To describe the clinical profile of patients with asthma and to identify possible risk factors for its development in subjects over the age of 12.
Patients and methodsA multicenter study of cases and controls. Recruited for inclusion were case subjects between the ages of 12 and 40 diagnosed with asthma, with an onset of symptoms after the age of 12. Control subjects were selected, with ages between 12 and 40, who did not have childhood asthma and did not present symptoms of asthma at the time of the study.
ResultsWe evaluated 923 subjects: 247 cases and 671 controls, 54.9% were women. Mean age of the cases was 28.3±8.2; mean age of controls was 30.8±7.1 (P<.001). In the logistic regression analysis, it was observed that the determining factors for the of the presence of asthma were hypersensitivity to animals or other allergens, presence of rhinitis, family history of asthma, occupational risk/exposure to irritants and the hypersensitivity/intolerance to NSAIDs. In said analysis, it was also demonstrated that age was a protection factor, as well as level of education.
ConclusionsThe risk factors for the development of asthma at an adult age are hypersensitivity to animals or other allergens, rhinitis, family history of asthma, occupational risk/exposure to irritants and the hypersensitivity/intolerance to NSAIDs, while age and level of education are protection factors.
Describir el perfil clínico de los pacientes con asma e identificar posibles factores de riesgo para su desarrollo en sujetos mayores de 12 años.
MétodosEstudio multicéntrico de casos y controles. Se reclutó como casos a sujetos entre 12 y 40 años con diagnóstico de asma, con inicio de los síntomas después de los 12 años. Se seleccionó como controles a sujetos entre 12 y 40 años que no tenían asma durante la infancia y que no presentaban síntomas de asma en el momento de realizar el estudio.
ResultadosSe evaluó a 923 sujetos, 247 casos y 671 controles. El 54,9% de ellos eran mujeres. La media de edad de los casos era 28,3±8,2 y la de los controles, 30,8±7,1 años (p<0,001). En el análisis de regresión logística se observó que los factores determinantes de la presencia de asma fueron la hipersensibilidad a animales o a otros alérgenos, la presencia de rinitis, los antecedentes familiares de asma, la profesión de riesgo/exposición a irritantes y la hipersensibilidad/intolerancia a AINE. En dicho análisis se demostró también que la edad era un factor de protección, así como el nivel de estudios.
ConclusionesLos factores de riesgo para el desarrollo de asma en la edad adulta son la hipersensibilidad a animales o a otros alérgenos, la rinitis, los antecedentes familiares de asma, la profesión de riesgo/exposición a irritantes y la hipersensibilidad/intolerancia a AINE, mientras que la edad y el nivel de estudios son factores protectores.
Asthma is a heterogeneous entity that is the result of complex interactions between environmental and genetic factors. The expression of the disease can vary with age, sex, airway inflammation patterns or severity, association with atopy or other triggering factors.1 In recent years, various clinical subtypes of asthma have been described and to date it is still unknown whether they are variations of a same disease or whether, contrarily, they are different diseases that run their course with similar symptoms.2
World-wide, asthma affects approximately 300 million people and, despite the notable therapeutic advances, it currently causes around 250,000 deaths per year. In Spain, according to the results of the European Community Respiratory Health Survey, the prevalence is high, although there is a clear geographical variation, ranging from 5% in Galdácano to 14.5% in Huelva.3
Given the high prevalence and the incidence of said affectation in the western world and its socioeconomic consequences, there is a need to identify risk factors in order to define possible primary prevention strategies.1,4 Currently, there are a very limited number of recommendations to prevent the development of asthma with sufficient scientific evidence.5
The current understanding about risk factors for developing asthma is mostly based on studies carried out in children; meanwhile, those involved in the development of the process in adults are less defined.4 In adults, atopy has been associated with the development of asthma as have the exposure to possible allergens present in the home and environment, such as pollen and grasses.6
A close relationship has also been reported between rhinitis and asthma. Both entities coexist in a large majority of patients and they share common risk factors, as is the case of atopy.7 Allergic rhinitis tends to precede asthma, and some authors propose it as an independent risk factor for the development of asthma, even in persons over the age of 50.8 Furthermore, if smoking and chronic sinusitis are excluded, the risk factors for the development of asthma in adults have not been clarified.
The objective of this study is to describe the clinical profile of asthma patients and identify possible risk factors for the development of asthma in subjects over the age of 12.
Material and MethodsStudy DesignFor the FENASMA (Fenotipos de Asma–Asthma Phenotypes) study, a multi-center study of cases and controls was designed and carried out between May 13 and October 21, 2009. The study was reviewed and approved by the Clinical Assay Committee of the Hospital General Universitario Gregorio Marañón in Madrid.
Study Population and SettingIn order to recruit the patients, we relied upon volunteer physicians who are specialists in pulmonology and allergology, distributed throughout the Spanish territory.
The inclusion criteria for the cases included subjects between the ages of 12 and 40 years of age who were seen in the consultation for any reason. The patients were those who had been diagnosed with asthma in the last 12 months and not necessarily at the time of their inclusion in the study. For the diagnosis of bronchial asthma, a compatible clinical history was necessary. Also needed were lung function tests demonstrating a reversible and variable airflow limitation (meeting the criteria of international guidelines4) and an onset of the symptoms after the age of 12. The inclusion criteria of the control subjects were defined as subjects between the ages of 12 and 40, with no history of asthma during childhood and who did not present subjective symptoms of the disease at the time of the study, who were seen in the consultation regardless of the reason, and who had no history of a chronic respiratory disease. The control subjects were included within 7 following the identification of each case. In addition, they could have no family relationship with the cases.
Excluded from the study were those patients who presented chronic obstructive pulmonary disease (COPD) or spirometry compatible with a mixed or restrictive pattern, individuals under the age of 12, those who had experienced an exacerbation in their lung disease in the previous 4 weeks, patients with any type of physical or mental impediment that would make it difficult to carry out the diagnostic tests and persons who did not give their consent to participate in the study.
The sample size was calculated by means of a multistage stratified probability sampling without replacement. The sample was obtained from the 17 autonomous regions of Spain. The first phase consisted of selecting specialists in pulmonology and allergology from each health-care region. The number of participating specialists was chosen proportionally to the population of each region. The probability of selection for each clinic/hospital was related to the population of the area of influence of said health-care center. The complete list of the health-care centers where the specialists were ascribed is given in detail in the acknowledgements section. In the second phase, a specialist was randomly selected at each center from among the professionals with experience in clinical research as well as epidemiology. The third phase was the patient selection. Patients were selected through a systematic sampling obtained from the daily list of patients who had appointments with each specialist participating in the study. These patients were required to meet all the inclusion criteria and none of the previously mentioned exclusion criteria.
