Immune checkpoint inhibitors have opened an era of lung cancer therapy. However, a notable disparity exists in the efficacy of immunotherapy among individual patients. The tertiary lymphoid structure (TLS) is an ectopic lymphocyte aggregation that appears under pathological conditions and is the primary site of action for anti-tumor immunity. It is commonly reported that the presence of TLS within the tumor microenvironment (TME) relates to a favorable clinical prognosis and an excellent response to immunotherapy in lung cancer patients. A thorough understanding of TLS and its dynamic changes in TME has become an attractive focus for optimizing immunotherapy strategies for lung cancer. In this review, we comprehensively generalize the composition, formation, mechanism, detection methods of TLS, and summarize the role of TLS in lung cancer immunotherapy. Finally, induction of TLS is also discussed, which may provide more effective therapeutic strategies for lung cancer therapy.
The highest incidence and mortality rate of lung cancer caused heavy social and economic burden for global nations.1 In recent years, the advent of immune checkpoint blockade (ICBs) provided patients with non-small cell lung cancer access to a whole new therapeutic option. ICBs targeted the immunosuppression receptor and blocked the involved signaling pathways to reactivate the activity of immune cells, thereby rapidly strengthen anti-tumor ability.2,3 As the key mediator of tumor progression and clinical outcomes, the tumor microenvironment (TME) exerts a significant influence on the extent of the response to immunotherapy. In recent years, elucidating the intricacies of the tumor microenvironment to explore personalized therapeutic strategies and identify efficacy-associated biomarkers has emerged as a focal point in the realm of immunotherapy research.4–6 The tertiary lymphoid structure (TLS), lymphocyte aggregates present in the tumor microenvironment, has been identified as the principal sites of anti-tumor immunity and strongly connected with positive clinical outcomes and responsiveness to immunotherapy across a spectrum of malignancies.7–9 In this review we comprehensively delve into the structural composition, formation mechanisms, detection techniques of TLS in the lung cancer tumor microenvironment. Moreover, it elucidated the clinical implications and therapeutic potential of TLS in lung cancer.
TLS in the Tumor MicroenvironmentThe Structure and Components of TLSTertiary lymphoid structures represent ectopic lymphocyte aggregations usually observed in pathological contexts such as infectious diseases and malignant tumors.10–12 TLS is characterized by an internal CD20+ B cell cluster and is encircled by external parts composed of CD3+ T cells. It could also be observed that T cells and B cells are mixed together in early TLS.13,14 Due to the absence of capsules, TLS exposed to antigens produces a more quick and effective immune response than secondary lymphoid organs (SLOs).15,16 In addition, the presence of CD4+ follicular helper T (Tfh) cells, CD4+ helper T cells (Th), CD8+ cytotoxic T cells (CTL) and regulatory T cells (Treg) could be detected in the CD3+ T cell subsets. Dendritic cells (DCs) with antigen presentation effects are scattered in the T cell area. In the inner zone of CD20+ B cells, different percentages of naive B cells, germinal center B cells, memory B cells and plasma cells are demonstrated in tumors.17 Besides, high endothelial venule (HEV), follicular reticular cells (FRC), and follicular dendritic cells (FDCs) are also integral constituents of TLS architecture. HEV typically co-localizes with TLS and expresses peripheral lymph node vascular addressin (PNAd), which establish a specialized vascular framework for lymphocyte recruitment, thereby enhancing anti-tumor immune responses.18 FRC forms a network to support the T-cell and B-cell regions, while FDC facilitates antigen presentation to B cells and the production of high-affinity antibodies and characterizing maturation stages within TLS.19,20 The maturation of TLS serves as a critical determinant of its anti-tumor efficacy and typically involves the following phases: (1) early TLS (E-TLS), characterized by the presence of T cells and B cells while lacking germinal centers and CD21+ FDC cells; (2) primary follicle-like TLS (PFL-TLS), lacks of germinal center (GC) and characterized by the formation of a CD21+ FDC network structure within the B cell zone; (3) secondary follicle-like TLS (SFL-TLS), characterized by a CD21+CD23+FDC network structure and an active GC, where B cells with high-affinity B-cell receptors (BCRs) undergo selective differentiation and further maturation process. The mature B cells subsequently transformed into functionally distinct cells such as plasma cells and memory B cells to promote anti-tumor immunity.20,21
