonitis (fHP), fibrosing autoimmune ILDs, idiopathic fibrosing non-specific interstitial pneumonia (fNSIP) and unclassifiable ILD. The group of other ILDs that present progressive pulmonary fibrosis includes pneumoconiosis (including asbestosis), fibrosing sarcoidosis, smoking-related interstitial fibrosis, and pleuroparenchymal fibroelastosis. Other entities that may also, though less frequently, present progression are cryptogenic organizing pneumonia (COP), acute interstitial pneumonia (AIN), interstitial pneumonia induced by drugs or respiratory infection (including COVID-19), or fibrotic forms of pulmonary Langerhans cell histiocytosis (PLCH). Finally, monogenic or hereditary ILDs, which are extremely rare, can start as pulmonary fibrosis between the age of 20 and 50 before progressing, often involving other organs or systems.
Advances in genetics over the past 20 years have led to the identification of genetic variants that increase the risk of, or even cause, pulmonary fibrosis (among other organ or tissue diseases). As these variants can be inherited, they often occur in a familial context,14 and include Hermansky-Pudlak syndrome (albinism and pulmonary fibrosis), telomeric syndromes (dyskeratosis congenita, hematological and liver diseases, signs of premature aging, cancer and pulmonary fibrosis), and surfactant deficiency syndromes (infant respiratory distress syndrome, immunodeficiency-induced infections, cancer, alveolar proteinosis and pulmonary fibrosis).
Concept 2: Progressive pulmonary fibrosisDifferent terms have been used in recent years to describe patients with ILD who present worsening and fibrotic progression, such as progressive fibrotic ILD, progressive fibrosing phenotype, or progressive pulmonary fibrosis (PPF). We consider the term PPF to be the most appropriate as it simply and directly encompasses the complete definition of this condition while avoiding any possible scientific ambiguities associated with the term “phenotype”. PPF is a common evolutionary clinical characteristic of different types of fibrotic ILD, and is therefore not a diagnosis or clinical entity per se.5–14 It includes patients with IPF and also those with other non-IPF fibrotic ILDs that progress despite appropriate etiological treatment. The number of patients presenting PPF for each type of fibrotic ILD is unknown, but estimates made in recent studies vary from 2.2% to 28%, with PPF being more common in fibrotic uILD and less so in fibrosing sarcoidosis7,12 (Fig. 1). In clinical practice, we refer to patients who present with a specific ILD that meets PPF criteria (e.g., patient with fHP who meets criteria for progressive pulmonary fibrosis).
IPF is progressive and irreversible, and estimated median post-diagnosis survival is 4–5 years.1 Our understanding and treatment of this disease have progressed considerably after more than 3 decades of translational research. The most significant advances include1–4:
- 1)
Identification of fibrogenesis mechanisms that can be regulated in vitro and in vivo, and the development of new drugs that act on these pathogenic mechanisms to inhibit fibrosis.2 Nintedanib and pirfenidone, the only anti-fibrotic drugs currently indicated and approved for these patients, have shown benefit in controlled clinical trials.1
- 2)
Improvements in clinical management, including definitive diagnosis, identification of poor prognostic factors, optimized follow-up, and comprehensive treatment.1
PPF in non-IPF ILD shares clinical, prognostic, and even pathogenic features with IPF, so many of the advances made in IPF are applicable to non-IPF PPF.9–13 For many years now, the criteria for identifying progression of pulmonary fibrosis initially developed for IPF (decline in forced vital capacity [FVC] and diffusing capacity of carbon monoxide [DLCO], increased fibrotic signs on chest HRCT) have also been used to identify progression in other non-IPF ILDs.5,6,8 Decline in FVC is a validated criterion for progression and a predictor of mortality in IPF and other fibrotic ILDs, such as fHP10 and fibrotic ILD associated with rheumatoid arthritis or systemic sclerosis.11
A study comparing the evolution of patients in the placebo arm of the INPULSIS (patients with IPF) and INBUILD (patients with progressing non-IPF fibrotic ILD) clinical trials showed that the annual rate of decline in FVC (−221.0ml/year and −192.9ml/year, respectively; p=0.19) and the proportion of subjects presenting>10% or >5% decline in FVC at 12 months were very similar.11 In this context, patients with fHP, autoimmune ILD, fNSIP, among others, that can potentially improve or stabilize with appropriate management, may present PPF despite anti-inflammatory, immunosuppressive treatment or elimination of the causal agent5–10 (Fig. 1). Half of all patients with PPF included in the INBUILD clinical trial presented fHP or autoimmune ILD.