The sample size was calculated taking into account that the presence of risk factors in the control population should not be less than 5%, considering relevant an odds ratio (OR) of more than 2.5 in the cases compared with the controls, for a statistical power of 80% (with Yates correction) and a significance of 5%. In order to have 2 cases per control, it was necessary to evaluate at least 648 patients, out of whom 216 were cases and 432 were control subjects.
Data Collection Questionnaires (DCQ)Each participating specialist filled out a DCQ per patient at a single office visit. The DCQs collected data on sociodemographics, smoking, personal and family history, comorbidities, treatment, spirometric data and bronchial provocation testing, diagnosis and use of health-care resources. The data for the patients’ personal medical histories, family histories and comorbidities were reported by the patients and/or extracted from the clinical medical files.
In those cases in which it was necessary to carry out bronchial provocation tests, the recommendations of the European Respiratory Society and the American Thoracic Society9 were followed in order to guarantee the reproducibility of the data obtained. The skin prick test panel of pneumoallergens was decided upon at each center according to the aerobiological characteristics of each area.
In order to evaluate the control of the disease and quality of life, the following scales were also applied in all cases: ACT (Asthma Control Test),10 SGRQ (St. George's Respiratory Questionnaire)11 and AQ20 (Airways Questionnaire 20).12 These scales are validated internationally and are not only used in daily practice but also in asthma clinical trials. The ACT scale consists of 5 questions that are scored from 1 to 5. The total score is obtained from the sum of each of them (ranging from 5 to 25) in such a way that the higher the score, the better the asthma control. The SGRQ quantifies the impact of the disease on the state of health and well-being of the patients and reflects the changes in activity with the disease. It is made up of 50 items divided into three dimensions: symptoms (frequency and severity), activity (limitations produced by the dyspnea) and impact (problems related with social and psychological functions). The scores obtained range from 0 to 100, this latter value representing the maximum alteration in quality of life. Last of all, the AQ20 scale is an abbreviated quality-of-life questionnaire consisting of 20 items that is scored from 0 to 20, with the highest score representing a poorer quality of life.
Statistical AnalysisThe population that was used for the statistical analysis included all the selected patients who met the inclusion criteria and none of the exclusion criteria.
The DCQs presented lack of information (not filled in by the researcher) for several analysis variables. Therefore, the results that are shown were calculated based on the number of subjects who presented the data. The sample sizes may oscillate in the different variables and be lower than the sample size of the evaluable population.
A descriptive statistical analysis was carried out with all the parameters, which included measurements of the central tendency and dispersion, with the 95% two-tailed confidence interval for the quantitative variables as well as absolute and relative frequencies for the qualitative variables.
We studied the distribution of the quantitative variables and evaluated the fit with the Gaussian distribution using the Kolmogorov–Smirnov test. If the data did not meet the assumed normality for the analysis, non-parametric statistical methods were used.
In the comparison of the independent data (cases vs controls), the odds ratio was obtained with the 95% confidence interval. The statistical significance was calculated using the χ2 test. In the case of quantitative variables, either the Student's t-test was used or, in cases that did not follow a normal distribution, the Mann–Whitney U test.
For the comparison of the results obtained using the ACT scale and the SGRQ questionnaire, the Pearson's correlation coefficient was used.
In order to determine the sociodemographic and clinical predictive factors associated with the control of asthma, a multivariate logistic regression model was completed. The statistical tests were carried out with a level of significance of 5%, and they were bilateral. The SAS® version 8.2 statistical package was used for all the statistical analyses.
ResultsA total of 369 researchers recruited 1475 subjects. Out of these, 552 (37.6%) were excluded for not meeting the selection criteria. In the final sample, 918 subjects were included (62.6%), out of which 247 were cases (26.9%) and 671 controls (73.1%). In total, 490 cases and 62 controls were excluded.
The reasons for exclusion were: the diagnosis of asthma more than 12 months before (467); the onset of symptoms indicating asthma before the age of 12 (69); being younger than 12 or older than 40 years of age (59); having a spirometry compatible with a restrictive or mixed pattern (4); incomplete data (1) and/or lack of informed consent (1). The control subjects were excluded for being under the age of 12 or older than 40 (56); presenting some type of chronic respiratory disease (6); incomplete data (1) and/or lack of informed consent (1).
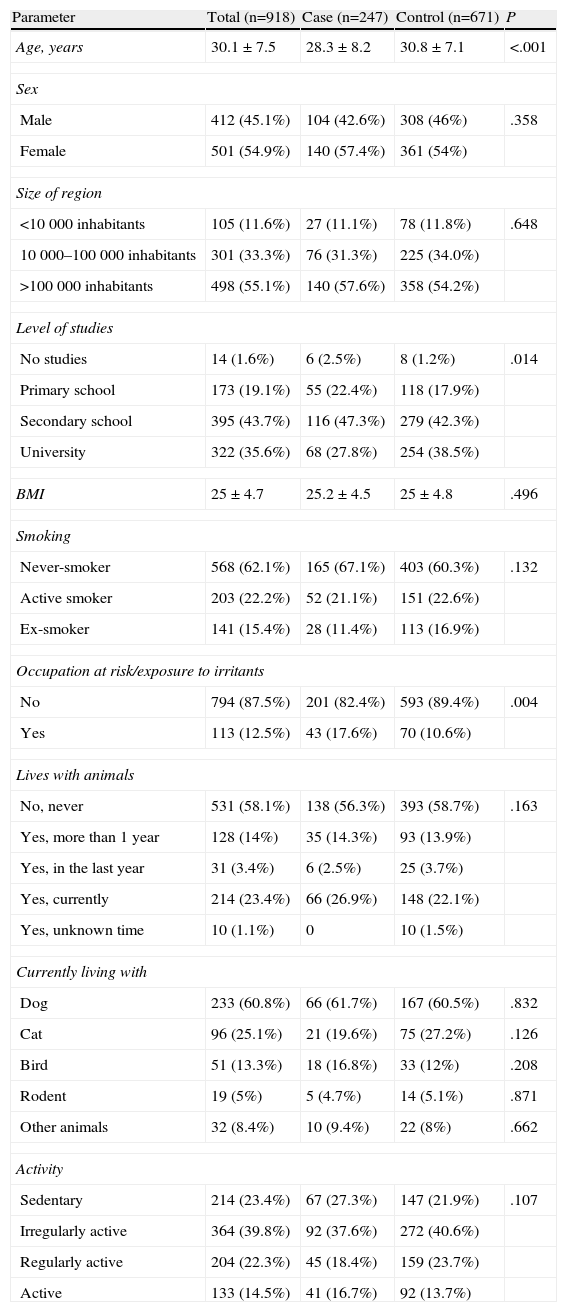
The characteristics of the patients are reflected in Table 1, 54.9% were women. Mean age was 30.1±7.5. In the case group, the mean age was 28.3±8.2 (P<.0001), 43.7% of the subjects had secondary school education, 35.6% university studies and 19.1% primary studies.
