Although bacteria are the main pathogens involved in community-acquired pneumonia, a significant number of community-acquired pneumonia are caused by viruses, either directly or as part of a co-infection. The clinical picture of these different pneumonias can be very similar, but viral infection is more common in the pediatric and geriatric populations, leukocytes are not generally elevated, fever is variable, and upper respiratory tract symptoms often occur; procalcitonin levels are not generally affected. For years, the diagnosis of viral pneumonia was based on cell culture and antigen detection, but since the introduction of polymerase chain reaction techniques in the clinical setting, identification of these pathogens has increased and new microorganisms such as human bocavirus have been discovered. In general, influenza virus type A and syncytial respiratory virus are still the main pathogens involved in this entity. However, in recent years, outbreaks of deadly coronavirus and zoonotic influenza virus have demonstrated the need for constant alert in the face of new emerging pathogens. Neuraminidase inhibitors for viral pneumonia have been shown to reduce transmission in cases of exposure and to improve the clinical progress of patients in intensive care; their use in common infections is not recommended. Ribavirin has been used in children with syncytial respiratory virus, and in immunosuppressed subjects. Apart from these drugs, no antiviral has been shown to be effective. Prevention with anti-influenza virus vaccination and with monoclonal antibodies, in the case of syncytial respiratory virus, may reduce the incidence of pneumonia.
Aunque las bacterias son los principales patógenos involucrados en la neumonía adquirida en la comunidad, algunos virus son responsables directos o en coinfección de un importante número de neumonías adquiridas en la comunidad. La clínica de estas neumonías puede ser muy similar, en el caso de los virus afectan más frecuentemente a la población infantil y geriátrica, con frecuencia no elevan la cifra de leucocitos, la fiebre es inconstante y frecuentemente se acompañan de síntomas de vías respiratorias altas. Característicamente no elevan la procalcitonina. Durante años el diagnóstico ha recaído en cultivos celulares y en detección de antígenos; desde la incorporación en la clínica de la PCR, la identificación de estos patógenos ha aumentado, descubriéndose nuevos microorganismos como el bocavirus. En general, el virus influenza A y el virus respiratorio sincitial siguen siendo los principales virus implicados. Sin embargo, la irrupción en los últimos años de epidemias con alta letalidad de coronavirus y de zoonosis de virus influenza hace que sea necesario mostrarse alerta ante estos nuevos patógenos emergentes. Los inhibidores de la neuraminidasa para neumonías víricas han demostrado disminuir la transmisión en casos expuestos y mejorar la evolución clínica en pacientes en Cuidados Intensivos; su uso en infecciones banales no está recomendado. La ribavirina ha sido utilizada en niños con infecciones por virus respiratorio sincitial, así como en inmunodeprimidos. Fuera de estos fármacos, ningún otro antiviral ha probado su eficacia. Las medidas de prevención con vacunación para virus influenza y con anticuerpos monoclonales para virus respiratorio sincitial podrían disminuir la incidencia de neumonía.
According to WHO estimates for 2012, around 450 million cases of pneumonia occur worldwide every year, causing 3 million deaths, and accounting for 5.5% of overall mortality worldwide.1,2 It is the fourth cause of death worldwide, and is a particularly a serious threat to children and the elderly.1,3
Bacterial infections, being more common, have been more extensively studied. In contrast, research into viral community-acquired pneumonia (CAP), despite its growing epidemiological significance in developing countries and in the pediatric population, has been limited.4 Assuming the rate of diagnosis to still be lower than real incidence, around 200 million cases of viral pneumonia occur annually throughout the world, half of which are in children.5 Viral pneumonia is of great interest due to its impact on infant mortality, its role as a facilitator of bacterial infections (co-infections), and its ease of transmission, a factor which has transformed it into a worldwide threat.
In this review, we will focus on CAP caused by respiratory viruses in immunocompetent patients.
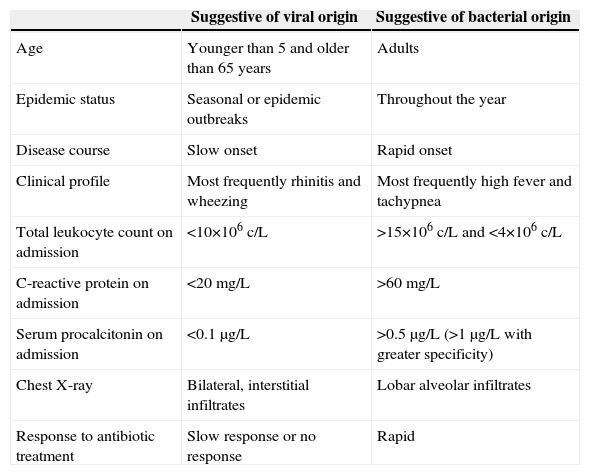
Differentiating Between Viral and Bacterial PneumoniaIt is important to distinguish between CAP of viral and bacterial origin. Clinical, radiological and laboratory variables that are commonly used to distinguish between these entities are listed in Table 1.