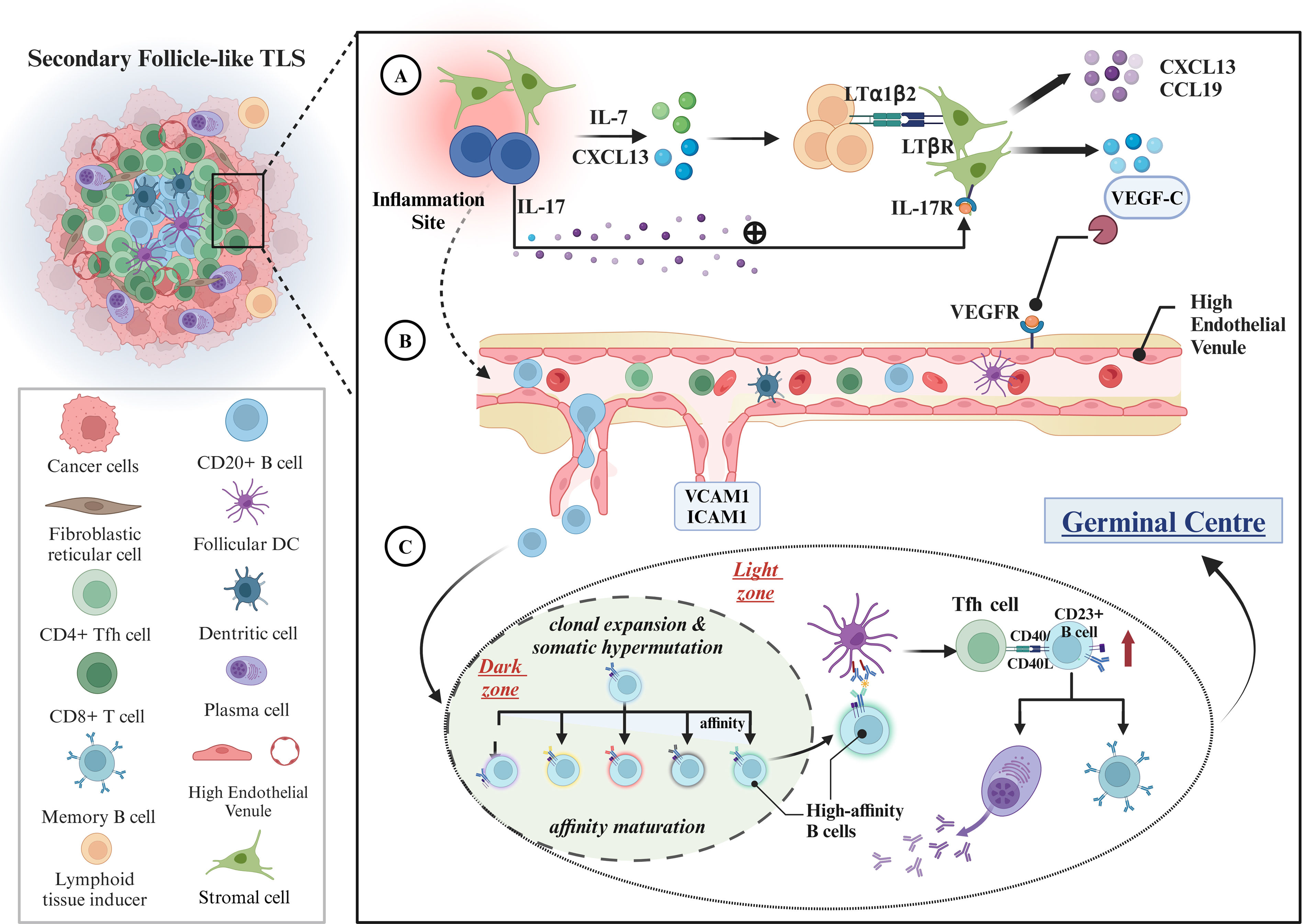
The Mechanisms of TLS Formation and MaturationAs shown in Fig. 1, the interaction of hematopoietic cells with nonlymphoid stromal cells under the influence of cytokines results in the formation of TLS. Lymphocytes or stromal cells release chemokines, CXCL13 and IL-7, when exposed to antigens. The chemokines trigger the CXCL13/CXCR5 signaling pathway, attracting CXCR5+ lymphocytes to tumor sites and recruiting lymphoid tissue inducer (LTi) cells to promote the development of lymphatic structures.22,23 LTi cells express lymphotoxin-α1β2 ligand (LTα1β2), which mediates the secretion of vascular endothelial growth factor C (VEGFC) by binding the lymphotoxin β receptor (LTβR) on the surface of stromal cells, thereby encouraging HEV formation. After HEV formation in TLS, adhesion molecules like ICAM1 and VCAM1 are continuously released, facilitating the homing of various immune cells.14 Notably, TLS formation is influenced by both lymphotoxins produced by antigen-activated immune cells and interleukin-17 (IL-17) secreted by T cells. It was reported that IL-17 could induces stromal cells to express CXCL13 and CCL19, which facilitated the formation of lymphoid aggregates in lung tissues in mouse. However, lymphoid aggregates often lack entry of HEVs due to the absence of lymphotoxins, resulting in their classification as early TLS that does not progress to a mature state.24,25 TLS maturation is often followed by B-cell activation and maturation, with FDCs, GC, and Tfh cells playing essential roles. It may be useful to contrast it to the maturation of SLOs. B cells with specific affinity enter the GC, undergo clonal expansion, and undergo somatic hypermutation (SHM) under co-stimulatory signals from CD4+ Th cells, which enhances the affinity of B-cell receptors (BCRs) and enables competitive binding to antigens presented on FDC surfaces. Subsequently, B cells interact with Tfh cells, presenting antigen through MHC II class molecules, and undergo class transformation through CD40−CD40L signaling, differentiating into memory B cells or antibody-secreting plasma cells.26 Additionally, previous studies indicated that FDCs were activated during TLS maturation, with CD23 expression upregulation being a significant sign of their activation and adaptation to the GC response.27 As a result, the FDC with CD23+ positive expression was defined as a crucial criterion for determining the maturation status of TLS.
Formation and maturation mechanisms of TLS. (A) Recruitment of immune cells and initial establishment of lymphoid-like architecture: within sites of chronic inflammation, stromal cells or lymphocytes release chemokines, including CXCL13 and IL-7, which attract lymphoid tissue-inducing (LTi) cells to the inflammatory locus. The binding of LTα1β2 from LTi cells to the LTβR on stromal cells triggers the secretion of VEGFC, thus facilitating the formation of high endothelial venules (HEVs). Additionally, lymphocytes secrete IL-17, which activates stromal cells via IL-17R, further augmenting the production of chemokines such as CXCL13 and CCL19. (B) HEV function and immune cell homing: following the establishment of HEVs, the secretion of adhesion molecules, such as ICAM1 and VCAM1, facilitates the recruitment of immune cells, including B and T cells, to the TLS, thereby reinforcing local immune responses. (C) B-cell activation and maturation: during TLS maturation, B cells undergo clonal expansion and somatic hypermutation within the germinal center (GC) dark zone with the assistance of CD4+ T cells to enhance BCR affinity. Subsequently, the high-affinity B cells interact with FDC and TFH cells in the bright zone and complete the antibody class switch via the CD40/CD40L pathway. This process leads to B cell maturation (e.g., increased expression of CD23) and differentiation into memory B cells or antibody-secreting plasma cells, thereby exerting their anti-tumor immune functions.