Sociodemographic Data of the Patients Included for Study. A Descriptive Statistical Analysis was Done for all the Parameters, Which Included Measurements of the Central Tendency and Dispersion With the 95% Two-Tailed Confidence Interval for the Quantitative Variables, as Well as Absolute and Relative Frequencies for the Qualitative Variables.
| Parameter | Total (n=918) | Case (n=247) | Control (n=671) | P |
| Age, years | 30.1±7.5 | 28.3±8.2 | 30.8±7.1 | <.001 |
| Sex | ||||
| Male | 412 (45.1%) | 104 (42.6%) | 308 (46%) | .358 |
| Female | 501 (54.9%) | 140 (57.4%) | 361 (54%) | |
| Size of region | ||||
| <10000 inhabitants | 105 (11.6%) | 27 (11.1%) | 78 (11.8%) | .648 |
| 10000–100000 inhabitants | 301 (33.3%) | 76 (31.3%) | 225 (34.0%) | |
| >100000 inhabitants | 498 (55.1%) | 140 (57.6%) | 358 (54.2%) | |
| Level of studies | ||||
| No studies | 14 (1.6%) | 6 (2.5%) | 8 (1.2%) | .014 |
| Primary school | 173 (19.1%) | 55 (22.4%) | 118 (17.9%) | |
| Secondary school | 395 (43.7%) | 116 (47.3%) | 279 (42.3%) | |
| University | 322 (35.6%) | 68 (27.8%) | 254 (38.5%) | |
| BMI | 25±4.7 | 25.2±4.5 | 25±4.8 | .496 |
| Smoking | ||||
| Never-smoker | 568 (62.1%) | 165 (67.1%) | 403 (60.3%) | .132 |
| Active smoker | 203 (22.2%) | 52 (21.1%) | 151 (22.6%) | |
| Ex-smoker | 141 (15.4%) | 28 (11.4%) | 113 (16.9%) | |
| Occupation at risk/exposure to irritants | ||||
| No | 794 (87.5%) | 201 (82.4%) | 593 (89.4%) | .004 |
| Yes | 113 (12.5%) | 43 (17.6%) | 70 (10.6%) | |
| Lives with animals | ||||
| No, never | 531 (58.1%) | 138 (56.3%) | 393 (58.7%) | .163 |
| Yes, more than 1 year | 128 (14%) | 35 (14.3%) | 93 (13.9%) | |
| Yes, in the last year | 31 (3.4%) | 6 (2.5%) | 25 (3.7%) | |
| Yes, currently | 214 (23.4%) | 66 (26.9%) | 148 (22.1%) | |
| Yes, unknown time | 10 (1.1%) | 0 | 10 (1.5%) | |
| Currently living with | ||||
| Dog | 233 (60.8%) | 66 (61.7%) | 167 (60.5%) | .832 |
| Cat | 96 (25.1%) | 21 (19.6%) | 75 (27.2%) | .126 |
| Bird | 51 (13.3%) | 18 (16.8%) | 33 (12%) | .208 |
| Rodent | 19 (5%) | 5 (4.7%) | 14 (5.1%) | .871 |
| Other animals | 32 (8.4%) | 10 (9.4%) | 22 (8%) | .662 |
| Activity | ||||
| Sedentary | 214 (23.4%) | 67 (27.3%) | 147 (21.9%) | .107 |
| Irregularly active | 364 (39.8%) | 92 (37.6%) | 272 (40.6%) | |
| Regularly active | 204 (22.3%) | 45 (18.4%) | 159 (23.7%) | |
| Active | 133 (14.5%) | 41 (16.7%) | 92 (13.7%) | |
The mean body mass index (BMI) was 25±4.7. According to the classification of the WHO, 54.2% of the patients had a normal body weight, while the rest presented obesity at varying degrees.13 No statistically significant differences were observed between the different groups.
22.2% of the subjects evaluated were active smokers, with no significant differences between the groups. The average number of daily cigarettes in the sample was 12.9±8, which was less in the case group at 11.4±7.8, while in the control group the amount was 13.4±8.1 (P=.047). The average number of years smoking was 11.3±6.2, with no differences observed. The pack-years index of the cases was 6.1±5.6 and that of the controls was 8.4±7.4 (P=.004).
12.5% of the individuals studied had an occupation at risk or were exposed to environmental irritants, which was more frequent in the case group (P=.004). Among the irritating agents which the patients reported being exposed to in the work setting were cleaning products (26.1%), smoke/fumes (26.2%) and chemical agents (15.3%).
The allergens due to which the cases were sensitized were mainly inhalants: pollens 61.5%, dust mites in el 59.8% and animal epithelial cells in 25.5%, respectively. Only the allergy to dust mites (P=.026) and animals (P<.001) showed significant differences between the cases and controls.
