To describe a 10-year experience of inserting Ultraflex™ self-expanding metal stents (SEMS) under sedation using flexible bronchoscopy for the treatment of malignant tracheobronchial stenosis in a tertiary referral center.
MethodsMedical notes were retrospectively reviewed for all patients who underwent SEMS insertion between 1999 and 2009.
ResultsA data analysis of 68 patients who had Ultraflex™ SEMS inserted under sedation was completed. Thirty-three males and 35 females with a mean age of 67.9 years (range 35–94) presented with features including dyspnea/respiratory distress (39 patients), stridor (16 patients), and hemoptysis/dyspnea (13 patients). Etiology of stenosis included lung cancer (46 patients), esophageal cancer (14 patients), and other malignancies (8 patients). Mean dose of midazolam administered was 5mg (range 0–10mg). The trachea was the most common site of stent insertion followed by the right and left main bronchus, respectively. Adjuvant laser therapy was applied at some stage in 31% of all cases, and chemotherapy and/or radiotherapy was administered to at least 64% of patients with malignant disease.
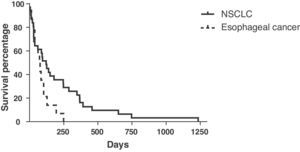
Hemoptysis and stent migration were the most frequent complications (5 and 4 patients, respectively). The mean survival time of stented non-small cell lung cancer (NSCLC) patients was 214 days (range 5–1233) and that of esophageal malignancy was 70 days (range 12–249). Mean pack-year history of individuals with lung cancer requiring stent insertion was 37 (range 2–100).
ConclusionUltraflex stents offer a safe and effective therapy for patients who are inoperable or unresectable that otherwise would have no alternative therapy. It has an immediate beneficial effect upon patients, not only through symptom relief but also, in some, through prolongation of life. Survival data are no worse than other studies using different varieties of stents and insertion techniques indicating its longer-term efficacy. Moreover, this report highlights the feasibility of performing this procedure successfully in a respiratory unit, without the need for general anesthesia.
Describir la experiencia de 10 años con la implantación de prótesis metálicas autoexpansibles (PMAE) Ultraflex™ bajo sedación, con el empleo de broncoscopia flexible para el tratamiento de las estenosis traqueobronquiales malignas en un centro de referencia terciario.
MétodosSe revisaron retrospectivamente las historias clínicas de todos los pacientes a los que se implantó una PMAE entre 1999 y 2009.
ResultadosSe llevó a cabo un análisis de los datos de 68 pacientes a los que se implantó una PMAE Ultraflex™ bajo sedación. Un total de 33 varones y 35 mujeres, de una media de edad de 67,9 años (rango: 35-94), presentaron manifestaciones clínicas consistentes en disnea/dificultad respiratoria (39 pacientes), estridor (16 pacientes) y hemoptisis/disnea (13 pacientes). La etiología de la estenosis fue la siguiente: cáncer de pulmón (46 pacientes), cáncer de esófago (14 pacientes) y otras enfermedades malignas (8 pacientes). La dosis media de midazolam administrada fue de 5mg (rango: 0-10mg). La tráquea fue la localización más frecuente de la implantación de la prótesis, seguida del bronquio principal derecho e izquierdo, respectivamente. Se utilizó laserterapia adyuvante en alguna fase en el 31% del total de casos y se usó quimioterapia y/o radioterapia en al menos el 64% de los pacientes con enfermedades malignas.
La hemoptisis y la migración de la prótesis fueron las complicaciones más frecuentes (5 y 4 pacientes, respectivamente). La media de tiempo de supervivencia en los pacientes con cáncer de pulmón no microcítico a los que se implantaron prótesis fue de 214 días (rango: 5-1.233 días), y la de los pacientes con cáncer esofágico fue de 70 días (rango: 12-249 días). La media de consumo de tabaco en paquetes-años en los individuos con cáncer de pulmón en los que fue necesaria la implantación de una prótesis fue de 37 (rango: 2-100).
ConclusiónLas prótesis Ultraflex proporcionan un tratamiento seguro y eficaz para los pacientes inoperables o con tumores inextirpables en los que no se dispone de ninguna otra terapia alternativa. Tienen un efecto inmediato que proporciona una acción beneficiosa a los pacientes, no solo por el alivio de los síntomas, sino también en algunos casos por la prolongación de la vida. Los datos de supervivencia no son peores que los presentados en otros estudios en los que se han utilizado variedades diferentes de prótesis y distintas técnicas de implantación, lo cual indica su eficacia a más largo plazo. Además, este análisis subraya la viabilidad de prestar este servicio de manera satisfactoria en una unidad respiratoria, sin necesidad de anestesia general.