There are various methods of detection and labeling of TLS. Hematoxylin–eosin (HE) staining of tissue sections remains the common method, wherein round-like lymphocyte aggregates indicate the presence of TLS. Quantification of TLS can be achieved by determining the number of TLS per unit area; however, this approach limited insight into TLS characteristics. At present, immunohistochemistry (IHC) or multiple immunofluorescence staining (mIF) has emerged as the mainstream method of TLS research. Multiple immunofluorescence staining allows for simultaneous labeling of multiple cellular antibodies, enabling comprehensive exploration of spatial biological information related to TLS, including spatial distance analysis and TLS maturation assessment. For instance, the tight co-localization of CD20+ B cells and CD4+ T cells within GCs often indicates a high level of TLS activity; With the change of TLS maturity, FDC expression decreased in CD21, while CD23 expression gradually increased.14,28 Gradually, with the development of spatial transcriptomics, revealing the spatial transcriptional features of TLS represents a significant advancement.29 Researchers used spatial transcriptomics to quantify 25 immune proteins linked to immune cells in melanoma, disclosing the molecular characteristics of T and B cells.9 Wu et al. developed a TLS-50 signature to accurately locate tertiary lymphoid structures, revealing their composition is influenced by their distance to tumor cells, which further enriched the cognition of TLS.30 HE staining, IHC, and mIF are essential for detecting TLS, but their reliance on pathological samples is notable. Therefore, efforts have been directed towards employing non-invasive imaging techniques such as positron emission tomography (PET), single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI) for TLS detection.31,32 Our previous findings demonstrated that visual punctate solid components on lung CT were correlated with TLS and verified by pathology.33 Additionally, Li et al. predicted the presence of TLS in tumors by identifying CT image features linked to TLS, such as intertumoral vascular signs.32 A recent study employed radiomics to extract TLS biomarkers from CT images developed a TLS score to predict TLS presence and immunotherapy efficacy in ovarian cancer patients.34 However, there are inherent limitations in detecting TLS through imaging techniques. Imaging can detect TLS as lymphocyte aggregates, but it primarily reveals dense immune cell populations and lacks specificity and accuracy, making it primarily used for correlation studies. The clinical application of TLS imaging remains a pressing challenge. With advancements in biomaterials technology, research on TLS imaging combining nanoparticles and radionuclides for diagnosis is gaining continuous attention. Future molecular imaging techniques with improved resolution, sensitivity, specificity, and signal to noise ratios hold promise for accurately imaging TLS in tumor tissues.35,36
Clinical Implications of TLS in Lung Cancer ImmunotherapyPrognostic Potential of TLS for Patients With Lung Cancer ResectionTLS is strongly correlated with favorable clinical prognosis for patients with lung cancer resection.37 The relationship between TLS abundance, location, maturation, and prognosis was focal point for researchers. TLS abundance was linked to the expression of genes involved in adaptive immune response and is recognized as an independent prognostic indicator for lung cancer patients.38 Previously, Germain et al.17 identified tumor-induced bronchus-associated lymphoid tissue (Ti-BALT) in 74 postoperative histopathological samples from early-stage lung adenocarcinoma patients, characterized by a sustained immune response. They identified LAMP+CD208+DCs as markers of TLS and found that TLS abundance could recognize patients with early-stage NSCLC at high risk of recurrence. Dieu-Nosjean et al. research further investigated the potential connection between TLS and the local humoral immune response in NSCLC patients, which proved TLS as a significant prognostic predictor following NSCLC surgery.39 Research investigated the relationship between TLS and clinicopathologic features, gene expression, and prognosis in 112 patients diagnosed with stage IB lung adenocarcinoma.40 The study found no significant association between TLS and PD-L1 expression, but patients with presence of TLS had longer RFS, with TLS being an independent prognostic factor. Furthermore, gene and related pathway analysis highlighted positive modulation of the humoral immune response, such as antigen receptor-mediated signaling, in TLS-positive individuals. Except for early-stage NSCLC, patients with stage II–III NSCLC have also shown clear positive links between high TLS expression and a favorable prognosis after surgery.41 Wang et al.’s42 multicenter study utilized artificial intelligence to accurately quantify TLS density in lung adenocarcinoma (LUAD) patients using digital pathology images. Their results demonstrated that computerized TLS density serves as an independent prognostic indicator, and its integration with clinicopathological factors can aid in personalized clinical decision-making. Additionally, Siliņa et al.’s43 study on 138 resected lung squamous cell carcinomas (LUSC) found that high TLS abundance significantly improved prognosis, including progression-free survival (PFS), disease-free survival (DFS), and overall survival (OS). Tang et al.44 found that lung cancer patients without chronic obstructive pulmonary disease (COPD) had significantly higher TLS abundance compared to those with COPD. Interestingly, patients with higher levels of TLS and B cells showed a favorable 10-year survival, regardless of underlying COPD.
Several studies have reported the impact of TLS location on prognosis, particularly in intrahepatic cholangiocarcinoma, breast cancer and melanoma, while it was rarely explored in lung cancer.45–48 A study of 167 breast cancer patients found that patients with abundant peritumoral TLS density had the worst DFS and OS.45 Ding et al.46 proposed a TLS scoring system based on intratumoral (T score) and peritumoral (P score) regions in intrahepatic cholangiocarcinoma and found that the high T score was correlated with a favorable clinical prognosis, while the high P score suggested poorer survival outcomes. In lung cancer, research on the correlation between peritumoral TLS and prognosis is limited, with a main focus on intratumoral TLS exploration.47 Zhang et al.48 integrated transcriptomic data and digital pathology images to evaluate immune hotspots’ impact on lung cancer prognosis in 935 patients, finding peritumoral hotspots significantly associated with favorable survival. The results also indicated that immune cells preferentially homed to the peritumoral region, which reflect an immune-supportive niche compared with intratumoral immune hotspots. Additionally, peritumoral hotspots showed higher CD20+CXCR5+ and CD79b+ cell abundance. CXCR5 and CD79b are markers linked to TLS, which also suggests a positive correlation between peritumoral TLS and favorable lung cancer prognosis. Taken together, the relationship between TLS location and prognosis displays tumor type-dependent heterogeneity. It is critically necessary to conduct further research on the relationship between TLS distribution and prognosis of patients with lung cancer.