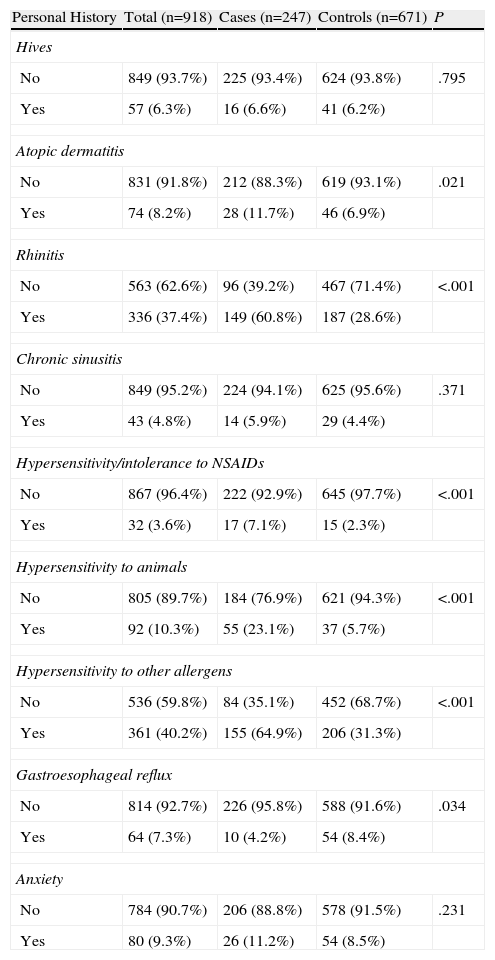
As for their personal medical histories (Table 2), the mean age at onset was 24.1±11.2 in the case of atopic dermatitis, rhinitis at 18.9±8, hypersensitivity or intolerance to non-steroidal anti-inflammatory drugs (NSAIDs) at 24.8±10.7 and anxiety disorders at 25.6±7.6. Statistically significant differences were found in atopic dermatitis, rhinitis, hypersensitivity or intolerance to (NSAIDs), hypersensitivity to other allergen and gastroesophageal reflux disease.
Personal Medical History.
| Personal History | Total (n=918) | Cases (n=247) | Controls (n=671) | P |
| Hives | ||||
| No | 849 (93.7%) | 225 (93.4%) | 624 (93.8%) | .795 |
| Yes | 57 (6.3%) | 16 (6.6%) | 41 (6.2%) | |
| Atopic dermatitis | ||||
| No | 831 (91.8%) | 212 (88.3%) | 619 (93.1%) | .021 |
| Yes | 74 (8.2%) | 28 (11.7%) | 46 (6.9%) | |
| Rhinitis | ||||
| No | 563 (62.6%) | 96 (39.2%) | 467 (71.4%) | <.001 |
| Yes | 336 (37.4%) | 149 (60.8%) | 187 (28.6%) | |
| Chronic sinusitis | ||||
| No | 849 (95.2%) | 224 (94.1%) | 625 (95.6%) | .371 |
| Yes | 43 (4.8%) | 14 (5.9%) | 29 (4.4%) | |
| Hypersensitivity/intolerance to NSAIDs | ||||
| No | 867 (96.4%) | 222 (92.9%) | 645 (97.7%) | <.001 |
| Yes | 32 (3.6%) | 17 (7.1%) | 15 (2.3%) | |
| Hypersensitivity to animals | ||||
| No | 805 (89.7%) | 184 (76.9%) | 621 (94.3%) | <.001 |
| Yes | 92 (10.3%) | 55 (23.1%) | 37 (5.7%) | |
| Hypersensitivity to other allergens | ||||
| No | 536 (59.8%) | 84 (35.1%) | 452 (68.7%) | <.001 |
| Yes | 361 (40.2%) | 155 (64.9%) | 206 (31.3%) | |
| Gastroesophageal reflux | ||||
| No | 814 (92.7%) | 226 (95.8%) | 588 (91.6%) | .034 |
| Yes | 64 (7.3%) | 10 (4.2%) | 54 (8.4%) | |
| Anxiety | ||||
| No | 784 (90.7%) | 206 (88.8%) | 578 (91.5%) | .231 |
| Yes | 80 (9.3%) | 26 (11.2%) | 54 (8.5%) | |
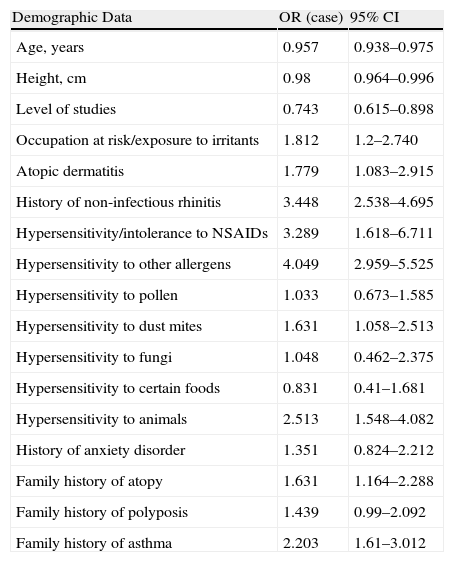
When we correlated the odds ratio and 95% CI according to analysis groups, it was found that the patients with a history of non-infectious rhinitis had 3.4 times more risk for developing asthma. Those with hypersensitivity or intolerance to NSAIDs showed 3.3 times more risk, and those with hypersensitivity to other allergens (pollen, dust mites, fungi, animals) had 4 times more (Table 3). Among the variables analyzed, age (OR=0.969; 95% CI 0.948–0.991) and level of studies (OR=0.684; 95% CI 0.546–0.856) were demonstrated to be protective factors. Out of the comorbidities researched, only hives, chronic sinusitis and anxiety disorders showed no relationship with the diagnosis of bronchial asthma.
Odds Ratio (OR) and 95% CI According to Analysis Groups. An OR Higher than 1 Indicates that the Patients With the Characteristic Analyzed are at a Higher Risk for Asthma than those who do not Present it.
| Demographic Data | OR (case) | 95% CI |
| Age, years | 0.957 | 0.938–0.975 |
| Height, cm | 0.98 | 0.964–0.996 |
| Level of studies | 0.743 | 0.615–0.898 |
| Occupation at risk/exposure to irritants | 1.812 | 1.2–2.740 |
| Atopic dermatitis | 1.779 | 1.083–2.915 |
| History of non-infectious rhinitis | 3.448 | 2.538–4.695 |
| Hypersensitivity/intolerance to NSAIDs | 3.289 | 1.618–6.711 |
| Hypersensitivity to other allergens | 4.049 | 2.959–5.525 |
| Hypersensitivity to pollen | 1.033 | 0.673–1.585 |
| Hypersensitivity to dust mites | 1.631 | 1.058–2.513 |
| Hypersensitivity to fungi | 1.048 | 0.462–2.375 |
| Hypersensitivity to certain foods | 0.831 | 0.41–1.681 |
| Hypersensitivity to animals | 2.513 | 1.548–4.082 |
| History of anxiety disorder | 1.351 | 0.824–2.212 |
| Family history of atopy | 1.631 | 1.164–2.288 |
| Family history of polyposis | 1.439 | 0.99–2.092 |
| Family history of asthma | 2.203 | 1.61–3.012 |
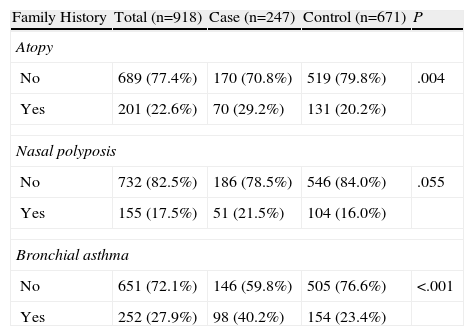
Table 4 shows the data for the family histories, 29.2% of the patients with asthma had a family history of atopy. Of these, 80.6% were immediate family members, with no differences between the groups. The most frequent history finding was bronchial asthma, present in 27.9% of the subjects. A family history of asthma was present in 40.2% of the cases, compared with 23.4% of the controls (P<.001). This factor was detected more frequently in the immediate family members of the cases (77.6%) compared with the controls (64.9%) (P=.0333).
