The concept of “remission” in asthma has been around for a long time and it has been a controversial topic. Despite the attempts of some studies to characterize this entity, the discussion continues.
In the case of asthma there is still no clear definition, either in terms of its meaning or the parameters that should be included or whether it should be divided into clinical or complete remission.
To help defining these controversial concepts, SEPAR has advocated the multidisciplinary working group REMAS (REMission in ASthma). Following the Delphi methodology and with the involvement of more than 120 specialists in asthma management, this group has arrived at a consensus on the definitions of remission in asthma and establishing the criteria and characteristics that will be of use in future studies evaluating the efficacy or effectiveness of treatments.
The advent of biological therapies for severe asthma and the results obtained in both clinical trials and routine clinical practice settings have reopened the debate on the definition of disease remission and other associated aspects.1,2 Despite the attempts of some studies to characterize this entity,1 the discussion continues.
The concept of “remission” in asthma is nothing new: it has been a controversial topic since the first attempts to define it were made in the 1980s, when Bronnimann and Burrows3 used the term to describe the absence of asthma attacks or symptoms in a patient with no asthma medication use for ≥1 year. Another concept is that of complete remission, which requires not only the prolonged absence of symptoms, but also the objective demonstration of normal airway function and negative bronchial hyperresponsiveness (BHR), no evidence of bronchial inflammation, and even the absence of any airway pathology suggestive of asthma.4,5
The following predictive markers for remission have been proposed: mild asthma; better lung function; better asthma control; younger age; early onset of the disease; shorter duration of asthma; milder BHR; no or few comorbidities; and no history of smoking.6
The current concept of remission is based on experience from other inflammatory diseases, such as rheumatoid arthritis, and the effect of treatment. However, in the case of asthma, while guidelines have begun to implement the term,7 there is still no clear definition, either in terms of its meaning or the parameters that should be included (clinical, functional, inflammatory, BHR, etc.) or whether it should be divided into different concepts (clinical or complete remission). It is also unclear how long it takes to be able to speak of remission, or whether the expression should be used in patients on or off treatment.
It is equally uncertain how a definition might contribute to the long-established concept of disease control, the assessment of treatment response using the scores and indices that are currently accepted or under validation (FEOS [FEV1, Exacerbations, Oral corticosteroids, Symptoms]8 and EXACTO scores [Exacerbations, ACT, Corticosteroids and Obstruction-FEV1]9), or the concept of super-responders to biological therapies.10
A number of validated tools are used to assess asthma symptoms, although they have not been specifically designed to determine disease remission. This is the case with the Asthma Control Questionnaire (ACQ) and the Asthma Control Test (ACT), which have been used in some studies to assess the response to monoclonal drugs and define the concept of clinical remission, using different cut-off points. For these purposes, the ACQ applies a cut-off point of <1, lower than that generally used,11 while the ACT habitually uses scores of >20 (PROSPERO), and even 25, as cut-off points.12,13
Other parameters to consider as remission criteria are the absence of exacerbations and no need for systemic steroids for a set period of time.1
In type 2 inflammation, different diseases often coexist that share a common underlying inflammatory pathophysiological mechanism, such as atopic dermatitis or chronic rhinosinusitis with nasal polyposis, leading to the concept of asthma as a type 2 systemic disease.14 Consequently, it may be important to include evaluation of the upper airway in the concept of united airway remission.15
To analyze these proposals, taking into account their relevance in current asthma management options, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) has advocated the multidisciplinary working group REMAS (REMission in ASthma) through the Autonomic Asthma Forum (FORASMA) and the working group of the Spanish Asthma Management Guidelines (GEMA). This group has set itself the objective of reaching consensus on the definitions of remission in asthma and establishing criteria and characteristics that will be of use in future studies evaluating the efficacy or effectiveness of treatments.
MethodsThis study was carried out following the consensus methodology developed by the RAND/UCLA.16 The Recommendation Development Group (RDG) was composed of 26 experts (respiratory medicine specialists, allergy specialists, family physicians, pediatricians, and pharmacists) with experience in the management of asthma patients. The first meeting held in January 2023 defined the concepts on which consensus was to be developed.
Based on the concepts, a non-systematic review of the literature related to complete and/or clinical remission in asthma was carried out in the PubMed databases (data closure: June 2023). The RDG was able to add studies they considered pertinent and subsequently performed a critical reading of the publications. The group then formulated the statements relating to the concepts previously defined to submit them to a vote by the panelists, proposing a total of 42 statements.