Differential Factors Between Viral Pneumonia and Bacterial Pneumonia.
| Suggestive of viral origin | Suggestive of bacterial origin | |
|---|---|---|
| Age | Younger than 5 and older than 65 years | Adults |
| Epidemic status | Seasonal or epidemic outbreaks | Throughout the year |
| Disease course | Slow onset | Rapid onset |
| Clinical profile | Most frequently rhinitis and wheezing | Most frequently high fever and tachypnea |
| Total leukocyte count on admission | <10×106c/L | >15×106c/L and <4×106c/L |
| C-reactive protein on admission | <20mg/L | >60mg/L |
| Serum procalcitonin on admission | <0.1μg/L | >0.5μg/L (>1μg/L with greater specificity) |
| Chest X-ray | Bilateral, interstitial infiltrates | Lobar alveolar infiltrates |
| Response to antibiotic treatment | Slow response or no response | Rapid |
Epidemiological studies have been published by many authors. Ruiz-González et al.6 included patients with viral and intracellular bacterial pneumonia in the same group. They concluded that pneumonia caused by intracellular pathogens affected older patients, had a more insidious disease course, and often did not produce leukocytosis. Johnstone et al.7 found that patients with viral pneumonia had more cardiac comorbidities and were older; Ma et al.8 found that institutionalization led to a greater risk of viral CAP. Liu et al.9 reported that viral pneumonia caused more cough and less pleuritic pain, while Jennings et al.10 found myalgia to be the symptom most commonly associated with the viral disease. Despite the many publications, predicting the viral etiology from clinical parameters is difficult and often inaccurate, as affirmed recently by Viasus et al.11
With regard to radiological changes, focal alveolar infiltrates have been traditionally associated with the bacterial entity, and bilateral, interstitial infiltrates with the viral form.12 However, recent studies using chest computed tomography (CT) have shown that a viral etiology cannot be ruled out by the appearance of localized alveolar infiltrates, and these may even signal the onset of many viral pneumonias.13
Viral infections do not usually affect the number of leukocytes, so the use of acute phase reactants, such as procalcitonin, as biomarkers may be of great help in reaching a diagnosis.14 Procalcitonin production depends on the presence of circulating tumor necrosis factor (TNF-α); in viral infections, macrophages produce interferon-α that can inhibit TNF-α, suppressing the elevation of procalcitonin, thus suggesting a viral origin.15
Despite these assertions, there is no gold standard for differentiating the etiology of pneumonia.16 Moreover, we must not forget that CAP, whether viral or bacterial, is a dynamic entity: differences in biomarker values or the appearance of infiltrates on radiology are only snapshots of an active process that can vary widely from day to day.
Types of VirusesThe list of viruses that can cause respiratory infection is long (Table 2). In this review, we will focus particularly on seasonal respiratory viruses.
Viruses Related with Community-Acquired viral Pneumonia in Children and Adults.
| Syncytial respiratory virus |
| Rhinovirus |
| Influenza A, B and C virus |
| Human metapneumovirus |
| Parainfluenza virus type 1, 2, 3 and 4 |
| Human bocavirus |
| Coronavirus type 229E, OC43, NL63, HKU1, SARS and MERS-CoV |
| Adenovirus |
| Enterovirus |
| Varicella zoster virus, Epstein–Barr virus, human herpesvirus 6 and 7, cytomegalovirus |
| Hantavirus |
| Parechovirus |
| Mimivirus |
| Measles virus |
Other viruses that have recently received considerable media attention, such as H5N1 influenza virus or coronaviruses (responsible for severe acute respiratory syndrome or the Middle East respiratory syndrome, MERS-CoV) will be examined in less depth.