Family History.
| Family History | Total (n=918) | Case (n=247) | Control (n=671) | P |
| Atopy | ||||
| No | 689 (77.4%) | 170 (70.8%) | 519 (79.8%) | .004 |
| Yes | 201 (22.6%) | 70 (29.2%) | 131 (20.2%) | |
| Nasal polyposis | ||||
| No | 732 (82.5%) | 186 (78.5%) | 546 (84.0%) | .055 |
| Yes | 155 (17.5%) | 51 (21.5%) | 104 (16.0%) | |
| Bronchial asthma | ||||
| No | 651 (72.1%) | 146 (59.8%) | 505 (76.6%) | <.001 |
| Yes | 252 (27.9%) | 98 (40.2%) | 154 (23.4%) | |
The mean age at the onset of asthma symptoms was 24±8 and the mean age at diagnosis was 27.4±8.2. Intermittent asthma was presented by 31.9% of the patients, mild persistent asthma by 41.5% and moderate persistent asthma by 25.8%. Only 0.9% presented severe persistent asthma.
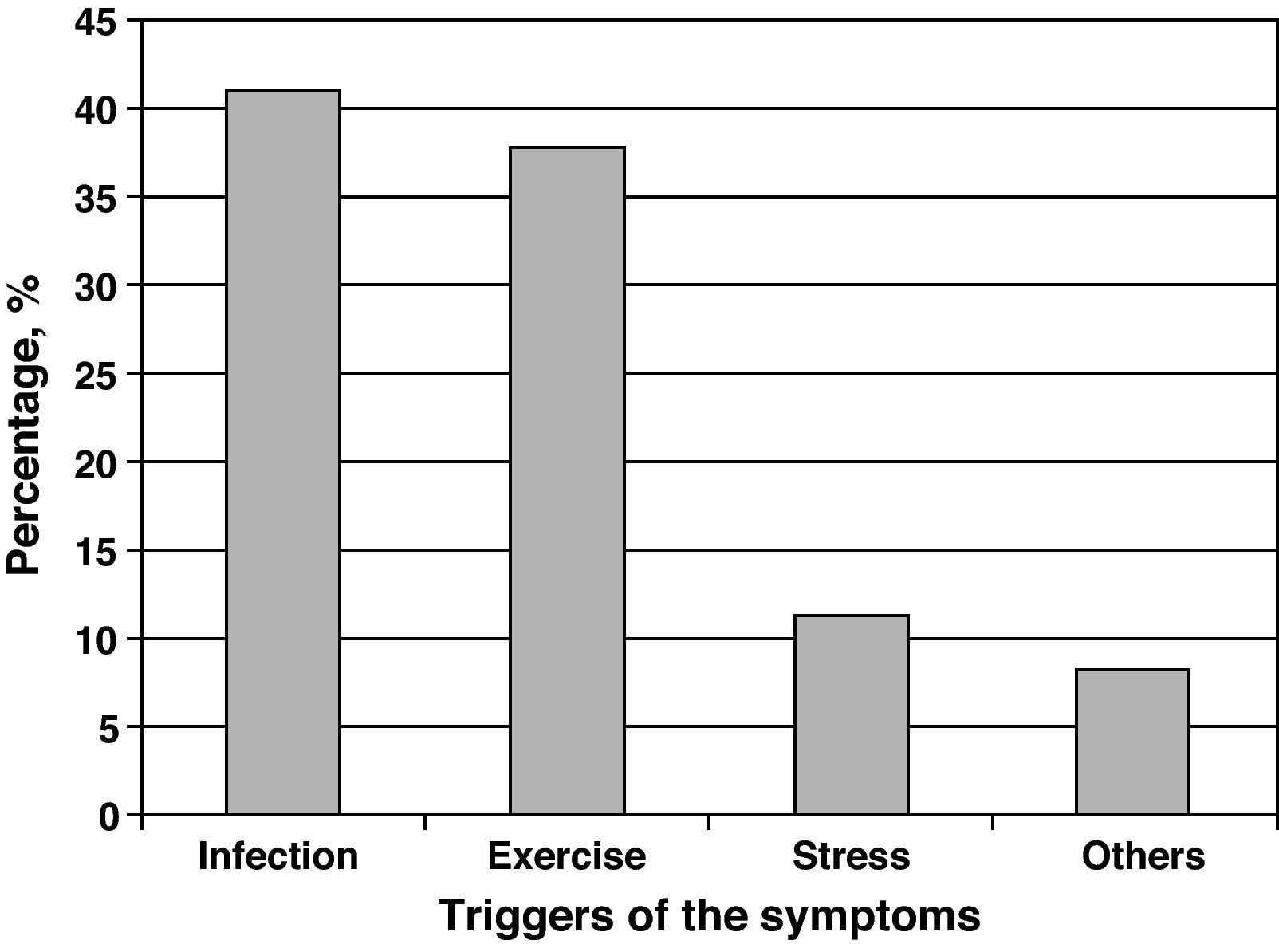
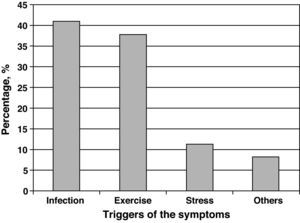
The agents most frequently reported as precipitants of asthma exacerbations were respiratory infections (40.9%), followed by physical exercise (37.7%) and emotional stress (11.3%) (Fig. 1).