The 42 proposed statements were evaluated using a 2-round iterative Delphi process according to a 9-point Likert scale (1: strongly disagree; 9: strongly agree) using an online questionnaire. The RAND/UCLA methodology was used for analysis of consensus in Delphi panels.16 Each item in the questionnaire was classified according to the level of agreement and the median score of the panel as “appropriate” (median in the 7–9 range), “uncertain” (median in the 4–6 range or any median with disagreement) or “inappropriate” (median in the 1–3 range). Agreement was reached if at least one third of the sample responded within the same score range as the median. Disagreement was considered to occur if the median score was at either of the 2 extremes and more than one third of the sample responded in the opposite extreme range, or if the median was in the central range, and at least one third of the sample responded in one of the other 2 ranges. If the assessment of the statement did not meet any of the previous criteria, it was considered neutral. Of the 139 panelists invited, 123 completed the first round (88% response rate) and 120 completed the second round (97% response rate) (a detailed description of professional activity can be found in the Supplementary Figs. 1–4).
After the 2 rounds of voting, the expert panel reached consensus on the agreement or disagreement of 71.5% of the statements (a total of 30 of the 42 proposed). The distribution of the voting ranges can be found in Supplementary Fig. 5. Below is the rationale for each point raised and the results obtained, which are summarized in the respective tables.
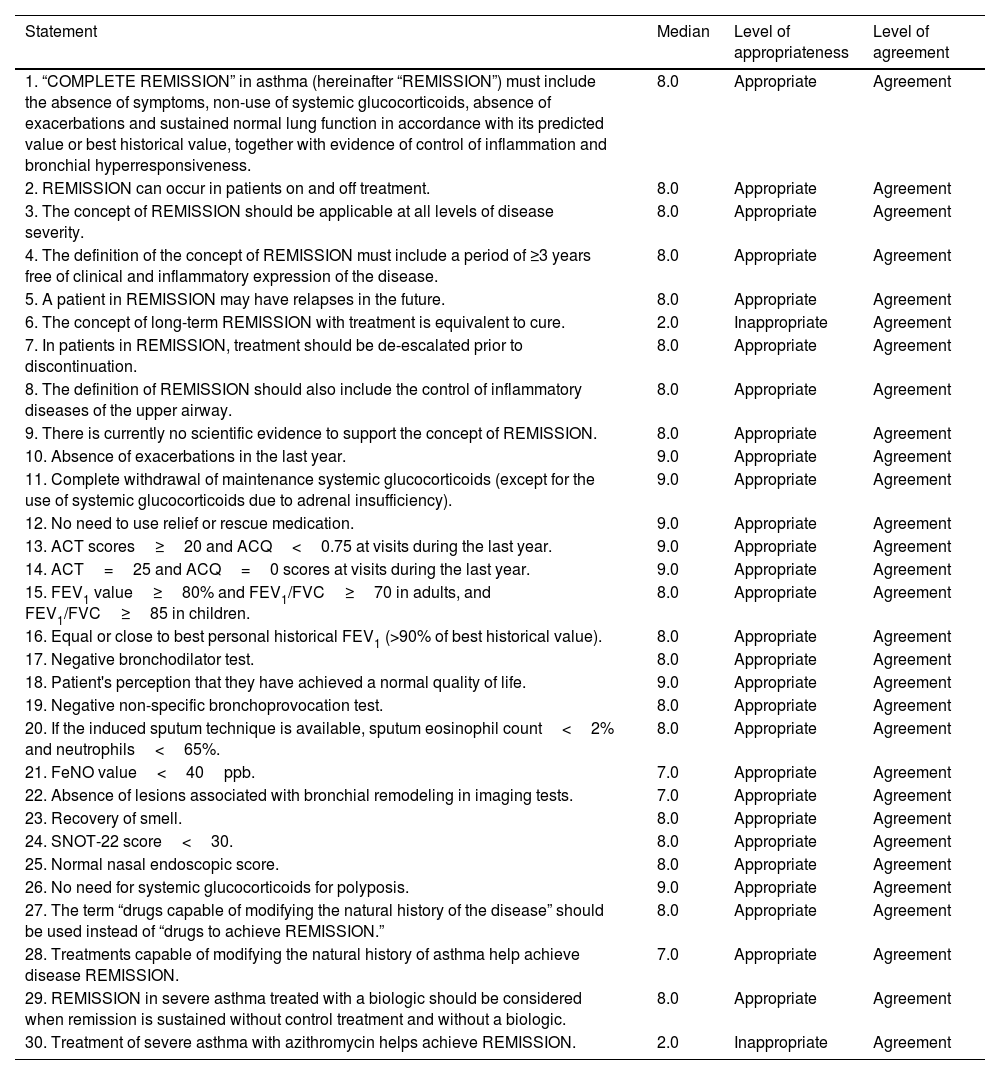
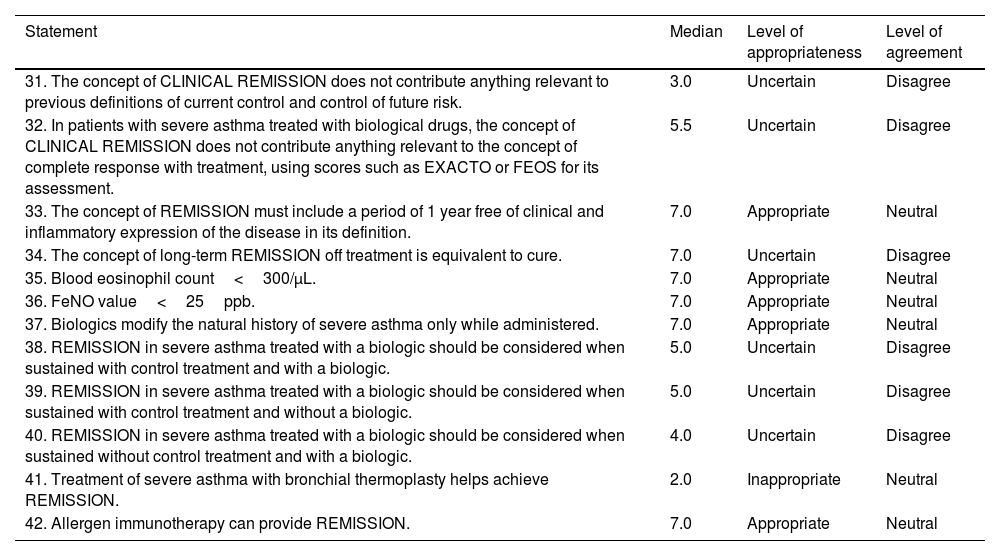
ResultsConcept of remissionThe panel approved a definition for the term “complete remission” (Table 1, statement 1) and a series of definitions that included the possibility of using this concept in patients on and off treatment, at any level of disease severity, the possibility of treatment de-escalation, and the inclusion of the upper airway. Agreement was reached on the likelihood of future relapses and how remission is not comparable to that of cure. Finally, it was agreed that the definition should include a period of ≥3 years free of clinical and inflammatory expression of the disease, while stating the absence of scientific evidence (Table 1, statements 2–9). Previous definitions for which no consensus was reached are presented in Table 2 (statements 31–34). It is interesting to note that uncertainty was expressed surrounding the statement that the concept of clinical remission does not contribute anything relevant to the concepts of disease control or treatment response using the current response assessment indices or scores.
Statements agreed by the panel.
| Statement | Median | Level of appropriateness | Level of agreement |
|---|---|---|---|
| 1. “COMPLETE REMISSION” in asthma (hereinafter “REMISSION”) must include the absence of symptoms, non-use of systemic glucocorticoids, absence of exacerbations and sustained normal lung function in accordance with its predicted value or best historical value, together with evidence of control of inflammation and bronchial hyperresponsiveness. | 8.0 | Appropriate | Agreement |
| 2. REMISSION can occur in patients on and off treatment. | 8.0 | Appropriate | Agreement |