The maturation of TLS significantly influences plasma cells and antibody production, which is crucial for the clinical prognosis of tumor patients.49 Previous research has underscored that matured TLS correlates with improved immune response, prolonged PFS and OS of patients.37 He et al.50 analyzed a follow-up cohort of 616 patients with NSCLC, and a correlation was observed between the maturation of TLS and long-term survival after surgery. Specifically, patients with mature GC+TLS had a significantly lower risk of recurrence in both univariate and multivariate analyses compared to those without mature GC−TLS. Furthermore, they suggested that combination of TLS abundance and maturation could promote assessment of prognosis of patients. Similarly, the positive correlation between mature TLS and favorable survival outcomes was observed in other tumor types. In non-metastatic colorectal cancer,51 patients with a high proportion of early TLS and a low proportion of PFL-TLS displayed a tendency toward increased recurrence risk. According to competing risk analysis, individuals harboring GC+TLS experienced a 70% lower 3-year recurrence risk compared to GC−TLS patients, indicating the importance of TLS maturation as a prognostic marker. In another cohort of 650 patients with esophageal squamous carcinoma,52 the analysis of surgical resection samples indicated that the 5-year OS rates for patients with mature TLS, immature TLS, and TLS-negative patients were 66.5%, 50.0%, and 50.2%, respectively (p=0.001), and DFS rates were 55.6%, 39.9%, and 46.3%, respectively (p=0.003). Significantly superior survival outcomes were observed among patients with mature TLS compared to those with immature TLS and TLS-negative status (p<0.05), while there was no difference of survival between individuals with immature TLS and TLS-negative status. Mature TLS typically contains a higher abundance of immune cells. The GC response creates a specialized microenvironment for immune cell interactions, promoting effective anti-tumor activity and improving tumor prognosis in patients. On the whole, the abundance, localization, and maturation of TLS in patients with resected lung cancer significantly impact clinical outcomes. TLS within tumor microenvironment enhances the anti-tumor immune response, making it a crucial prognostic predictor for primary lung cancer patients undergoing surgical resection. Further rational individualized prediction models for TLS will hold promise in guiding more precise prognostic management for lung cancer.
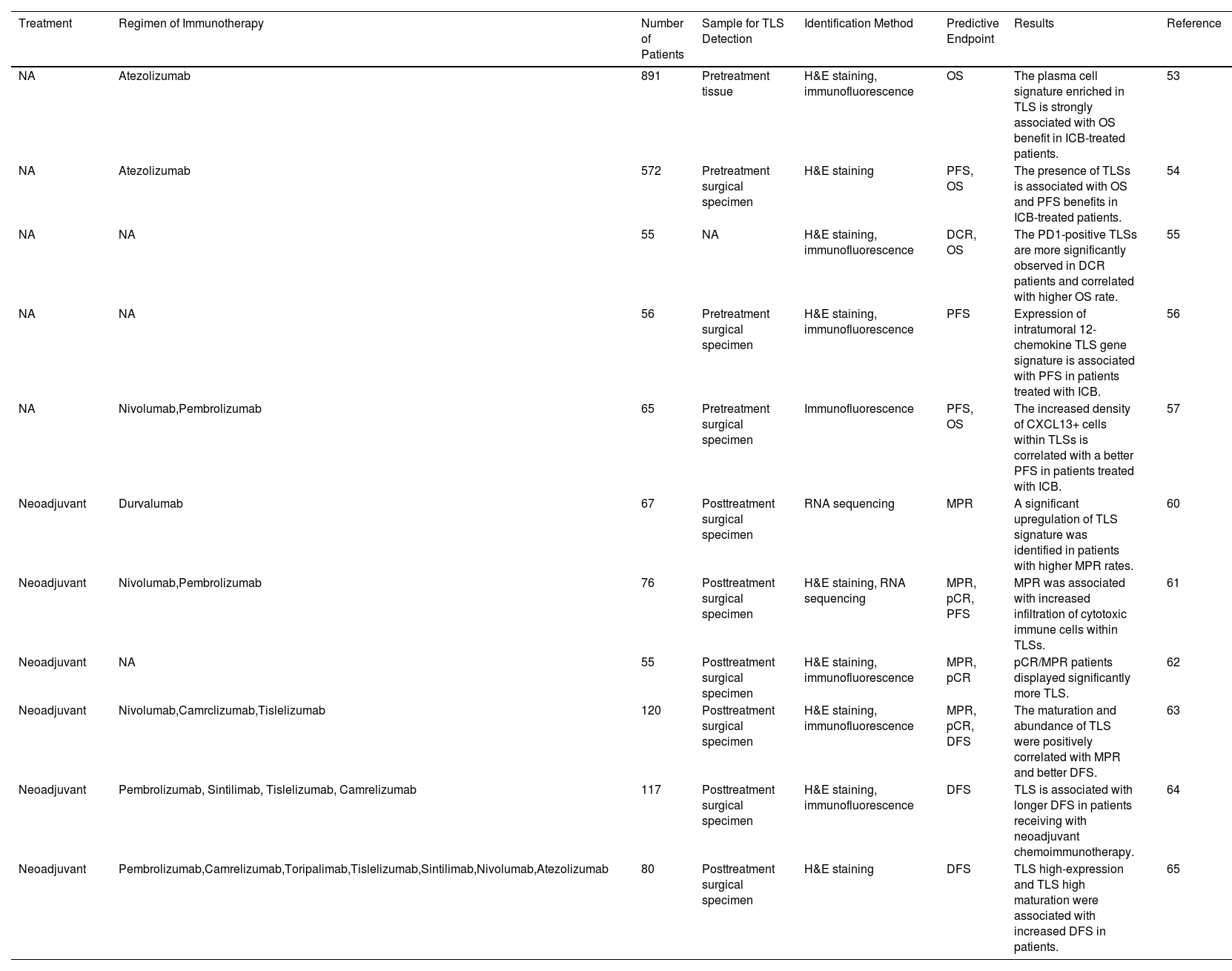
Predictive Potential of TLS for the Efficacy of Immunotherapy in Lung CancerAs a pivotal immune site in the TME, TLS initiates antigen-specific adaptive immune responses effectively and is crucial in predicting the effectiveness of immunotherapy in lung cancer, as summarized in Table 1. In patients with advanced NSCLC, the presence of TLS synergistically enhanced the effect of immune checkpoint inhibitors, which help patients benefit from treatment. Patil et al.53 analyzed tumors H&E from two randomized clinical trials of atezolizumab versus chemotherapy for exploring the association between presence of TLSs and survival. The results showed that TLS-positive patients had a more favorable OS when treated with atezolizumab. Furthermore, they demonstrated that B and plasma cell expression genes strongly correlate with TLS presence, and increased plasma cell signatures significantly predict OS in immunotherapy-treated patients. The IMpower110 phase 3 clinical trial data indicates that the presence of TLS in NSCLC patients enhances their immunotherapy responsiveness.54 Specifically, patients treated with atezolizumab showed an improvement in overall survival compared to chemotherapy, with an extended overall survival of 7.1 months (p=0.01). In patients with recurrent NSCLCs, the TLS and its lymphocyte distribution were also associated with durable clinical effect (DCR).55 Raju Paul et al.56 characterized the NSCLC TME and demonstrated that expression of intratumoral 12-chemokine TLS gene signature is associated with PFS in patients with ICIs. Assessment of CXCL13+ cells density and localization in NSCLC suggested that increased CXCL13+ cells within TLSs facilitated antigen presentation to T cells and led to a better prognosis.57 Notably, substantial evidence indicates that CXCL13 is related to the formation of TLSs and the recruitment of B cells, TFH cells, and dendritic cells to the TLSs.14 In addition, a recent clinical study has shown that excessive clearance of lymph nodes can hinder the effectiveness of postoperative adjuvant immunotherapy.50 The mechanism may be attributed to the destruction of tumor-draining lymph nodes, which consequently destroy the source of TLS immune cells, inhibiting their formation and maturation, and attenuating immunotherapy response.50,58
Predictive Functions of TLSs in Immunotherapy-treated Lung Cancer Patients.