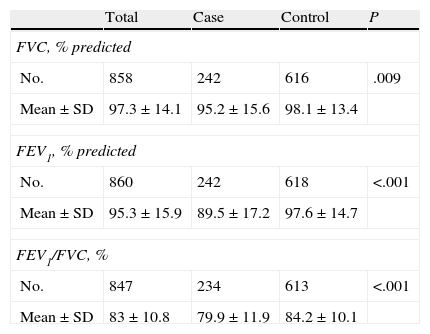
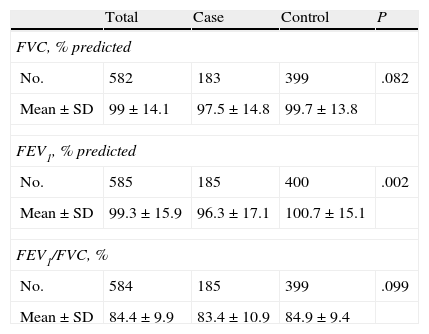
In Tables 5 and 6, spirometry levels are given both before and after bronchodilation, respectively. The ratio between forced expiratory volume in one second and forced vital capacity (FEV1/FVC) presented statistically significant differences (P<.001). The FEV1/FVC ratio in the spirometry after bronchodilation did not show differences that were statistically significant. When correlating the odds ratio and the 95% CI by analysis groups, it was observed that the patients with a lower pre-bronchodilator FEV1/FVC ratio had 1.04 times more risk for developing asthma and those with lower post-bronchodilator FEV1 had 1.02 times more risk for having asthma.
Bronchial challenge tests with methacholine or histamine were done in 15.7% of the patients in the case group and 5.5% of the subjects of the control group at the age of 26.8±7.5 and 28.9±8.3, respectively. The result was positive in 43 of the 68 patients who underwent the bronchial challenge (63.2%), more frequently in the case group (94.4%) than in the control group (P<.001). The patients who underwent a provocation test with methacholine or histamine were 3.2 times more likely to develop asthma, and those in whom the result was positive had 43.5 times more risk.
All the patients who underwent the bronchial provocation test had a previous bronchodilator test, therefore the number of subjects who underwent all the studies was 38 cases and 35 controls.
Another of the aspects evaluated in this study were the asthma control and quality of life scales. The mean score of the ACT scale in the group of patients with this disease was 20.5±3.8. Insufficient asthma control was presented by 30.9%. As for the SGRQ, the mean score for the “symptoms” domain was 41.2±17.4, for the “activity” domain it was 26.7±21.7, for “impact” 34.4±12.1 and the total mean score was 33.1±13.7. Last of all, the mean total score on the AQ20 scale was 6±3.9.
DiscussionThis study shows that the variables associated with the presence of asthma in the population between the ages of 12 and 40 are hypersensitivity to animals, level of studies, hypersensitivity to other allergens, rhinitis, age, family history of asthma, occupational exposure to irritants and hypersensitivity or intolerance to NSAIDs. While older age and the higher level of studies behave as protective factors for the appearance of bronchial asthma, hypersensitivity to animal epithelia and other allergens, rhinitis, family history of asthma, work exposure to irritants and hypersensitivity or intolerance to NSAIDs behave like independent risk factors.
One of the most interesting findings among the subjects of our sample is the fact that the risk for presenting asthma diminishes with the age of the patients. This finding agrees with the data from previous studies, such as those carried out in the United Kingdom and Australia, which performed follow-ups until the ages of 33 and 35, respectively, with children who had started to have asthma at early ages.14,15
According to the results of our study, the lower the cultural level of the patient, the greater the risk for presenting asthma. With regards to this fact, some authors have argued that the lower socioeconomic status and cultural level could explain the greater prevalence of asthma in certain ethnic groups, such as Puerto Ricans who reside in the United States.16 However, there are currently no consistent data that establish a clear connection between the risk for having asthma and socioeconomic level.17
The sensitization to animal epithelia seems to have a clear relationship with asthma in adults. It has been demonstrated that the aeroallergens from dogs and cats are associated with smaller-sized particles than other allergenic sources, which facilitate their penetration into the lower airways and increase their pathogenic potential.18 But, paradoxically, the presence of animals in the home does not seem to be related with the diagnosis of asthma, which concurs with the notifications made regarding the innocuity of dog epithelium, and even its protective role. In the case of cats, the results to date have been more disparate.19
In the subjects evaluated in the present study, there is a notable incidence of rhinitis and nasal polyposis in patients with asthma, which at the same time conditions a greater severity of the latter. Prospective studies with up to 20 years of follow-up affirm that rhinitis is a risk factor for the development and severity of asthma. Numerous studies show that both entities often coexist in the same patient, and that a large part of asthmatics present symptoms of rhinitis. Rhinitis appears in 75% of patients with allergic asthma and in 80% of those with non-allergic asthma.20,21 A multicenter Spanish study about the coexistence of rhinitis and asthma corroborates the high prevalence of the referred to association in allergic patients, affecting 89.5% of a total of 942 included in the study.22
What is evident in our study is the importance of family history as a risk factor for the diagnosis of asthma in adults. Even though to date this tendency towards family aggregation leaves little room for doubt,23–25 the attempts made at establishing a pattern of inheritance have been unfruitful, probably due to the fact that bronchial asthma can be the phenotypic expression of different genotypes.26
With regards to the exposure to certain irritating agents in the environment, this present study has demonstrated that it is a risk factor for the development of asthma in adults. In the case of occupational asthma, it has been confirmed that the continuous exposure to the causal agent is associated with a poorer prognosis and with a more accelerated decrease in lung function.27,28
The relationship between adult asthma and intolerance to NSAIDs is well known. Between 5% and 10% of asthmatic adults present exacerbations after the administration of acetylsalicylic acid,29 associated in the characteristic ASA triad (asthma, intolerance to NSAIDs and nasal polyposis). Our finding corroborates this relationship as other studies have previously done.29–31
In agreement with numerous previous studies, we have also been able to verify that in our patients respiratory infections can exacerbate bronchial asthma as they are known to be capable of increasing the degree of bronchial hyperreactivity32 and to produce various alterations in the airways that compromise their maintained function.33
Even though in our study smoking showed no statistically significant differences between cases and controls, the greater asthma severity in active smokers is well known.34 Likewise, both smoking and rhinitis predict a great risk for developing bronchial asthma in the future.35
Even though it is accepted that low physical activity is closely related to the risk for developing obesity, in our study no statistically significant differences were observed between the cases and controls in this aspect. This fact contrasts with the data of the National Asthma Survey36,37 regarding the level of sedentarism and excess weight or obesity and their influence in asthma, which established a relationship between obesity and severity of asthma in American adults. In this same direction, in a multicenter study recently published in the United States with a population of 368 adolescents between the ages of 12 and 20 with moderate-severe asthma, it was observed that adiposity is associated with poorer asthma control only in women, as there was evidence of a better control of the disease in males due to influx by adiponectin.38 However, in 2002 Phelan et al. published a study on risk factors in adult asthma carried out in monozygotic and dizygotic twins in which a protective effect was found for sedentary lifestyles with regards to developing asthma, indicating that excessive physical activity would be considered a risk factor for a subject to have episodes of bronchospasm.39 This could be related to our finding.