| 3. The concept of REMISSION should be applicable at all levels of disease severity. | 8.0 | Appropriate | Agreement |
| 4. The definition of the concept of REMISSION must include a period of ≥3 years free of clinical and inflammatory expression of the disease. | 8.0 | Appropriate | Agreement |
| 5. A patient in REMISSION may have relapses in the future. | 8.0 | Appropriate | Agreement |
| 6. The concept of long-term REMISSION with treatment is equivalent to cure. | 2.0 | Inappropriate | Agreement |
| 7. In patients in REMISSION, treatment should be de-escalated prior to discontinuation. | 8.0 | Appropriate | Agreement |
| 8. The definition of REMISSION should also include the control of inflammatory diseases of the upper airway. | 8.0 | Appropriate | Agreement |
| 9. There is currently no scientific evidence to support the concept of REMISSION. | 8.0 | Appropriate | Agreement |
| 10. Absence of exacerbations in the last year. | 9.0 | Appropriate | Agreement |
| 11. Complete withdrawal of maintenance systemic glucocorticoids (except for the use of systemic glucocorticoids due to adrenal insufficiency). | 9.0 | Appropriate | Agreement |
| 12. No need to use relief or rescue medication. | 9.0 | Appropriate | Agreement |
| 13. ACT scores≥20 and ACQ<0.75 at visits during the last year. | 9.0 | Appropriate | Agreement |
| 14. ACT=25 and ACQ=0 scores at visits during the last year. | 9.0 | Appropriate | Agreement |
| 15. FEV1 value≥80% and FEV1/FVC≥70 in adults, and FEV1/FVC≥85 in children. | 8.0 | Appropriate | Agreement |
| 16. Equal or close to best personal historical FEV1 (>90% of best historical value). | 8.0 | Appropriate | Agreement |
| 17. Negative bronchodilator test. | 8.0 | Appropriate | Agreement |
| 18. Patient's perception that they have achieved a normal quality of life. | 9.0 | Appropriate | Agreement |
| 19. Negative non-specific bronchoprovocation test. | 8.0 | Appropriate | Agreement |
| 20. If the induced sputum technique is available, sputum eosinophil count<2% and neutrophils<65%. | 8.0 | Appropriate | Agreement |
| 21. FeNO value<40ppb. | 7.0 | Appropriate | Agreement |
| 22. Absence of lesions associated with bronchial remodeling in imaging tests. | 7.0 | Appropriate | Agreement |
| 23. Recovery of smell. | 8.0 | Appropriate | Agreement |
| 24. SNOT-22 score<30. | 8.0 | Appropriate | Agreement |
| 25. Normal nasal endoscopic score. | 8.0 | Appropriate | Agreement |
| 26. No need for systemic glucocorticoids for polyposis. | 9.0 | Appropriate | Agreement |
| 27. The term “drugs capable of modifying the natural history of the disease” should be used instead of “drugs to achieve REMISSION.” | 8.0 | Appropriate | Agreement |
| 28. Treatments capable of modifying the natural history of asthma help achieve disease REMISSION. | 7.0 | Appropriate | Agreement |
| 29. REMISSION in severe asthma treated with a biologic should be considered when remission is sustained without control treatment and without a biologic. | 8.0 | Appropriate | Agreement |
| 30. Treatment of severe asthma with azithromycin helps achieve REMISSION. | 2.0 | Inappropriate | Agreement |
ACT, asthma control test; ACQ, 5-item asthma control questionnaire; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; EXACTO, exacerbations, ACT, systemic corticosteroids and obstruction-FEV1; SNOT-22, 22-item sinonasal outcome test.
Statements not agreed by the panel.