| Treatment | Regimen of Immunotherapy | Number of Patients | Sample for TLS Detection | Identification Method | Predictive Endpoint | Results | Reference |
|---|---|---|---|---|---|---|---|
| NA | Atezolizumab | 891 | Pretreatment tissue | H&E staining, immunofluorescence | OS | The plasma cell signature enriched in TLS is strongly associated with OS benefit in ICB-treated patients. | 53 |
| NA | Atezolizumab | 572 | Pretreatment surgical specimen | H&E staining | PFS, OS | The presence of TLSs is associated with OS and PFS benefits in ICB-treated patients. | 54 |
| NA | NA | 55 | NA | H&E staining, immunofluorescence | DCR, OS | The PD1-positive TLSs are more significantly observed in DCR patients and correlated with higher OS rate. | 55 |
| NA | NA | 56 | Pretreatment surgical specimen | H&E staining, immunofluorescence | PFS | Expression of intratumoral 12-chemokine TLS gene signature is associated with PFS in patients treated with ICB. | 56 |
| NA | Nivolumab,Pembrolizumab | 65 | Pretreatment surgical specimen | Immunofluorescence | PFS, OS | The increased density of CXCL13+ cells within TLSs is correlated with a better PFS in patients treated with ICB. | 57 |
| Neoadjuvant | Durvalumab | 67 | Posttreatment surgical specimen | RNA sequencing | MPR | A significant upregulation of TLS signature was identified in patients with higher MPR rates. | 60 |
| Neoadjuvant | Nivolumab,Pembrolizumab | 76 | Posttreatment surgical specimen | H&E staining, RNA sequencing | MPR, pCR, PFS | MPR was associated with increased infiltration of cytotoxic immune cells within TLSs. | 61 |
| Neoadjuvant | NA | 55 | Posttreatment surgical specimen | H&E staining, immunofluorescence | MPR, pCR | pCR/MPR patients displayed significantly more TLS. | 62 |
| Neoadjuvant | Nivolumab,Camrclizumab,Tislelizumab | 120 | Posttreatment surgical specimen | H&E staining, immunofluorescence | MPR, pCR, DFS | The maturation and abundance of TLS were positively correlated with MPR and better DFS. | 63 |
| Neoadjuvant | Pembrolizumab, Sintilimab, Tislelizumab, Camrelizumab | 117 | Posttreatment surgical specimen | H&E staining, immunofluorescence | DFS | TLS is associated with longer DFS in patients receiving with neoadjuvant chemoimmunotherapy. | 64 |
| Neoadjuvant | Pembrolizumab,Camrelizumab,Toripalimab,Tislelizumab,Sintilimab,Nivolumab,Atezolizumab | 80 | Posttreatment surgical specimen | H&E staining | DFS | TLS high-expression and TLS high maturation were associated with increased DFS in patients. | 65 |
NA, not available; ICB, immune checkpoint blockade; OS, overall survival; PFS, progression-free survival; DCR, durable clinical effect; MPR, major pathological response; pCR, pathologic complete response; DFS, disease-free survival.