This present study has some limitations that should be kept in mind. As it is a cross-sectional study, we cannot establish causal relationships nor can we give a temporary evaluation in the conclusions. On the other hand, as we have selected patients between the ages of 12 and 40, we have not evaluated a high percentage of asthma patients, and therefore the conclusions obtained may only be extrapolated to this age group.
Likewise, the study is limited by the fact that the recruitment of cases depends upon the fact that the patients assure not having presented symptoms before the age of 12. In this point, there may be an evident memory bias as patients, especially with a history of asthma at early ages, may not be able to properly relate it during the medical interview. This takes on more importance when we consider that 31% of the sample presented intermittent symptoms, among whom memory bias would be more frequent.
In addition, the fact that not all the patients underwent the bronchodilation test and the bronchial provocation test with methacholine or histamine could make the study fall into a selection bias, as patients considered asthmatic may not be, despite having compatible symptoms. This seems not to affect the group of control subjects as they did not have subjective symptoms of bronchial asthma.
In conclusion, there are different variables associated with the development of asthma in adults. Their identification may help prevent the appearance of this disease in said population. Therefore, longitudinal studies are required in order to clarify the role of these factors in the development of asthma in adults.
FundingPfizer.
Conflict of InterestsVerónica Sanz de Burgoa Gómez-Piñán is an employee of Pfizer. The rest of the authors have no conflict of interests.
The authors of the study would like to thank the following institutions for their collaboration in recruiting the cases and controls: Hospital USP La Esperanza (Álava), Centro de Salud Amurrio (Álava), Hospital General de Almansa (Albacete), Hospital del Perpetuo Socorro (Albacete), Hospital General de Albacete (Albacete), Hospital Virgen de la Altagracia (Albacete), Hospital de Levante (Alicante), Hospital General de Elda (Alicante), CSI Villena (Alicante), Hospital General Universitario de Elche (Alicante), Hospital General de Alicante (Alicante), Hospital Universitario San Juan de Alicante (Alicante), Hospital Vega Baja (Alicante), Hospital de Alcoy (Alicante), Hospital San Vicente (Alicante), Hospital Torrevieja Salud (Alicante), Hospital de Denia (Alicante), Hospital Torrecárdenas (Almería), Hospital del Poniente (Almería), Hospital La Inmaculada (Almería), Hospital Valle del Nalón (Asturias), Hospital de Avilés (Asturias), Hospital de San Agustín (Asturias), Hospital Universitario Central de Asturias (Asturias), Hospital Alvarez Buylla (Asturias), Hospital de Cabueñes (Asturias), Hospital de Jove (Asturias), CE Ávila Estación (Ávila), Hospital Infanta Cristina (Badajoz), Hospital Comarcal de Zafra (Badajoz), Hospital Infanta Cristina (Badajoz), CS San José de Almendralejo (Badajoz), Hospital de Llerena (Badajoz), Corporación Parc Taulí (Barcelona), Hospital ABS Sant Fèlix (Barcelona), CAP Pare Claret (Barcelona), Hospital Santa Creu i Sant Pau (Barcelona), CAP Central (Barcelona), Hospital de Mataró (Barcelona), Hospital de Viladecans (Barcelona), Hospital de Sant Boi (Barcelona), Hospital General de Granollers (Barcelona), Hospital General de Vic (Barcelona), CAP Sant Feliu (Barcelona), Hospital Germans Trias i Pujol (Barcelona), CS Can Mora (Barcelona), Hospital ABS Numancia (Barcelona), CAP Poble Nou (Barcelona), Hospital General de Hospitalet (Barcelona), Hospital Espirit Sant (Barcelona), Hospital Universitario de Bellvitge (Barcelona), Clínica d’Especialitats (Barcelona), CAP Pineda de Mar (Barcelona), CAP Sant Andreu (Barcelona), Hospital Vall d’Hebron (Barcelona), Hospital Sant Joan de Déu (Barcelona), Hospital del Mar (Barcelona), CS Gava (Barcelona), Hospital de Terrassa (Barcelona), Centro Médico Cataluña (Barcelona), CAP Maragall (Barcelona), CAP Roger de Flor (Barcelona), Complejo Asistencial Burgos (Burgos), Hospital General Yagüe (Burgos), Hospital Ciudad de Coria (Cáceres), Hospital Navalmoral de la Mata (Cáceres), Hospital Virgen del Puerto (Cáceres), Hospital San Pedro de Alcántara (Cáceres), CS Argel (Cáceres), Ambulatorio de Especialidades San Benito (Cáceres), Hospital Puerta del Mar (Cádiz), CS Barrio Bajo (Cádiz), Hospital de La Línea (Cádiz), Hospital Virgen del Camino (Cádiz), Hospital Punta Europa (Cádiz), Hospital General de la Defensa San Carlos (Cádiz), Centro Médico API (Cádiz), Hospital Marqués de Valdecilla (Cantabria), Hospital Sierrallana (Cantabria), Hospital General de Castellón (Castellón), Hospital Comarcal de Vinaroz (Castellón), Complejo Hospitalario Provincial de Castellón (Castellón), Hospital Ingesa (Ceuta), Hospital General de Ciudad Real (Ciudad Real), Hospital General de La Mancha (Ciudad Real), Hospital Santa Bárbara (Ciudad Real), Hospital Universitario Reina Sofía (Córdoba), Hospital Infanta Margarita (Córdoba), Hospital Virgen de la Luz (Cuenca), Hospital de