| Statement | Median | Level of appropriateness | Level of agreement |
|---|---|---|---|
| 31. The concept of CLINICAL REMISSION does not contribute anything relevant to previous definitions of current control and control of future risk. | 3.0 | Uncertain | Disagree |
| 32. In patients with severe asthma treated with biological drugs, the concept of CLINICAL REMISSION does not contribute anything relevant to the concept of complete response with treatment, using scores such as EXACTO or FEOS for its assessment. | 5.5 | Uncertain | Disagree |
| 33. The concept of REMISSION must include a period of 1 year free of clinical and inflammatory expression of the disease in its definition. | 7.0 | Appropriate | Neutral |
| 34. The concept of long-term REMISSION off treatment is equivalent to cure. | 7.0 | Uncertain | Disagree |
| 35. Blood eosinophil count<300/μL. | 7.0 | Appropriate | Neutral |
| 36. FeNO value<25ppb. | 7.0 | Appropriate | Neutral |
| 37. Biologics modify the natural history of severe asthma only while administered. | 7.0 | Appropriate | Neutral |
| 38. REMISSION in severe asthma treated with a biologic should be considered when sustained with control treatment and with a biologic. | 5.0 | Uncertain | Disagree |
| 39. REMISSION in severe asthma treated with a biologic should be considered when sustained with control treatment and without a biologic. | 5.0 | Uncertain | Disagree |
| 40. REMISSION in severe asthma treated with a biologic should be considered when sustained without control treatment and with a biologic. | 4.0 | Uncertain | Disagree |
| 41. Treatment of severe asthma with bronchial thermoplasty helps achieve REMISSION. | 2.0 | Inappropriate | Neutral |
| 42. Allergen immunotherapy can provide REMISSION. | 7.0 | Appropriate | Neutral |
FEOS, FEV1, exacerbations, oral corticosteroids, symptoms score; FeNO, fractional exhaled NO.
The statements proposed to the panelists on the specific parameters for establishing the diagnosis of remission were based on those included in various studies, such as the evaluation of symptoms with commonly used tests such as the ACQ and ACT, questioning the specific cut-off points; the presence or absence of exacerbations; lung function, with specific cut-off points for FEV1 (forced expiratory volume in the first second) and FEV1/FVC (forced vital capacity); the need to perform a challenge test; and the study of inflammation using FeNO cut-off points or the induced sputum cell count, if available. The parameters that the panelists found appropriate for establishing the diagnosis of remission can be found in Table 1 (statements 10–22). Cut-off points for ACT>20 and ACQ<0.75 are highlighted (although the possibility of more stringent values, with cut-off points of ACT=25 and ACQ=0, was also agreed). Agreement was reached in terms of lung function, both an FEV1>80% and an FEV1 equal to or close to patient's personal best historical FEV1 (>90% of their historical best), a negative bronchodilator test, and a negative non-specific provocation test. The FeNO cut-off point was set at <40ppb. The possibility of considering the absence of lesions associated with remodeling in imaging tests was also mentioned. Parameters that did not reach the appropriate level of consensus are listed in Table 2 (statements 35–36). Notably, no agreement was reached with regard to eosinophil count<300/μL or FeNO value<25ppb.