In terms of neoadjuvant approaches, TLSs also play a significant predictive role in the efficacy of immunotherapy. Previously, Cottrell et al.59 identified histopathological characteristics of immunotherapy-mediated tumor regression in 20 cases, including TLS formation, cholesterol necrosis, foam cell infiltration, proliferative fibrosis, and neovascularization. Multiplatform immune profiling analysis from the first randomized neoadjuvant immunotherapy trial, NeoCOAST, demonstrated that improved MPR rates were associated with TLS formation markers and systemic functional immune cell activation.60 A real-world investigation on 76 NSCLC patients with neoadjuvant immunochemotherapy found significant differences in immune cell infiltration between patients with MPR and non-MPR, suggesting a correlation between MPR and increased cytotoxic immune cells.61 The TME features of patients with neoadjuvant immunotherapy showed that CD3, CD20, and CD56 cell subsets and TLS were more frequently observed in pCR/MPR patients.62 Sun et al.63 conducted a study focusing on the correlation between TLS abundance, maturation, and the efficacy of neoadjuvant immunotherapy in resectable NSCLC. Their findings indicated a relationship between TLS abundance and the rate of pathological response, with patients achieving MPR showing higher TLS maturation. Multivariate regression analysis further identified TLS maturity as an independent predictor of DFS following neoadjuvant immunotherapy. Similarly, a retrospective study conducted by Xu et al.64 also demonstrated that TLS were independent prognostic factors in NSCLC patients undergoing neoadjuvant chemoimmunotherapy and radical surgery. Especially the prognosis of the patients with TLS high maturation was significantly better than that of low maturation.65
Taken together, studies consistently show a strong correlation between TLS and neoadjuvant immunotherapy for lung cancer, highlighting TLS's predictive role in determining immunotherapy efficacy and prognosis. Immunotherapy stimulates TLS formation and immune cell activation in the TME, which is a significant possible mechanism of action for the treatment. A study involving 24 uroepithelial carcinoma patients indicated a significant increase in TLS levels after neoadjuvant immunotherapy,66 particularly in MPR patients, which suggests that immunotherapy may induce TLS formation and activate immune cells. In another study on NSCLC, single-cell transcriptome TCR sequencing analysis showed that neoadjuvant immunotherapy stimulated TLS formation in 12 patients and increased the diversity of immune cell subsets within TLS.67
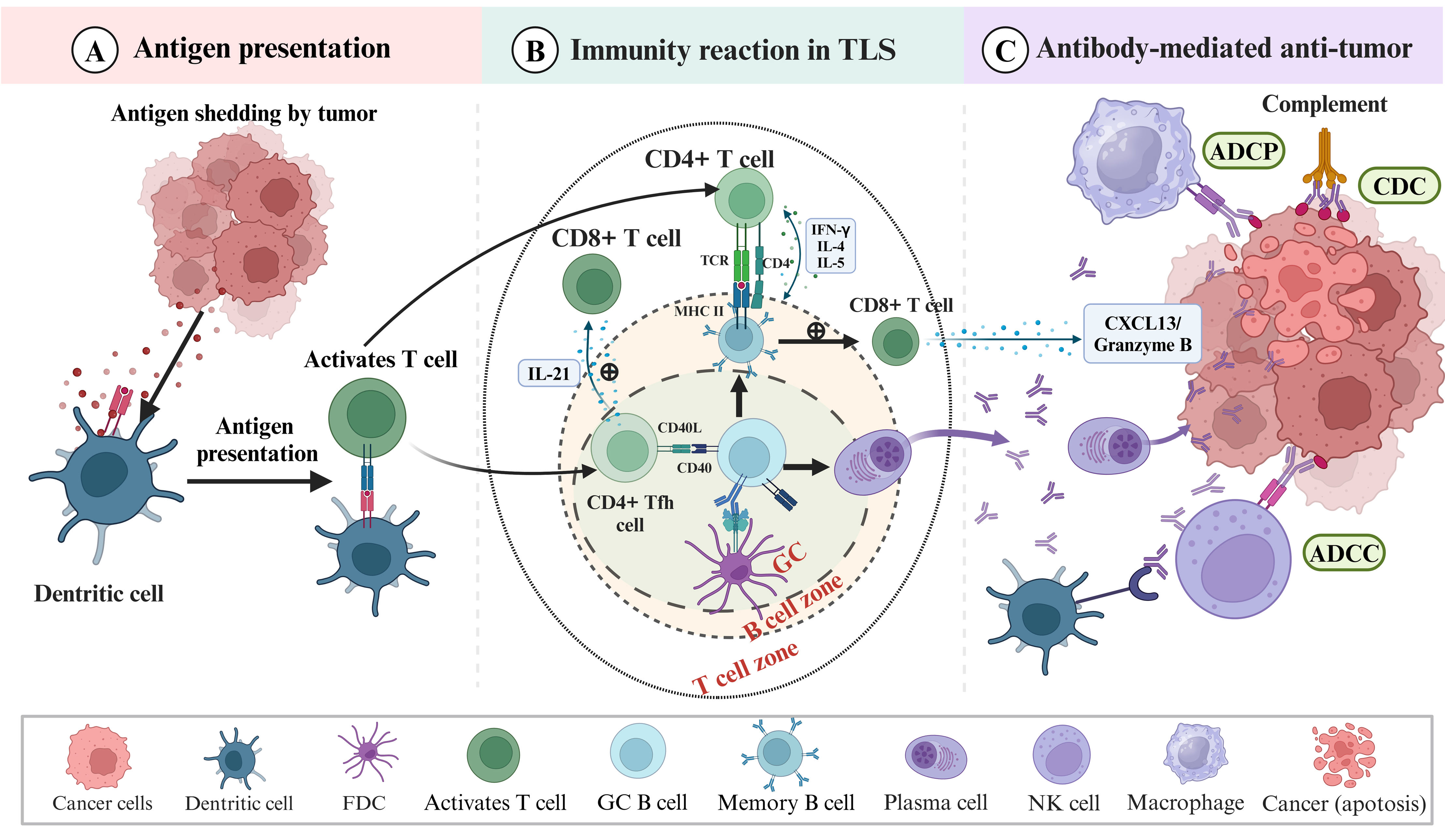
Mechanisms of Anti-tumor Immunity in TLSsGiven the critical role of TLS in tumor prognosis and immunotherapeutic response, exploring its anti-tumor mechanisms has emerged as a central focus in tumor immunotherapy.38 In this review, we elaborated on the immune mechanisms underlying TLS's anti-tumor effects, with a focus on immune response pathways and interconnections (Fig. 2). Genetic mutations trigger the production of tumor antigens, recognized by antigen-presenting cells (APCs), which activate immune cells, initiating the immune response. Dendritic cells act as the main antigen-presenting cells, uptaking and internalizing degradation of antigens, then forming peptide complexes with MHC molecules and transporting to the membrane surface to trigger CD4+ T-cell responses. Recent investigations revealed that DCs within TLS can directly activate tumor-specific T cells, accelerating the immune response by avoiding long distances from DCs to lymph nodes.68 FDCs within TLS can capture and retain antigens for extended periods, enabling B cell activation and maturation by presenting them to select high-affinity B cell populations. Additionally, B cells, the primary constituents of TLS have also been found to function as antigen-presenting cells.38 B cells at various developmental stages participate in distinct antigen presentation processes69: B cells in the germinal center can acquire antigens from FDCs, internalize them into antigenic peptides, and present them to CD4+ Tfh cells. Concomitantly, these B cells receive co-stimulatory signals from CD4+ Tfh cells, culminating in the activation and differentiation of B cells. While memory B cells, transformed by mature B cells, could quickly recognize surrounding low-concentration of antigen and present it to nearby T cells to achieve rapid immune response.