Progression of pulmonary fibrosis or PPF can appear at any time; it can be observed from diagnosis, or the patient can appear to be stabilized, or even show some improvement, months or years later present progression.
Although the criteria for defining progression have yet to achieve international consensus, PPF is usually diagnosed when the patient presents at least 2 of the following changes:
- 1)
Worsening of respiratory symptoms (cough/dyspnea), with no another cause or added dysfunction.
- 2)
Absolute 5–10% decline in forced vital capacity (FVC) and/or absolute≥10% decline in diffusing capacity of carbon monoxide (DLCO).
- 3)
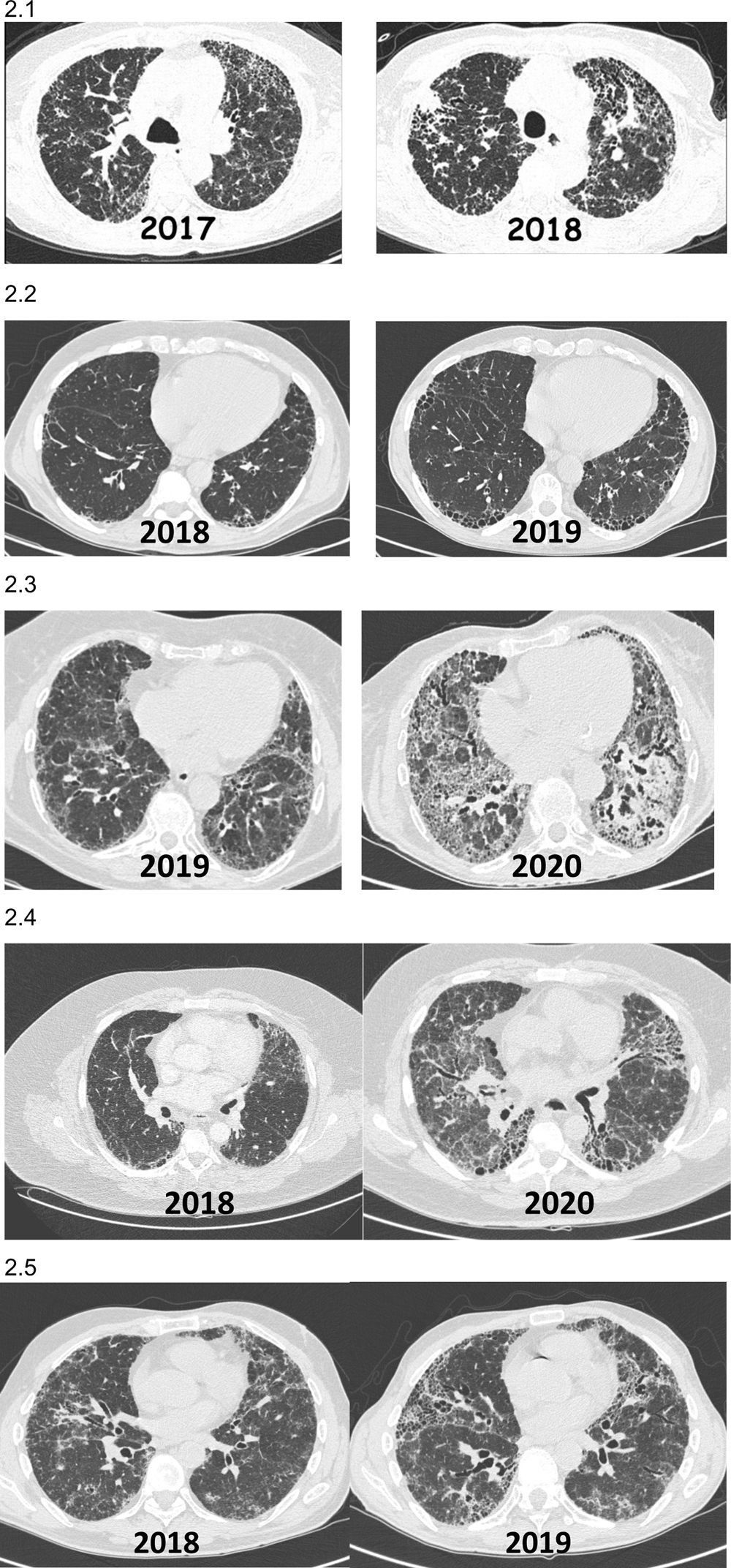
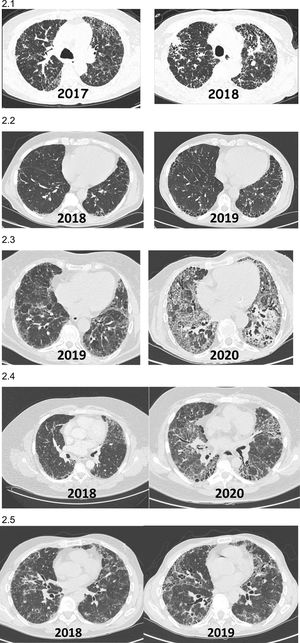
Increased extent of fibrosis on imaging study and/or appearance of previously unobserved fibrosis on imaging study (Fig. 2).
Fig. 2.Images (chest HRCT) of patients with non-IPF fibrotic ILD who present PPF during their clinical course. (2.1) Patient with hypersensitivity pneumonitis diagnosed in 2017 who presented a significant progression on HRCT and reduced pulmonary function after 1 year of follow-up. (2.2) Patient with rheumatoid arthritis-ILD (UIP) diagnosed in 2018 in whom 1-year HRCT showed progression of fibrosis with increased respiratory symptoms, despite being in remission from arthritis with methotrexate+abatacept. (2.3) Patient with Sjögren's syndrome-ILD diagnosed in 2019 in whom 1-year HRCT showed progression of fibrosis and loss of lung function despite mycophenolate mofetil+prednisone. (2.4) Patient with pulmonary fibrosis secondary to uILD who presented fibrosis progression 24 months after diagnosis. (2.5) Patient with chronic fibrosing hypersensitivity pneumonitis that worsened clinically and radiologically 12 months after diagnosis, despite avoiding exposure and taking corticosteroids and mycophenolate.
The follow-up period in which these changes should be observed to determine PPF has yet to be clearly established, but in most cases, worsening is observed at 6–12 months, although fibrotic progression may appear even earlier. Either way, it is important to identify progression as soon as possible.