When there is an obstruction of the central airway, patients present respiratory difficulty after hours or days of breathlessness. Although surgical resection with reconstruction is the reference treatment, in many patients extirpation is not possible or there are important comorbidities that are an impediment for performing this method of treatment. Interventional bronchoscopy with stent insertion in the airways is an alternative treatment that may save the lives of these patients.
There are several interventional bronchoscopy techniques that provide palliation for central airway obstruction. In general, benign disease is treated with balloon dilation, with or without laser therapy.1 Malignant disease is treated with laser vaporization, electrocautery, brachytherapy, cryotherapy, resection of the internal part of the tumor or photodynamic therapy with or without implantation of prosthesis.1 The insertion of stents in the airways also has a useful application in stenoses secondary to an extrinsic compression by a tumor, goiter, lymph nodes or vascular anomalies.
In our unit, we have used Ultraflex™ stents. These are nitinol (nickel and titanium alloy) self-expanding stents that have an elastic flexibility superior to that of steel and respond better to tensions, such as that caused by coughing. The stent, which resists longitudinal elongation when compressed externally due to its mesh design, is also easier to deploy, adapt, and extract than other metallic stents.1–3 In this paper, we describe our experience in the insertion of this type of prosthesis (coated or uncoated) using flexible bronchoscopy and sedation, and we highlight the beneficial effects of its use in the management of inoperable stenoses of the central airways.
Material and MethodsThe Department of Respiratory Medicine at Northern General Hospital in Sheffield offers tertiary reference health care for the evaluation and management of patients with tracheobronchial stenoses who are not candidates for surgery. We reviewed all the cases of self-expanding metallic stent (SEMS) placement in our department since 1999. This review included medical files, pathology reports and bronchoscopy reports of each patient in order to identify key data, such as the demographic characteristics of the patient, clinical presentation, indication for stent placement, location and size of the prosthesis, sedation administered, adjuvant treatment, initial complications, and survival time.
In our unit, our patients first underwent flexible bronchoscopy to examine the location and the degree of the stenosis before stent insertion. All patients were sedated with intravenous midazolam, and topical xylocaine aerosol was applied to the pharynx. The patients received additional oxygen with nasal cannula (2–6l/min), and oxygen saturation was monitored by an oximeter coupled with a digital catheter. The bronchoscope was introduced into the mouth through a mouth protector and situated above the stenosis or the obstructive lesion of either the trachea or the main bronchus.
Afterwards, a flexible guide was pushed through the biopsy canal of the bronchoscope until a position that was distal to the stenotic region. Then, the bronchoscope was withdrawn over the guide, which was carefully maintained in position within the tracheobronchial tree. The bronchoscope was once again introduced through the mouth and placed proximally to the stenosis. The application catheter, which maintained the prosthesis compressed, was introduced along the guide until situated in the area of the stenosis. Once in place, the stent was released from its application catheter by means of traction of the nylon thread and the circular knotted mesh of the stent was uncovered.3 At this time, the catheter was withdrawn from the application system. The diameter of the stent required was calculated based on the thoracic computed tomography (CT) images prior to bronchoscopy. Adjuvant therapy was used, such as laser therapy, either before or after the stent insertion, as necessary.
The position of the prosthesis was verified immediately by bronchoscopy during the intervention and once again 24h later by chest radiography. Afterwards, follow-up was carried out at regular intervals in our center.
ResultsThe medical files of 68 patients were reviewed: 33 males and 35 females, with a mean age of 67.9±12.7, all of whom received Ultraflex™ stents between 1999 and 2009.
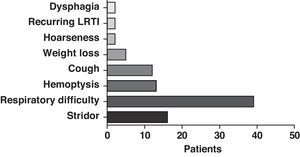
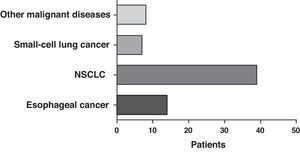
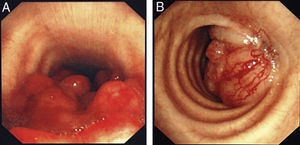
The most frequent form of presentation was one of the following manifestations: dyspnea/respiratory distress (36 patients), stridor (15 patients) or hemoptysis/dyspnea (13 patients) (Fig. 1). All the cases had inoperable disease. The WHO functional state was 0–2 in all cases, except for 4 patients with a WHO level 3. In the majority of the patients, there was no information available about the stage, but in those who did have such information, approximately 60% had tumor metastases at the time of the stent placement. The most frequent cancer responsible for the stenoses was non-small-cell lung cancer (NSCLC) (39 patients), followed by spinocellular carcinoma of the esophagus (14 patients), small-cell lung cancer (SCLC) (7 patients) and one case each of other malignant diseases (8 patients), including pleomorphic adenoma, mesothelioma, thyroid cancer, non-Hodgkin's lymphoma (NHL), atypical carcinoid, melanoma, adenoid cystic carcinoma, and metastasis of intestinal adenocarcinoma (Fig. 2). The bronchoscopic aspect corresponded with external compression in 18 patients, 6 of whom also had esophageal tumor infiltration in the airways. In the other patients there was endoluminal tumor growth that was important enough to cause respiratory symptoms (Fig. 3). Although the exact degree of endoluminal affectation was measured and commented on at the multidisciplinary clinical sessions, in the retrospective review of the medical files this information was not made available to us.