In the concept of united airway remission, the question arose regarding specific data on the clinical course of nasal polyposis. Consensus was reached on the recovery of smell, a sinonasal outcome test 22 (SNOT-22) score<30, normal nasal polyp endoscopy score, and no need for systemic glucocorticoids (Table 1, statements 23–26).
Effects of medication in relation to remissionTaking into account the specific evidence on the effect of discontinuing biologics, a series of statements were proposed. Those approved by the panel can be found in Table 1 (statements 27–29). It is interesting to note that the panelists found it preferable to use the term “drugs capable of modifying the natural history of the disease” instead of “drugs to achieve remission” and how precisely treatments capable of modifying the natural history of the disease would allow remission to be achieved. The report also highlights that asthma treated with biologics should be considered in remission when remission is maintained without control treatment and without a biologic. No consensus was reached on the other options, as can be seen in Table 2 (statements 37–40).
The panelists reach consensus on the disagreement that azithromycin does help achieve remission (Table 1, statement 30). Conversely, no consensus was reached on the disagreement regarding whether thermoplasty helps achieve remission (Table 2, statement 41), nor was consensus reached on the item referring to allergen immunotherapy (AIT) and remission (Table 2, statement 42).
Based on the above results, the RDG agreed on a concept of clinical remission that involves fulfilling all of the following conditions: disease control (ACT=20–25), absence of exacerbations, no need for systemic steroids, lung function with FEV1≥80% predicted or, if previous spirometries are available, a value equal to or close to FEV1>90% of the patient's best historical value, and a negative bronchodilator test. This situation must be maintained for at least 12 months, and it should be specified whether it is with treatment or once treatment has been discontinued.
DiscussionAs the repeated attempts described in the literature and the results of this consensus reveal, the definition of remission in asthma is controversial. Nevertheless, it can and should be the ultimate goal in the asthmatic patient.
One of the milestones of this study was to reach consensus on a definition for complete remission and on the period it must span to be considered as such, in addition to other important factors. Thus, in order to consider a patient in remission, in addition to the complete absence of symptoms and exacerbations and no need for oral steroids, they must have sustained normal lung function and show evidence of control of inflammation and negative BHR. The panelists agreed that this situation must be maintained for at least 3 years. This is clearly a stricter definition than those established so far in the majority of recent articles.17 On the other hand, although a consensus could not be reached on the concept of clinical remission, there was disagreement on the proposal that this concept does not contribute anything to the existing concepts of disease control or response to treatment determined with indices such as the FEOS8 and EXACTO scores.9 Therefore, in the absence of further evidence, the need to use a concept derived from the cut-off points with which there is agreement was suggested, for which the RDG established a concept of clinical remission that included these clinical and functional parameters.
In one of the earliest references on clinical remission in asthma, the criterion used for its definition was no asthma attacks or symptoms in the absence of treatment for 1 year.3 Several articles were subsequently published in which this concept was used, usually defining it as the absence of symptoms for a long period of time. In addition, the common denominator of most definitions includes not using medication and a period of at least 1 year, which in some articles can be as long as 2 or even 3 years.3,18–23The prevalence of clinical remission according to this concept is estimated to be between 2% and 52% of patients, depending on the study and the definition, in most cases these were not very stringent and population samples were small.12,18–20,24–33 Follow-up in these studies ranges between 5 and 70 years.12,18–20,24–33 However, the natural history of asthma must be taken into account: the disease changes throughout life, so childhood asthma cannot be compared with that of adults. This factor would explain the different remission rates observed in studies conducted in children (6–52%), compared with adults (2–17%).34
Menzies-Gow et al.35 recently proposed different types of definitions for remission:
- -
Clinical remission, defined as the absence of symptoms and exacerbations for at least 12 months; optimization and stabilization of lung function; absence of use of corticosteroids for exacerbation or asthma control; and agreement between the patient and physician regarding disease remission.
- -
Complete remission, when patients also present no BHR or bronchial inflammation.35
Furthermore, both these concepts are considered on and off treatment.