Pathogenesis of progressive pulmonary fibrosisThe cellular and molecular mechanisms involved in the progression of pulmonary fibrosis are still under investigation. Regardless of the origin or cause of pulmonary fibrosis, once it is established and has progressed, a positive feedback loop is created that can drive progression even when the causative factors have been treated or avoided.15,16 Several environmental and host risk factors are thought to play an important role in fibrosis.17–30 Initial studies suggest that air pollution levels could contribute to the progression of these diseases, although the exact mechanism remains unclear.36 Some of the primary patient-related factors are genetic alterations and the mechanisms associated with accelerated aging.17,19–29 Aging is a complex biological process characterized by various changes, including genomic instability, cellular senescence, telomere shortening, mitochondrial dysfunction, and epigenetic reprogramming.19 Several of these mechanisms have been described in fibrotic ILD, and are associated with fibrotic progression.2,20,21 A recent experimental study showed that senescence of alveolar epithelial type II cells is sufficient to trigger PPF characterized by pathological changes similar to usual interstitial pneumonia (UIP).22 Fibroblasts can also become senescent, a process that could help these cells evade apoptosis and promote other profibrotic characteristics.23,24 A novel single-cell RNA sequencing technique has identified activated subpopulations of profibrotic epithelial cells, fibroblasts and macrophages in PPF that could drive progression to the point of no return.25–27 Finally, several studies have shown that gradual stiffening of the extracellular matrix at sites of injury plays a key role in progression.28,29 This rigid matrix stimulates fibroblast activity by generating positive feedback loops that involve the activation and release of TGF-β, which in turn activates several signaling pathways that stimulate expression of profibrotic genes, resulting in a progressive profibrotic circuit.28–30
Management of progressive pulmonary fibrosisRisk factorsIdentifying predictive factors for PPF continues to be the subject of intense research. There is no way of accurately predicting which patients will present PPF after a confirmed diagnosis of ILD; however, certain clinical and biological features have been associated with an increased risk of progression, such as active smoking, the presence of an HRCT or histological UIP pattern, the existence of familial aggregation in the clinical history, and telomere dysfunction.17,31–42
An imaging pattern consistent with UIP includes the presence of subpleural reticular thickening, traction bronchiectasis and honeycombing predominantly in the lower lobes, and scant or no ground glass opacification.1 This HRCT pattern is typical of IPF, but can also be found in other fibrotic ILDs.1 UIP in rheumatoid arthritis-associated ILD is a predictor of likely progression and a poor outcome.31,32 A recent study in 1330 patients with fibrotic ILD of various causes, including fHP, autoimmune ILD, IPF and uILD, showed that honeycombing, present in 42.0%, 41.9%, 37.6% and 28.6% of patients, respectively, was associated with higher mortality.33 Some years ago, an abundance of fibroblastic foci in lung biopsy, which is characteristic of UIP but can also be found in other histological patterns such as fHP, was proposed as a prognostic factor.34 However, there is no solid evidence to confirm this suggestion.35
Familial aggregation or familial pulmonary fibrosis is defined as the existence of 2 or more patients with pulmonary fibrosis in the same family, and is associated with a greater likelihood of genetic alterations, telomere dysfunction, and possible inheritance of the disease.36 Telomeres are nucleoprotein complexes found at the ends of chromosomes that shorten with each cell division; their progressive shortening is associated with aging.36–40 Accelerated or premature telomere shortening contributes to the development of degenerative diseases such as IPF.36–38 A severe reduction in telomere length correlates with a worse prognosis not only in IPF, but also in interstitial pneumonia with autoimmune features (IPAF), autoimmune ILD, and fHP.36–42 The presence of mutations in telomere-related genes in patients with fibrotic ILDs is associated with an increased risk of mortality, irrespective of the etiology of the underlying disease.38 Similarly, a recent study has shown that patients with non-IPF fibrotic ILD and familial aggregation have similar survival to patients with IPF, which is significantly worse than patients with sporadic non-IPF fibrotic ILD.17
Serum biomarkers in progressive pulmonary fibrosisAt present, PPF can only be identified after a series of functional and HRCT studies have been performed and compared over a period of several months,5 and there is therefore a pressing need for biomarkers that can identify the turning point of fibrotic progression, or even predict the likelihood of progression at the time of diagnosis.30 Some studies in patients with PPF, mostly retrospective with small sample sizes and limited validation, have suggested serum markers that could be helpful in identifying patients with non-IPF fibrotic ILD with a higher risk of progression (Table 2).43–50 A recent study found that serum levels of Krebs von den Lungen-6 (KL-6), a glycoprotein located in alveolar epithelial cells, are significantly elevated in patients with different fibrosing ILDs who present PPF, and suggested that a baseline KL-6 value of ≥800U/ml could be an independent risk factor.47 Another study that evaluated patients with systemic sclerosis-ILD also found greater lung function decline in patients with elevated KL-6 levels.48 In other studies, serum epididymal glycoprotein 4 (HE4) levels of over 238pmol/l have been associated with higher mortality.49 Likewise, low serum levels of the soluble receptor for glycation end products (sRAGE) in patients with fHP and IPF have been associated with greater alveolar epithelial injury and greater progression.50
Serum markers that have shown some association with pulmonary fibrosis progression variables.