A wide variety of stent sizes was used, from 2 to 8cm long and between 8 and 20mm wide. 94% of the prostheses were uncoated (Fig. 4) instead of coated.
Adjuvant therapy with laser was used in 21 patients (31%). In at least 44 patients (64.7%) with malignant diseases, treatment was established with chemotherapy and/or radiotherapy. These data are an underestimation, as we did not have all the oncology data due to the tertiary reference center character of our participation.
For the insertion of the stents, the mean dose of midazolam used in the course of this analysis was 5mg (range: 0–10mg). After deploying the stent, satisfactory expansion and dilation of the airway was obtained in all cases. The symptoms of the patients improved immediately after the expansion of the prosthesis in all cases (except those who experienced early complications–see below), especially dyspnea, stridor, and respiratory difficulty. This effect was clearly determined by questioning the patients after the intervention, but it was also objectively observed by the improvement in oxygen saturation measured by oximetry.
The most frequent location for the implantation of the prosthesis was the trachea (25 patients), followed by the right main bronchus (22 patients), and the left main bronchus (21 patients).
Complications included hemorrhage originating in the area of the stent placement during the insertion itself (which required the use of local adrenalin in 1 patient), mild hemoptysis (5 patients), stent migration (4 patients), severe granulation tissue (3 patients), pneumonia (2 patients), odynophagia, respiratory failure, stent occlusion, and deployment failure (1 patient in each case). There were no episodes of fistula formations, lobe collapse, pneumothorax or sudden death.
The mild hemoptysis was resolved spontaneously in all the cases, with no need for other interventions. All the cases of stent migration occurred within the first 24h and required another flexible bronchoscopy to re-situate the prosthesis. The formation of severe granulation tissue required another flexible bronchoscopy and the ablation of this tissue with laser. The cases of respiratory failure and deployment failure were approached immediately in the same session, with satisfactory results. The occlusion of the stent by granulation tissue required the extraction and substitution of the stent using rigid bronchoscopy.
Mean survival of the patients with NSCLC treated with stent insertion was 214 days (range: 5–1233 days) (Fig. 5). The data available were limited for the survival of the patients with microcellular carcinoma: 2 patients continued to survive (>5 years); 2 patients had a mean survival of 101 days; and no data were available on the other 3 patients, as ours is a tertiary reference center. The mean survival of the patients with esophageal cancer treated with prosthesis was 70 days (range: 12–249 days).
A history of smoking was documented in 40 of the 46 patients with lung cancer. The mean pack-years smoked in these patients requiring stent placement was 37 (range: 2–100 pack-years).
DiscussionOur retrospective analysis of the use of SEMS with flexible bronchoscopy is the most extensive study to analyze the insertion of Ultraflex™ stents under sedation with flexible bronchoscopy. Previous studies have examined the use of rigid bronchoscopy under general anesthesia in this type of patients, or they analyzed different SEMS, like Gianturco or Wallstent.1,2,4–6
We observed that the presence of dyspnea/respiratory difficulty and stridor were the most frequent manifestations of presentation, and that vigilance is needed in patients with unexplained severe dyspnea. Saad et al.6 observed that the patients in whom they implanted SEMS (Wallstent and Ultraflex) presented predominantly dyspnea/respiratory difficulty (53%), stridor (22%), or the combination of both (19%).
Our analysis showed that the most frequent etiology of airway stenosis was NSCLC (39), followed by esophageal cancer (14) and SCLC (7).