Using criteria similar to those described for clinical remission, the same authors subsequently published a post hoc study of patients enrolled in the SIROCCO/CALIMA and ZONDA benralizumab clinical trials.36 The parameters were evaluated for 6 months in ZONDA and for 6–12 months in SIROCCO and CALIMA. Based on these criteria, the results from the ZONDA study showed 22.5% remission in the treatment group versus 7.5% in the placebo group, while for SIROCCO and CALIMA, the authors reported 14.5% remission in the benralizumab group versus 7.7% in the placebo group.36
More recently, in Italy, 80 panelists reached a consensus on the concepts of complete clinical remission and partial clinical remission. For complete clinical remission it was agreed, after the second round, that the following 4 criteria should be met: absence of asthma symptoms (ACT≥20 or ACQ<1.5); absence of asthma exacerbations or attacks; stable lung function; and no need for treatment with oral corticosteroids. On the point of functional stability, no consensus was reached on the value required to consider lung function stable (improvement of 100–200mL or FEV1≥80%). Another parameter evaluated was the normalization of asthma-related quality of life, although no consensus was reached on this item regarding the questionnaire cut-off point. With respect to the clinically relevant reduction in inflammatory parameters in asthma, the cut-off points of <300eosinophils and FeNO<25ppb were considered good markers of a reduction in inflammation, but consensus was not reached for this statement as a criterion for inflammatory remission. In the case of partial clinical remission, the need to meet the criterion of no need for oral corticosteroid treatment and 2 of the following 3 criteria was indicated: absence of symptoms, absence of exacerbations, and stable lung function. The time frame for considering clinical remission was set at ≥12 months.17
The concept of remission agreed in our study can thus be considered more stringent than previous proposals, in terms of the inclusion of normality in symptoms, lung function, BHR and inflammation, as well as a minimum period of 3 years. Furthermore, independently of the treatment, it considers the future possibility of including imaging tests to assess the absence of remodeling.
On the other hand, the term clinical remission agreed by the RDG is similar to that suggested by Menzies-Gow et al.,36 with the exception that clearer cut-off points are provided for lung function (current FEV1% and as it relates to the best historical value; negative bronchodilator test).
However, in our study, the required level of agreement was not reached to include a blood eosinophil count<300/μL as a parameter to be taken into account for the diagnosis of remission. The reasons given in the participants’ comments were that the eosinophil count could also be related to the presence of other conditions with a type 2 profile such as atopic dermatitis, allergic rhinitis and nasal polyposis.37 In this sense, a recent meta-analysis showed that the cut-off point for eosinophil levels could range between 157 and 280eosinophils/μL in asthma in general (22 studies) or 200–400eosinophils/μL in the case of severe asthma (8 studies); however, these values are affected by various factors, such as smoking, positive skin-prick test, elevated total IgE, comorbid allergic rhinitis, age≤18 years, male sex, spirometric asthma/chronic obstructive pulmonary disease, metabolic syndrome, and obesity.38
The appropriate level of agreement was not reached for the statement that biological treatments are capable of altering the natural history of the disease when administered. Although biological treatments have shown a reduction in exacerbations and improvements in disease control, quality of life and lung function, their impact in modifying the pathophysiology of asthma in a sustained way and, therefore, changing the natural history of the disease, remains to be demonstrated. Some promising results with new biological treatments in terms of controlling BHR,39 inflammation, and even remodeling parameters, such as mucous plugs40 have recently been published, but long-term studies are needed to evaluate these outcomes and whether they are maintained once treatment is withdrawn.
In relation to AIT, the subanalysis of allergy specialists did reach the level of agreement. The treatment of asthma with AIT in children and adolescents shows the possibility of modifying the natural history of the disease.41,42 However, this is yet to be demonstrated in populations treated at older ages, and the specific endophenotype in which it occurs must be identified.
The strengths of this study include the number of asthma experts who participated, its multidisciplinary nature, and the participation of international experts.
Regarding the limitations of this study, the type of professionals involved could be questioned, since there could be a bias toward 1 group. The fact that the consensus process was conducted under the auspices of SEPAR could lead, on the one hand, to a predominance of respiratory medicine specialists and, on the other, to a geographical constraint, since the exclusively Spanish setting may be a limitation for its international use. Furthermore, the definitions of remission, both complete and clinical, should be validated by clinical studies to demonstrate that achieving these goals is associated with better long-term outcomes.