Palamós (Gerona), Hospital Dr. Josep Trueta (Gerona), CS Santa Caterina (Gerona), Hospital de Blanes (Gerona), Hospital de Figueres (Gerona), CS Armilla (Granada), Hospital Universitario San Cecilio (Granada), Hospital Ruiz de Alda (Granada), Hospital Virgen de las Nieves (Granada), Hospital General de Guadalajara (Guadalajara), CS Ondarreta (Guipúzcoa), CS Lasarte (Guipúzcoa), CS Irún (Guipúzcoa), Policlínica Alto Aragón (Huesca), CE Virgen de la Cinta (Huelva), Policlínica Miramar (Baleares), Hospital Son Llàtzer (Baleares), Clínica Palma Planes (Baleares), Clínica Juaneda (Baleares), Hospital Son Dureta (Baleares), Hospital de Manacor (Baleares), Centro USP Sa Pobla (Baleares), EPH Alto Guadalquivir (Jaén), Ambulatorio Virgen de la Capilla (Jaén), Hospital de Úbeda (Jaén), Hospital Médico Quirúrgico (Jaén), Hospital de San Agustín (Jaén), CS Federico del Castillo (Jaén), Hospital Naval (La Coruña), Complejo Hospitalario de Ferrol (La Coruña), Hospital Modelo (La Coruña), Complejo Hospitalario Universitario A Coruña (La Coruña), Hospital Arquitecto Marcide (La Coruña), CHU A Coruña (La Coruña), Hospital Provincial de Conxo (La Coruña), Complejo Hospitalario Universitario de Santiago (La Coruña), Hospital San Pedro (La Rioja), Hospital Insular (Las Palmas), CS Maspalomas (Las Palmas), Hospital Dr. Negrín (Las Palmas), Centro Médico Finlay (Las Palmas), CS Casa del Mar (Las Palmas), CS Mogan (Las Palmas), Hospital Sur (Las Palmas), Hospital de León (León), Centro Médico Gran Vía (León), Hospital El Bierzo (León), CS Alcarras (Lérida), Hospital Sta. María de Lleida (Lérida), Hospital Xeral Calde (Lugo), CS Valadouro (Lugo), CS Foz (Lugo), Hospital Puerta del Hierro (Madrid), Hospital del Tajo (Madrid), Hospital Infanta Elena (Madrid), Hospital La Paz (Madrid), Hospital General Universitario Gregorio Marañón (Madrid), CEP Hermanos Sangro (Madrid), Hospital Príncipe de Asturias (Madrid), Ambulatorio Vicente Soldevilla (Madrid), Hospital 12 de Octubre (Madrid), Hospital de Fuenlabrada (Madrid), Hospital Ramón y Cajal (Madrid), CS Los Pedroches (Madrid), Hospital Severo Ochoa (Madrid), Hospital Clínico San Carlos (Madrid), Hospital de Móstoles (Madrid), Fundación Jiménez Díaz (Madrid), Hospital del Henares (Madrid), Hospital Clínico (Málaga), Hospital Axarquía (Málaga), CS Torre del Mar (Málaga), Hospital La Serranía (Málaga), Hospital Benalmádena (Málaga), Hospital Costa del Sol (Málaga), Hospital Virgen de la Victoria (Málaga), Hospital General Carlos Haya (Málaga), Hospital de Antequera (Málaga), Hospital Morales Meseguer (Murcia), Hospital de Yecla (Murcia), Hospital de Cieza (Murcia), Hospital Comarcal Noroeste de Caravaca (Murcia), Hospital General Reina Sofía (Murcia), Hospital Virgen de la Arrixaca (Murcia), Hospital Santa María del Rosell (Murcia), Hospital Los Arcos (Murcia), Hospital Rafael Méndez (Murcia), CS Fustiñana (Navarra), CS Tudela Este (Navarra), Hospital San Juan de Dios (Navarra), Clínica Universidad de Navarra (Navarra), Complejo Hospitalario Ourense (Orense), CS Novoa Santos (Orense), Hospital de Verin (Orense), Hospital de Cristal (Orense), Hospital Río Carrión (Palencia), Complejo Hospitalario de Palencia (Palencia), Hospital Provincial de Pontevedra (Pontevedra), Hospital Xeral Cíes (Pontevedra), Hospital Povisa (Pontevedra), Complejo Hospitalario de Pontevedra (Pontevedra), CS Pontevedra (Pontevedra), Hospital de la Santísima Trinidad (Salamanca), Hospital Los Montalvos (Salamanca), Hospital Nuestra Sra. de la Candelaria (Tenerife), Hospital Sur (Tenerife), Hospital Universitario de Canarias (Tenerife), CE Rumeo Hardisson (Tenerife), Hospital de Valme (Sevilla), Hospital Virgen Macarena (Sevilla), Ambulatorio María Auxiliadora (Sevilla), Hospital de la Merced (Sevilla), Mar Écija (Sevilla), Clínica Sagrado Corazón (Sevilla), Hospital Virgen del Rocío (Sevilla), CS Tomares (Sevilla), Clínica Andaluza de Alergia y Asma (Sevilla), Hospital Joan XXIII (Tarragona), Hospital San Joan de Déu (Tarragona), Clínica Monegal (Tarragona), Hospital de Tortosa (Tarragona), CAP Calafell (Tarragona), Hospital Ntra. Sra. del Prado (Toledo), Hospital Virgen del Valle (Toledo), Hospital Lluís Alcanyis (Valencia), Hospital Clínico (Valencia), Hospital de la Ribera (Valencia), Hospital General de Requena (Valencia), Hospital Dr. Peset (Valencia), Hospital General Universitario de Valencia (Valencia), Hospital Francesc de Borja (Valencia), Hospital de Sagunto (Valencia), Hospital de Manises (Valencia), Hospital General de Hellín (Valencia), Hospital de la Fe (Valencia), Hospital Arnau de Vilanova (Valencia), Hospital Clínico Universitario (Valladolid), Hospital de Basurto (Vizcaya), Hozpital de Zumárraga (Vizcaya), Hospital de Cruces (Vizcaya), Hospital de San Eloy (Vizcaya), CS Landako (Vizcaya), Hospital de la Cruz Roja (Zaragoza), Hospital Royo Villanova (Zaragoza), CAR Casar (Zaragoza), Consultorio Adeslas (Zaragoza), Hospital Clínico Universitario (Zaragoza), Hospital Miguel Servet (Zaragoza).
Please cite this article as: Pelta Fernández R, et al. Factores de riesgo de asma de inicio entre los 12 y 40 años. Resultados del estudio FENASMA. Arch Bronconeumol. 2011;47:433-40.