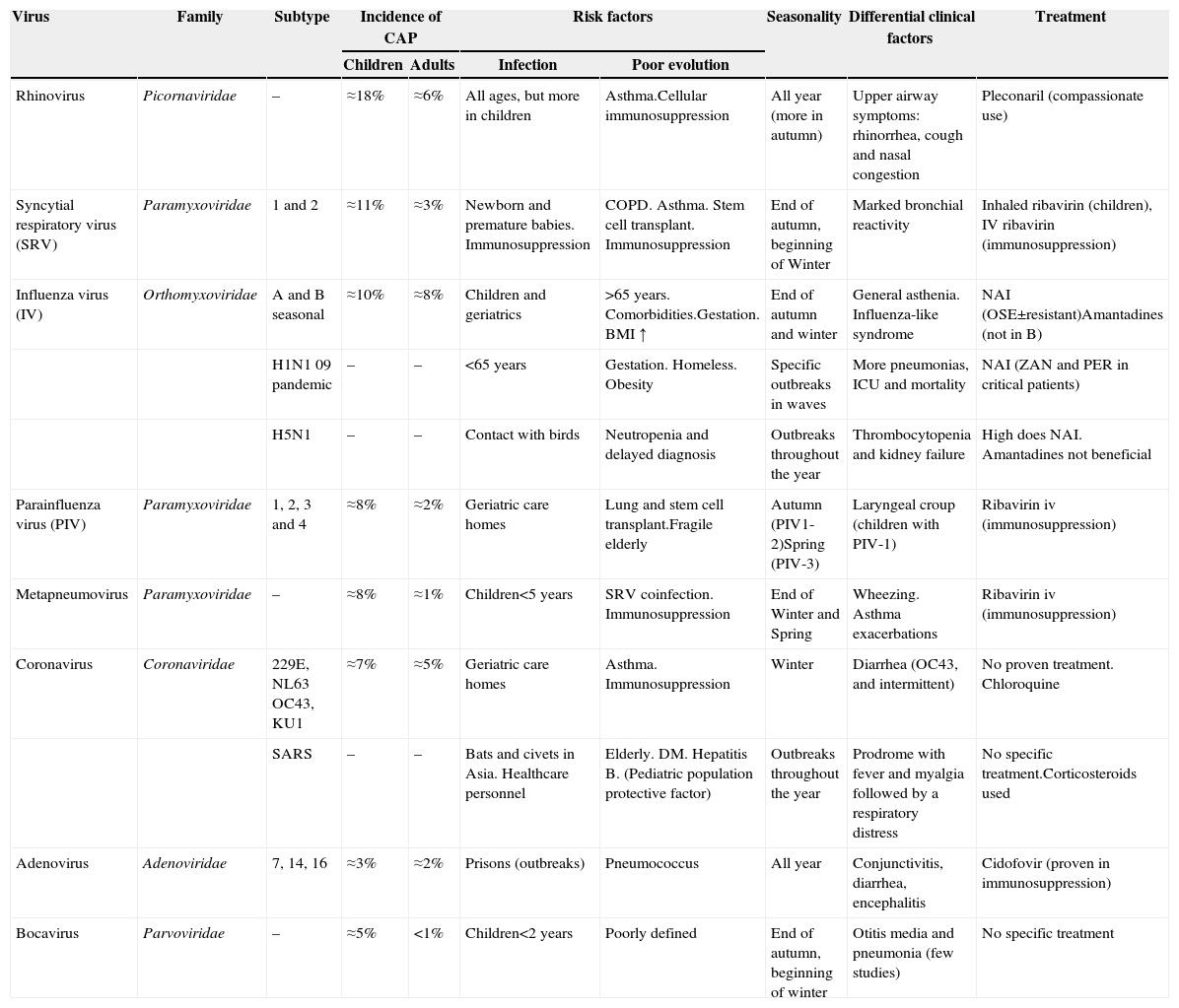
The relative incidence and clinical and epidemiological characteristics of the different respiratory viruses are listed in Table 3.
Characteristics of the Main Viruses Involved in Viral Pneumonia.