Mechanisms of TLS in anti-tumor immunity. (A) Tumor antigen presentation and lymphocyte activation: TLS facilitates efficient T cell activation and proliferation by tumor-associated antigens presentation of DCs and activation of lymphocytes. (B) TLS anti-tumor immune response: upon recognition of antigens presented by DCs, CD8+ T cells execute direct tumor cell killing. Furthermore, the interaction between germinal center B cells and FDCs, along with Tfh cells, as well as activation of the CD40/CD40L signaling pathway, facilitates B cell maturation and differentiation into memory B cells and antibody-secreting plasma cells. Moreover, the interaction between B cells and CD8+ T cells may enhance the activity of CD8+ T cells. Following antigen stimulation, CD4+ T cells differentiate into various subtypes, including Th1 and Tfh cells, which secrete cytokines such as IFN-γ, CXCL13, and IL-21, contributing to TLS formation and maintenance, and bolstering immunosurveillance against tumors. (C) Antibody-mediated tumor killing function: mature B cells generate targeted antibodies, such as IgG and IgA, which impede tumor growth and infiltration through triggering antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) mediated by NK cells and macrophages. Antibodies can also activate complement molecules through the complement-dependent cytotoxicity (CDC) pathway to lyse cancer cells.
Immune cells are activated in response to antigen stimulation, and the interaction between T cells and B cells is strengthened. On the one hand, sparsely distributed CD8+ T cells around TLS directly kill tumor cells by releasing granzyme, perforin and cytokines.70 B cells near CD8+ T cells within TLS can initiate secondary stimulation of CD8+ T cells.71 CD8+ T cells often display dysfunction due to tumor microenvironment suppression, but immune checkpoint inhibitors have successfully reversed this dysfunction and restored their ability to eliminate tumor cells.70 On the other hand, following antigenic stimulation, CD4+ T cells differentiate into different subsets and secrete a diverse range of cytokines. Th1 cells produce IFN-γ for cell-mediated responses, while Th2 cells stimulate B-cell proliferation and differentiation through cytokines like IL-4 and IL-5. Moreover, Tfh cells produce interleukin-21 (IL-21), indirectly enhancing CD8+ T cell-mediated tumor immunity. Tfh cells are also actively involved in the GC response, providing co-stimulatory signals for B cell activation and maturation via the CD40/CD40L axis, facilitating antibody class switch. Additionally, it has been evidenced that CXCR5+ memory Tfh cells offer enhanced support to B cells, accelerating the reinitiation of the memory immune response.72,73
Immune checkpoint inhibitors activate immune cells in TLS, disarming dysfunctional states and reactivating anti-tumor functionality, enabling more responses to immunotherapy in TLS-positive patients. B cells represent the predominant cellular component within TLS, and the robust anti-tumor immune activity of TLS relies heavily on the secretion of specific high-affinity antibodies by plasma cells. RNA-seq data analysis revealed somatically mutated immunoglobulin genes in tumors, indicating a B-cell-mediated response.74 A spatial transcriptomics investigation on renal cell carcinoma revealed matured B cells are forming plasma cells, which can migrate into tumors along the TLS network structure of fibroblasts and produce high-affinity IgG and IgA antibodies.75 In NSCLC, activated plasma cells capable of producing IgG and IgA antibodies against tumor-associated antigens could also be detected via ELISA.17 Researches indicated TLS-associated B-cell transformation and antibody production in breast cancer, melanoma, and other tumors, highlighting its role in anti-tumor mechanisms through B-cell antibody secretion.76,77 Tumor-specific antibodies function as anti-tumor immune mediators through various pathways, including antibody-dependent cellular phagocytosis (ADCP), complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC).78
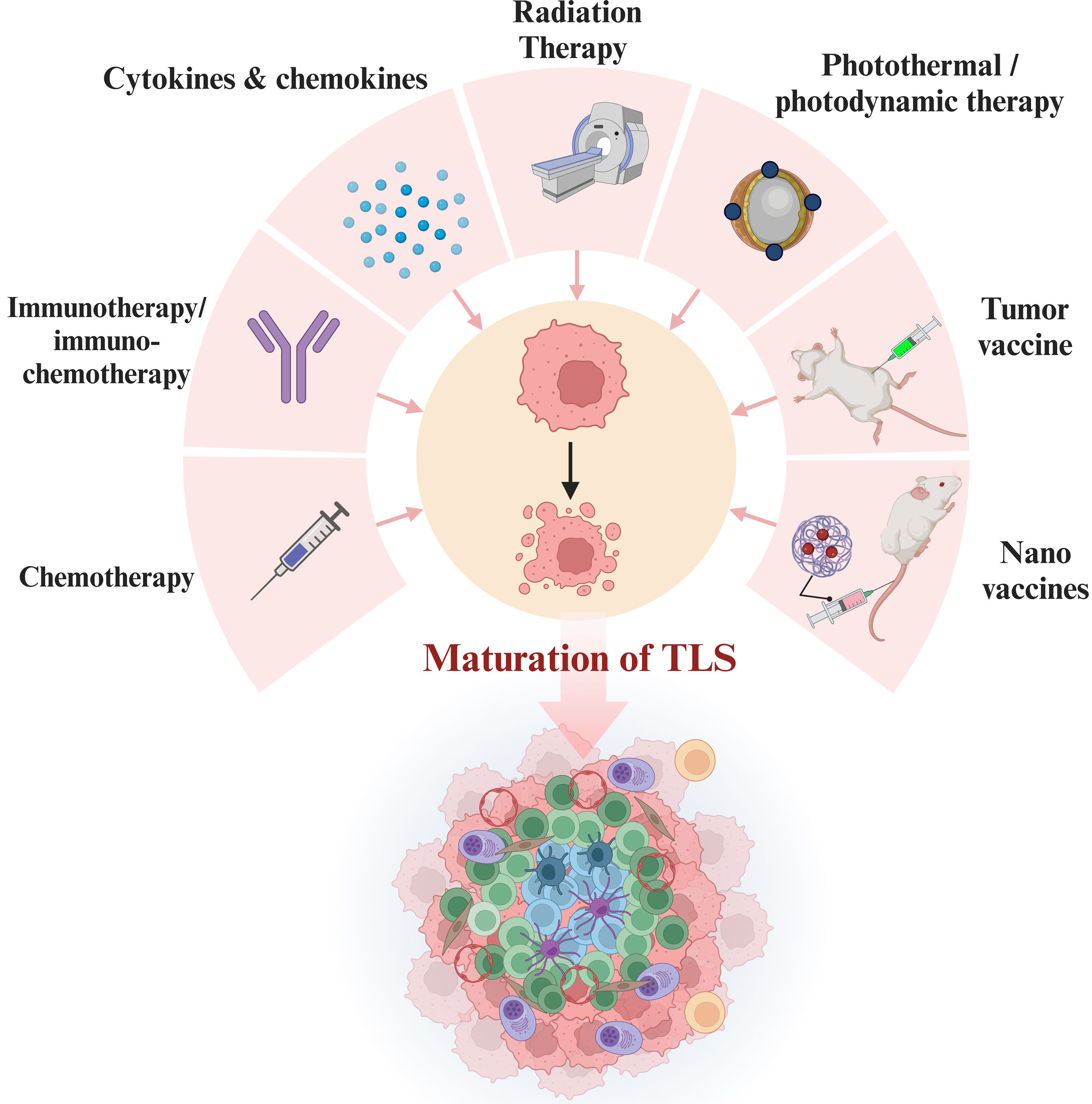