| Serum biomarkers | ILD evaluated | |
|---|---|---|
| Epithelial dysfunction | KL-6 | NSIP, fHP, autoimmune ILD |
| SP-A, SP-D, YKL-40 | Autoimmune ILD | |
| sRAGE | fHP | |
| ECM remodeling | MMP-7, periostin | fHP, fNSIP |
| MMP-12, TIMP-1, CCL16, tenascin-C, suPAR | Scleroderma | |
| CA19-9, CA125, VCAM1 | Autoimmune ILD, HP, sarcoidosis | |
| Immune dysregulation | S100A9 | NSIP |
| CCL2, CCL18, CXCL4, CXCL10, CX3CL1, IL6, IL2, CRP | Scleroderma | |
| CXCL13 | Autoimmune ILD, fHP | |
| Anti-MX1 | NSIP | |
| Chitotriosidase | Sarcoidosis | |
| Glycoprotein | ||
| Other | HE4 | PPF |
CA19.9 and CA125: nonspecific tumor markers; CCL: chemokine ligand; CRP: C-reactive protein; CXCL: CXC chemokine ligand; ECM: extracellular matrix; fHP: fibrosing hypersensitivity pneumonitis; HE4: human epididymis protein 4; ILD: interstitial lung disease; IL: interleukin; KL-6: Krebs von den Lungen-6; PPF: progressive pulmonary fibrosis; autoimmune ILD: autoimmune interstitial lung disease; MMP: metalloproteinase; MX1: Myxovirus 1; NSIP: non-specific interstitial pneumonia; suPAR: soluble urokinase plasminogen activator receptor; SP-A and SP-D: surfactant protein-A and D; sRAGE: soluble receptor for soluble receptor for glycation end products; S100A9: S100 calcium-binding protein A9; TIMP: tissue inhibitor of MMP; VCAM1: vascular cell adhesion molecule 1; YKL-40: chitinase-3-like protein 1.
However, no serum biomarker has yet been approved for clinical use in PPF. Research and international multicenter studies currently in progress will provide solid evidence and validation for serum biomarkers that can identify, or even predict, the biological progression of pulmonary fibrosis before these changes are observed on functional or HRCT studies.
PharmacotherapyThe management of PPF, which is the primary cause of death in these patients, is a clinical challenge. It is important to take a comprehensive approach to treatment, taking into account various prognostic factors (comorbidities, physical and mental status, vaccination, and protection measures against respiratory infections), nutritional and welfare requirements, and pharmacotherapy that specifically targets the fibrotic process.2,6 Drugs for the treatment of IPF have only been available for the past 10 years or so.2 The PANTHER clinical trial confirmed that the empirical therapy with high-dose corticosteroids and immunosuppressants that had been used for several years was not only ineffective, but was also associated with greater morbidity and mortality.51 Other clinical trials and meta-analyses, meanwhile, found that pirfenidone and nintedanib significantly slowed FVC decline at 12 months, and these antifibrotics were then approved for IPF.52,53 Nintedanib is a potent tyrosine kinase inhibitor that reduces the synthesis of profibrotic mediators such as fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) and vascular endothelial factor (VEGF). Pirfenidone inhibits the production of collagen and pro-fibrotic growth factors, such as transformer-beta (TGF-beta) and PDGF.52,53
Subsequent studies investigated the efficacy and safety of these drugs in patients with non-IPF fibrotic ILD who presented progression despite receiving appropriate etiological treatment. In this context, the INBUILD double-blind phase 3 trial evaluated the use of nintedanib 150mg/12h vs. placebo in 663 patients with non-IPF fibrotic ILD from different continents and ethnicities who had progressed at any time within the 24 months prior to inclusion.54 The patients were stratified according to the existence of a UIP pattern on imaging studies. The annual rate of decline in FVC was significantly lower among patients who received nintedanib, both in the overall population (−80.8ml with nintedanib versus −187.8ml with placebo) and in the subgroup with a UIP fibrotic pattern (−82.9ml with nintedanib versus −211.1ml with placebo). A subsequent post hoc analysis of the rate of lung function decline by ILD category found that the improvement in the rate of decline, though variable, was independent of the type of fibrotic ILD evaluated.55 The efficacy and safety of pirfenidone has also been investigated in non-IPF progressing fibrotic ILD.56,57 A phase II randomized clinical trial in 253 patients with progressive unclassifiable ILD evaluated the effect of pirfenidone at 6 months.56 Although the main objective, change in FVC (ml) measured by a home spirometer, did not show significant differences, the rate of decline in both FVC% and DLCO% measured in the hospital (secondary objectives) was significantly lower in the group that received pirfenidone.56 Despite its early termination, the RELIEF phase IIb randomized clinical trial evaluated 127 patients with fHP, autoimmune ILD, asbestosis and fNSIP who met the criteria for PPF. The results showed that pirfenidone reduced the rate of FVC decline (p=0.043) in all ILDs analyzed.57 Anti-fibrotics, therefore, reduce progression of PPF in both IPF and non-IPF fibrotic ILD. A certain number of patients with PPF develop pulmonary arterial hypertension (PAH), which is associated with decreased quality of life and early mortality.58 A recent randomized clinical trial in 326 patients with pulmonary fibrosis and severe PAH evaluated the effect of inhaled treprostinil, a prostacyclin analog,59 and found that a 16-week course improved exercise capacity (distance achieved in the 6-minute walk test) and reduced the risk of clinical worsening and exacerbations.59
Several clinical trials exploring different combination therapies for PPF have been planned or are already underway (Table 3).60
Ongoing or planned clinical trials in patients with progressive pulmonary fibrosis.
| Clinical trial (identifier) | Disease | Investigational drug | Primary outcome measure |
|---|---|---|---|
| NCT02958917 | Fibrotic HP | Pirfenidone | Change in FVC in 1 year |
| PirFS-NCT03260556 | Fibrotic sarcoidosis | Pirfenidone | Change in FVC in 1 year |
| NCT03820726 | Various ILDs | Nintedanib | Incidence of adverse effects (extension of INBUILD) |
| NCT03385668 (PIRFENIVAS) | ILD associated with anti-MPO | Pirfenidone | Change in FVC in 1 year |
| NCT03856853 | SS-ILD | Pirfenidone | Change in FVC in 1 year |
| NCT03857854 | Dm-ILD | Pirfenidone | Change in FVC in 1 year |
| NCT03313180 | SS-ILD | Nintedanib | Incidence of adverse effects |
| NCT02808871 (TRAIL 1) | RA-ILD | Pirfenidone | Change in FVC or mortality |
| NCT03221257 (SLS III) | SS-ILD | Pirfenidone and mycophenolate | Change in FVC in 18 months |
| NCT04193592 (PEARL) | Hermansky-Pudlak | Pirfenidone | Decline in FVC≥10% at 6 and 12 months |
| NCT04325217 | SS-ILD | Nintedanib | Incidence of adverse effects |
| NCT04161014 (NSIPPS) | Pneumoconiosis | Nintedanib | Change in FEV1 in 36 months |
Dm: dermatomyositis; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ILD: interstitial lung disease; MPO: myeloperoxidase; RA: rheumatoid arthritis; SS: systemic sclerosis.
PPF, a characteristic or progression of various fibrotic ILDs, is identified during patient follow-up using respiratory, clinical, physiological and imaging criteria. Accurately diagnosing the etiology of fibrotic ILD is the key to optimizing the initial therapeutic approach. A finding of PPF during follow-up should be treated with anti-fibrotics in order to slow progression (mild to moderate phase); advanced cases will require lung transplantation, if feasible.
Conflict of interestThe authors declare that they have no conflict of interest for the development of this document.