Husain et al.,5 who reported their experience with inserting Ultraflex SEMS in 66 patients with benign and malignant diseases, also indicated that non-small-cell carcinoma was the most frequently diagnosed type of lung cancer. It was approximately 12 times more common than SCLC (2 cases). Esophageal cancer was the cause in 8 of the cases that required stent implantation. Likewise, Wilson et al.4 observed that NSCLC had a frequency that was almost 10 times higher than small-cell cancer in their patients who needed stents.
The most frequent location of the stent placement was the trachea (27 patients), followed by the right main bronchus (25 patients) and the left main bronchus (23 patients). Wilson et al.4 used Gianturco SEMS with flexible bronchoscopy in patients with lung cancer, and they inserted the majority of the prostheses trachea, followed by the right and left bronchial tree. In contrast, Breitenbücher et al.2 implanted Ultraflex stents in 60 patients using rigid bronchoscopy and general anesthesia. The stents were inserted in the trachea in 5 patients, and in the right main bronchus (RMB) and the left main bronchus (LMB) in ten cases each. Stockton et al.7 implanted Gianturco SEMS with rigid bronchoscopy in the trachea in 47 of their patients, and in the right and left main bronchi in 43 and 38 patients, respectively. The size of the stents inserted in our patients agrees with that indicated in other SEMS studies. We mainly used uncoated stents, which have certain advantages over covered stents for maintaining airway permeability at the bifurcations and for allowing for neo-epithelization in the area where the prosthesis placed by the epithelial layer of the airway (providing a nearly normal mucociliary activity). Coated prostheses impede tumor growth in their interior, but they interrupt the mucociliary system, increasing the accumulation of mucus. They may also occlude the main bronchi, for instance if they are implanted in the lower trachea/main bronchus because they are necessary there, or after suffering migration (migration is easier than with the uncoated prosthesis). The majority of the stents were inserted before there were any recommendations published about the best type of prosthesis for certain disorders, such as the 2005 FDA Declaration on the use of SEMS in benign disease.5 Coated stents were used mainly in patients with esophageal cancer, especially in cases with fistula formation.
The mean dose of sedation was 5mg of midazolam, which clearly contrasts with the general anesthesia used for rigid bronchoscopy. The dose of sedatives used has not been commented in any other prior studies.
Five patients presented mild hemoptysis as a complication of the stent placement, and in another 4 patients there was stent migration. Pneumonia was a complication in 2 patients, and formation of severe granulation tissue in 3 cases. It is important to indicate that very little additional intervention was necessary in the total population treated. One patient who presented with mild hemoptysis after the intervention was treated with adrenalin at the hemorrhage spot during stent insertion. No other treatment was necessary for the minor hemoptysis, and the number of repeated interventional bronchoscopies necessary was low (3 due to granulation tissue, 4 due to stent migration). Rigid bronchoscopy was necessary in 1 patient with occlusion of the prosthesis. This was carried out by the cardiothoracic team that is permanently on call for any complication that may require rigid bronchoscopy.
The patients who survived for more than 3 years did not present the stent fractures that have been commented in another publication.5
These data indicate that, overall, the patients continued to enjoy a good quality of life after the immediate beneficial effects described after stent insertion. These beneficial effects are not cancelled out by the need for new hospitalizations or interventions associated with the known complications of stent insertion. It also seems clear that the use of Ultraflex™ SEMS does not increase the medical treatment load compared with other SEMS, not even with silicone prostheses, as reported here.
Major complications have been associated with the general anesthesia necessary for the use of rigid bronchoscopy when silicone stents or other types of metallic stents are implanted. Silicone prostheses have higher migration rates than those reported here, and they give rise to a mucociliary interruption that leads to the retention of secretions (reported in up to 27% of patients), recurring respiratory infections and cough, which certainly deteriorate patient quality of life.1
Madden et al.3 inserted Ultraflex stents under general anesthesia using rigid bronchoscopy, and the complications observed included halitosis (2 patients), sputum retention and/or infection (5 patients) and formation of granulation tissue (1 patient). Saad et al.6 implanted SEMS with rigid bronchoscopy (20% Ultraflex), and they observed that infectious tracheobronchitis was the most frequent complication (15.9%). The formation of obstructive granulomas in the interior of the prosthesis was the second most frequent complication (12 out of 82 patients, 14.6%). However, the rate varied depending on the underlying disorder. In cases of malignant lung disease, the formation of granulation tissue was produced in only 2 patients (4%). There was also hemoptysis in 12 out of 82 patients, while stent migration occurred in 4 of these patients. Madden et al.,8 in their patients treated with Ultraflex stent placement using rigid bronchoscopy due to benign disease, observed the formation of granulation tissue in 11 of the 31 patients. Saad et al.6 also observed significantly higher formation of granulation tissue in patients with benign diseases than in those from the malignant disease group (32% compared with 4%). Breitenbücher et al.2 inserted Ultraflex SEMS by means of rigid bronchoscopy in patients with malignant diseases, and they observed a complication of mucus plugging in 8% of the cases, as well as stent migration, the formation of granulation tissue and the re-stenosis of the tumor in 5% of cases, each.