ConclusionsIn this study, consensus was reached on the definition of complete remission in asthma (referred to as remission). The concept of remission should include the absence of symptoms, no need for systemic glucocorticoids, absence of exacerbations and sustained normal lung function, in accordance with its predicted value or best historical value, together with compliance with the parameters for control of inflammation and BHR. It can occur in patients on or off treatment, and it should be applicable at all levels of disease severity and include a period of ≥3 years free of clinical and inflammatory expression of the disease and control of upper airway inflammatory diseases (Table 1). Although the statements regarding the concept of clinical remission did not reach the necessary level of agreement (Table 2, statements 31 and 32), there was disagreement on the statement that this concept contributes nothing relevant to previous definitions of current control and control of future risk, or to the concept of complete response to treatment, using scores such as EXACTO or FEOS. Accordingly, the RDG states that the concept of clinical remission could be applied if all of the following conditions are met: disease control (ACT≥20), absence of exacerbations; no need for systemic steroids; lung function with FEV1≥80% predicted, or, if previous spirometries are available, a value equal to or close to FEV1>90% of the patient's best historical value; and a negative bronchodilator test. This situation should be maintained for at least 12 months, and it should be specified whether remission is associated with treatment or occurs once treatment has been discontinued (Table 3).
Concepts related to remission.
| Remission in asthma | |
| CLINICAL remission | - Controlled asthma (ACT≥20).- No need for relief or rescue medication.- No exacerbations and no need for systemic steroid cycles.- Spirometry with FEV1≥80%, or in previous tests, values >90% of your personal best.- Spirometry with negative bronchodilator test.- This situation must be maintained for ≥12 months, specifying whether it is with or without treatment. |
| COMPLETE remission | - All clinical remission criteria.- No evidence of systemic or bronchial inflammation (FENO<40ppb and eosinophils sputum<2%, if performed).- No bronchial hyperresponsiveness.- No bronchial remodeling lesions on imaging tests.- This situation must be maintained for ≥3 years, specifying whether it is with or without treatment. |
| Remission in asthma and CRSwNP (single airway) | |
| COMPLETE remission | - All criteria for complete remission in asthma.- Recovery of smell.- SNOT-22<30.- Normal nasal endoscopy.- This situation must be maintained for ≥3 years, specifying whether it is ±treatment. |
ACT, asthma control test; CRSwNP, chronic rhinosinusitis with nasal polyps; FENO, fractional-exhaled-nitric-oxide; FEV1, forced expiratory volume in 1s; SNOT-22, Sino-nasal Outcome Test.
In any case, prospective studies using remission as the study objective must be carried out to establish scientific evidence on remission (clinical or complete). In this way, the concept will obtain recognized scientific validity.
Conflict of interestFrancisco Javier Álvarez-Gutiérrez has received consulting fees from AstraZeneca, GSK, Sanofi, and
Bial has received speakers honoraria/manuscript support from AstraZeneca, Teva, GSK, Bial, Sanofi, and Orion Pharma and has received travel support from AstraZeneca, GSK, Bial, and Sanofi.
Fracisco Casas-Maldonado has received consulting fees from AstraZeneca, GSK, Grifols, Sanofi, and CSL Behring; has received speaker's honoraria and travel support from AstraZeneca, Boehringer-Ingelheim, GSK, Grifols, Sanofi, and CSL Behring.
Gregorio Soto-Campos has received speakers honoraria/manuscript support from AstraZeneca, Chiesi, Gebro, Sanofi, and GSK; has received travel support from Sanofi, and AstraZeneca.
Marina Blanco-Aparicio has received speakers honoraria/manuscript support from AstraZeneca, Sanofi, GSK, Teva, and Chiesi.
Julio Delgado Romero has received fees for advisory boards from Bial, has received speaker's honoraria from AstraZeneca, Bial, Chiesi, GlaxoSmithKline Novartis, and Sanofi, and received Grant/Research Support from AstraZeneca and Orion. He also received help assistance with meeting travel from Sanofi and Menarini.
Alicia Padilla-Galo has received consulting fees from AstraZeneca, Sanofi, GSK, Chiesi, Menarini, Alk-Abelló, and Bial, and has received travel support from AstraZeneca and Sanofi.
Santiago Quirce has received consulting fees and speaker's honoraria from ALK, Allergy Therapeutics, AstraZeneca, Chiesi, GSK, Mundipharma, Novartis, Sanofi, and Teva Pharmaceuticals.
Vicente Plaza has received consulting fees from AstraZeneca, Chiesi, GSK, and Medwell, and has received travel support from AstraZeneca.