Induction Strategies of TLSThe targeted induction of TLS formation in TME has emerged as a promising therapeutic strategy due to its reported anti-tumor effects (Fig. 3). Studies suggest that conventional chemotherapy can induce TLS generation but may disrupt TLS maturation, potentially leading to the loss of the GC. Conversely, immunotherapy was proved to have a positive promoting effect on TLS infiltration and maturation.63,69,79–81 For instance, research on stage IIIA NSCLC patients showed neoadjuvant immunochemotherapy significantly promoted TLS formation, increased B and CD4+ T cell numbers, and increased IL-21 levels in tumor response.82 In addition, new strategies for TLS induction are formulated based on its formation mechanism. For instance, a study targeted LIGHT expression in the glioma vasculature using adeno-associated viral vectors, resulting in the formation of tumor-associated HEV and T-cell-rich tertiary lymphoid structures.83 Similarly, in human papillomavirus infection negative head and neck squamous cell carcinoma mouse model, targeting tumor vasculature by LIGHT can induce TLS formation and establish TLS-enriched TME.84 Moreover, the presence of TLS-inducing factors such as CXCL13, CCL19, and CCL21 is attractive for TLS generation. In a trial of lung adenocarcinoma, individuals with smoking history showed high level of CCL21, which could activate CCL21/CCR7 signaling pathway and facilitated TLS formation.85 Additionally, the binding of CXCL13 to CXCR5 can induce B cells into the TLS, promoting TLS formation and maturation, as demonstrated in murine models.86,87 Recent studies showed stimulator of interferon genes (STING) agonists normalize tumor vasculature, increase TLS-inducing factor production, enhance immune cell infiltration, and facilitate local TLS neogenesis in a mouse model.88,89 The above novel TLS-inducing methods have shown promising results in laboratory settings, but further validation is needed to evaluate their efficacy and safety in clinical treatment. Besides, research has explored radiotherapy and photothermal/photodynamic therapy, aiming to further promote TLS generation by inducing immunogenic cell death and release antigens into the tumor microenvironment.90–92 Additionally, researchers have innovatively explored approaches such as tumor vaccines and dual-adjuvant nano vaccinates to induce TLS formation, which enhance local immune responses and inhibit tumor growth.93–96 Future research on TLS and its inducing modality will offer more opportunities for developing innovative anti-tumor therapeutic strategies.
TLS induction strategies. TLS can be induced by various personalized anti-tumor treatment regimens, including chemotherapy, immunotherapy/chemoimmunotherapy, targeted therapy against TLS-associated cytokines, radiotherapy, photothermal/photodynamic therapy, tumor vaccines, and nano vaccines.
The advancements in precision medicine and immunotherapy have led to revolutionary changes in lung cancer treatment approaches. The advancement emphasizes the crucial role of the immune system in tumor therapy and the significance of the TME, particularly TLS, in immune regulation in lung cancer. This article delves into the role of TLS in the immune microenvironment of lung cancer, mechanisms of anti-tumor and the latest advancements in lung cancer immunotherapy research. TLS characteristics, including abundance, location, and maturation, are crucial biomarkers for predicting treatment response and prognosis in lung cancer patients, offering prospects for the personalized application of immunotherapy. Future investigations should prioritize several key areas: firstly, elucidating the precise mechanisms of TLS regulation and its role in the immune response against lung cancer. Secondly, improving and developing methodologies for TLS detection and labeling to enhance its application in clinical prognostic assessment and guide therapeutic decision-making. Additionally, delineating the relationship between TLS attributes and immunotherapeutic efficacy across different histopathological types of lung cancer patients is important for making personalized treatment regimens. Further research should focus on exploring how to optimize the therapeutic effect of lung cancer by actively intervening in the immune response within TLS, so as to open a new chapter in lung cancer immunotherapy.
Ethical ApprovalNot applicable.
FundingThis study was supported by the National Natural Science Foundation of China (62176166), the Capital Medical University Affiliated Beijing Shijitan Hospital Talent Training Program during the ‘14th Five-Year Plan’ period (2023DTRXXY) and the Industry-University-Research Innovation Fund for Chinese Universities (2022IT241).
Authors’ ContributionsX.X. was in charge of the data and analysis content of the manuscript. X.X. and L.P. devoted to the excogitation and conceptualization of the research. M.X., X.L., and X.B. contributed to original draft preparation. M.X., X.L., X.B., X.M. J.S., X.Z., Y.L. and J.Y. contributed to writing, review and editing. M.X. and X.L. contributed to visualization; M.X., H.D. and X.B. contributed to manuscript revision. X.X. obtained funding. All authors have read and agreed to the published version of the manuscript.
Conflict of InterestAll authors have agreed to publish this manuscript.
Data Availability StatementNot applicable.
All figures were created with BioRender.com.