Overall, in comparison with silicone stents, Ultraflex stents have a lower rate of migration and hemoptysis, but with a higher rate of granulation tissue formation.2,9–11
We did not observe any episodes of pneumothorax, lobe collapse or operative death, which were described in other studies.4,7
Although the exact survival data of the patients with SCLC were incomplete, the mean survival in our study for the 39 patients with NSCLC was 214 days (range: 5–1233 days). Breitenbücher et al.2 observed a mean survival time of 160 days among their patients with lung cancer in whom Ultraflex SEMS were inserted with general anesthesia, although they did not indicate the histologic diagnosis. Wilson et al.4 indicated that, out of the 56 patients with lung cancer in whom Ultraflex stents were implanted under general anesthesia, 5 continued to survive after an average of 207 days (range: 135–274) and 51 had died, with a mean survival of 77 days (range: 1–477 days). Stockton et al.7 implanted Gianturco SEMS with flexible bronchoscopy in their patients with lung cancer and they observed a mean survival time of 142 days (range: 1–2571 days). Their study included a higher proportion of patients with SCLC (25%) compared to ours (<10%). It is important to point out that our rate of survival (mean: 214 days) was similar to or not lower than that of other studies using Dumon, Gianturco or Wallstents prostheses and rigid rigid broncoscopy,2,4,12–14 which suggests that our department is at least as least equally safe and effective with regard to selecting patients and the necessary resources.
The survival of esophageal cancer was 70 days (range: 12–249 days), which can be favorably compared with the numbers reported by other studies, almost all of which used rigid bronchoscopy and general anesthesia.15–19 Chan et al.18 and Belleguic et al.16 reported a mean survival of 61 and 107 days, respectively, using a treatment with rigid bronchoscopy for tracheobronchial stenoses of esophageal causes. Takamori et al.17 are the only other group to specifically describe the use of SEMS in the tracheobronchial stenoses of esophageal causes, and they have reported a mean survival of 35 days in 10 out of 12 patients. Mroz et al.15 presented the use of SEMS with flexible bronchoscopy in all their cases of central airway obstruction and they observed that the subgroup of patients with esophageal cancer had a mean survival of 27 days.
Obviously, the comparison of the survival data with those of other studies is extremely difficult because there are variables, such as tumor load, functional state and comorbidities that are not comparable. Nevertheless, our data indicate that the patients who undergo this intervention do not evolve any worse than those treated with other therapeutic methods.
The mean pack-year smoking history in the patients with lung cancer was 37. This aspect of the analysis has not been commented in any other study.
The limitations of this study are its retrospective character and the lack of a control population and more objective data regarding the quality of life/improved lung function. As for an untreated control group, we believe, as do other authors, that there are important ethical impediments to using a control group in studies about the palliative treatment of patients with advanced obstruction of the upper airways, which would put their lives’ at risk.5
Our retrospective study about the use of Ultraflex SEMS with flexible bronchoscopy is the most extensive about the insertion Ultraflex SEMS using flexible bronchoscopy for the treatment of all-cause inoperable tracheobronchial stenoses. It also highlights the importance of offering this service to patients who suffer respiratory difficulties and whose disease is inoperable. Stent insertion under sedation instead of general anesthesia has been an advancement in providing this service to these patients. Stent insertion in the airways does not substitute tracheobronchial resection for benign or malignant disease, but it does provide immediate palliation of the respiratory symptoms and respiratory difficulty, with an improvement in quality of life.1
There are few groups that have described the results of the insertion of Ultraflex stents specifically under sedation with flexible bronchoscopy, and we believe that its immediate effects provide patients with benefits, not only through the alleviation of the symptoms, but also by means of prolongation of life. In addition, this study emphasizes the viability of providing this service in a respiratory medicine unit instead of in a cardiothoracic unit, and in an outpatient setting. This makes it a much more accessible procedure, as only 6% of the pulmonologists in the United States are capacitated to perform rigid bronchoscopy, and training programs are limited.20,21
Please cite this article as: McGrath EE, et al. Implantación de prótesis metálicas autoexpansibles con broncoscopia flexible bajo sedación para estenosis traqueobronquiales malignas: análisis retrospectivo de un solo centro. Arch Bronconeumol. 2011;48:43–8.