The authors want to acknowledge the contribution of the following panelists: Carlos Almonacid Sánchez;María Álvarez Puebla; Rubén Andújar Espinosa; Jose Ángel Carretero; Aurelio Arnedillo Muñoz; Sonia de Arriba Méndez; Mario Bárcena Caamaño; Blanca Barroso García; Miguel Ángel Bergna; Irina Bobolea; Ana Boldova Loscertales; Carlos Bujalance Cabrera; Carlos Cabrera López; Concepción Cañete; Manuel Castilla Martínez; Jose Antonio Castillo Vizuete; Pilar Cebollero Rivas; Carlos Andrés Celis Preciado; Carolina Cisneros Serrano; Carlos Colas; Astrid Crespo; Elena Curto Sánchez; Alfredo De Diego Damiá; David Díaz Pérez; Javier Dominguez; Sandra Dorado; Teresa Dordal Culla; Ibon Eguiluz; Ana Isabel Enríquez Rodríguez; Luis Manuel Entrenas; Cleofe Fernández Aracil; Ana Fernández Tena; Jorge Ferreira; Rocío García; Luis García Marcos; Juan Luis Garcia Rivero; Juan José Garrido Romero; Ana Carmen Gil Adrados; Raúl Godoy Mayoral; Ana Gómez Bastero; José Antonio Gullón Blanco; Tamara Hermida Valverde; Luis Hernández Blasco; José Ignacio García; Hemily Izaguirre Flores; Alberto Levy Naón; María José Linares Serrano; Antolín Lopez Viña; Jaime Lozano Blasco; Antonio Martínez Gimeno; Carlos Martínez Rivera; Antonio Martorell Aragonés; Juan Francisco Medina Gallardo; Carlos Melero Moreno; Francisco Javier Michel De La Rosa; Francisco Javier Montoro Lacomba; Antonio Moreno Galdo; Mar Mosteiro Añon; Daniel Ocaña Rodríguez; Iñigo Ojanguren; José María Olaguíbel; Cristina Ortega Casanueva; Abel Pallarés; Antonio Parra Arrondo; Antonio Pereira Vega; Gerardo Pérez Chica; Francisco Pérez-Grimaldi; Francisco Javier Plaza Zamora; Manuel Pranea; José Ignacio Prieto Romo; Carolina Puchaes Manchón; Ana Pueyo; Jacinto Ramos Gonzalez; Manuel Rial; Fernando Rodriguez; Berta Román Bernal; Auxiliadora Romero; Pedro Romero Palacios; Francisco José Sáez Martínez; Silvia Sanchez; Sylvia Sanchez Cuellar; Fernando Sánchez-Toril López; Joaquín Sastre; Antonio Sebastián Ariño; Joan Serra Batlles; José Carlos Serrano Rebollo; Ana Isabel Sogo Sagardía; Agustín Sojo Gonzalez; Andrea Trisán; Isabel Inés Urrutia Landa; Borja Valencia Azcona; Antonio Valero; José Miguel Valero Pérez; Paz Vaquero Lozano; Jose Vega; Elisabeth Vera Solsona; Ana Viejo Casas; Ignacio Zabert
The authors would like to thank Antoni Torres-Collado, PhD and Blanca Piedrafita, PhD of Medical Statistics Consulting (MSC, Valencia) for methodological support during the Delphi consultation and editorial assistance.
- •
Respiratory Medicine: César Picado, Hospital Clínic, Barcelona
- •
Respiratory Medicine: Christian Domingo Ribas, Corporació Parc Taulí, Sabadell
- •
Respiratory Medicine: Francisco Javier González-Barcala, Hospital Clínico Universitario de Santiago de Compostela
- •
Respiratory Medicine: Miguel Perpiñá Torderá, Hospital Universitario y Politécnico La Fe, Valencia
- •
Respiratory Medicine: Xavier Muñoz Gall, Hospital Universitario Vall d’Hebron, Barcelona
- •
Respiratory Medicine: Eva Martinez Moragón, Hospital Universitario Dr Peset, Valencia
- •
Respiratory Medicine: Luis Pérez de Llano, Hospital Universitario Lucus Augusti, Lugo
- •
Allergology: Javier Domínguez-Ortega, Hospital Universitario La Paz, Madrid
- •
Allergology: Ignacio Dávila González, Hospital Universitario de Salamanca, Salamanca
- •
Allergology: Juan Carlos Miralles-López, Hospital Universitario Reina Sofia, Murcia
- •
Hospital Pharmacy: Noé Garin Escrivá, Hospital de la Santa Creu i Sant Pau, Barcelona
- •
Pharmacology: Antonio Gómez Outes, Agencia Española de Medicamentos y Productos Sanitarios (AEMPS), Madrid
- •
ALAT: Gabriel García, Centro de Investigación Respiratoria, CEPIR, La Plata, Argentina
- •
Pediatric Respiratory Medicine: Javier Korta Murua, Hospital Universitario Donostia, Donostia
- •
Pediatric Allergology: José Sanz, Hospital Católico Universitario Casa de Salud, Valencia
- •
Primary Care: Jesús Molina París, Centro de Salud Francia, Fuenlabrada
- •
Primary Care: Antonio Hidalgo Requena, Centro de Salud de Lucena, Córdoba
- •
Primary Care: Fernando Gómez Ruiz, Centro de Salud de Bargas. Toledo