| Virus | Family | Subtype | Incidence of CAP | Risk factors | Seasonality | Differential clinical factors | Treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| Children | Adults | Infection | Poor evolution | ||||||
| Rhinovirus | Picornaviridae | – | ≈18% | ≈6% | All ages, but more in children | Asthma.Cellular immunosuppression | All year (more in autumn) | Upper airway symptoms: rhinorrhea, cough and nasal congestion | Pleconaril (compassionate use) |
| Syncytial respiratory virus (SRV) | Paramyxoviridae | 1 and 2 | ≈11% | ≈3% | Newborn and premature babies. Immunosuppression | COPD. Asthma. Stem cell transplant. Immunosuppression | End of autumn, beginning of Winter | Marked bronchial reactivity | Inhaled ribavirin (children), IV ribavirin (immunosuppression) |
| Influenza virus (IV) | Orthomyxoviridae | A and B seasonal | ≈10% | ≈8% | Children and geriatrics | >65 years. Comorbidities.Gestation. BMI ↑ | End of autumn and winter | General asthenia. Influenza-like syndrome | NAI (OSE±resistant)Amantadines (not in B) |
| H1N1 09 pandemic | – | – | <65 years | Gestation. Homeless. Obesity | Specific outbreaks in waves | More pneumonias, ICU and mortality | NAI (ZAN and PER in critical patients) | ||
| H5N1 | – | – | Contact with birds | Neutropenia and delayed diagnosis | Outbreaks throughout the year | Thrombocytopenia and kidney failure | High does NAI. Amantadines not beneficial | ||
| Parainfluenza virus (PIV) | Paramyxoviridae | 1, 2, 3 and 4 | ≈8% | ≈2% | Geriatric care homes | Lung and stem cell transplant.Fragile elderly | Autumn (PIV1-2)Spring (PIV-3) | Laryngeal croup (children with PIV-1) | Ribavirin iv (immunosuppression) |
| Metapneumovirus | Paramyxoviridae | – | ≈8% | ≈1% | Children<5 years | SRV coinfection. Immunosuppression | End of Winter and Spring | Wheezing. Asthma exacerbations | Ribavirin iv (immunosuppression) |
| Coronavirus | Coronaviridae | 229E, NL63 OC43, KU1 | ≈7% | ≈5% | Geriatric care homes | Asthma. Immunosuppression | Winter | Diarrhea (OC43, and intermittent) | No proven treatment. Chloroquine |
| SARS | – | – | Bats and civets in Asia. Healthcare personnel | Elderly. DM. Hepatitis B. (Pediatric population protective factor) | Outbreaks throughout the year | Prodrome with fever and myalgia followed by a respiratory distress | No specific treatment.Corticosteroids used | ||
| Adenovirus | Adenoviridae | 7, 14, 16 | ≈3% | ≈2% | Prisons (outbreaks) | Pneumococcus | All year | Conjunctivitis, diarrhea, encephalitis | Cidofovir (proven in immunosuppression) |
| Bocavirus | Parvoviridae | – | ≈5% | <1% | Children<2 years | Poorly defined | End of autumn, beginning of winter | Otitis media and pneumonia (few studies) | No specific treatment |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; IV: intravenous; NAI: neuraminidase inhibitors (OSE: oseltamivir; PER: peramivir; ZAN: zanamivir).
Overall, syncytial respiratory virus (SRV) remains the primary causative agent of CAP in children, and the main cause of severe pneumonia in this population.17,18 Since the widespread introduction of PCR techniques, rhinoviruses are more often detected as the causative agent of viral pneumonia in children.19–22 However, because it is so frequently detected in asymptomatic individuals (15%), the etiological role of rhinovirus continues to be questioned, although it could be an indication of real, subclinical infections.23 Recently identified pathogens, such as metapneumovirus and human bocavirus,24–27 are less commonly encountered. Although the prevalence of viral pneumonia caused by adenovirus is low (2%–12%), it must be identified, as it can lead to necrotizing pneumonia.28 In this case, PCR techniques are much more sensitive than antigen detection techniques.29
In the case of adults, the most commonly detected viruses are influenza virus (IV), SRV and parainfluenza,5,10,30,31 although incidence varies depending on the diagnostic techniques used. These viruses are also the most important in Spain.32 Pneumonias caused by other viruses are more rarely reported and include outbreaks of rhinovirus,33,34 adenovirus35 (particularly serotype 14 in military institutions36), coronavirus,37 metapneumovirus,38 and even bocavirus (in immunocompromised patients).39
Co-infectionInfections involving both respiratory bacteria and viruses or 2 different viruses are common. The most widely accepted hypothesis is that the viral infection occurs first, followed by the bacterial form. Viral-mediated activation of proinflammatory molecules, such as interleukin-10, is thought to attract large numbers of neutrophils and macrophages to the lung. The arrival of these cytokines amplifies the immune response, causing inflammatory damage and preventing the proper clearance of bacteria.40 Bacterial superinfection worsens the prognosis of the original viral infection. Indeed, research into the influenza pandemics of 1918, 1957, and 1968 shows that most deaths were caused by a secondary bacterial infection.41 During the 2009 H1N1 pandemic, 4%–24% of cases presented secondary bacterial infection.42–44 In infections caused by other viruses, however, especially H5N1 avian influenza, the associated pneumonia appears to be caused (more frequently) by direct viral action.45
In clinical practice, this type of co-infection is particularly common in children (up to 45% of cases with CAP), and mainly involves pneumococcus,46–49 thus increasing clinical severity.50Mycoplasma pneumoniae and several species of Chlamydophila51 are also common,51 while simultaneous co-infection with 2–3 viruses is not unusual.52
CAP of mixed etiology has been characterized less in adults than in children, and prevalence is estimated at less than 5%.7,13 The most common combinations reported are rhinovirus+pneumococcus and influenza A virus+pneumococcus. More serious infections have been identified with the combination of viruses with Legionella pneumophila.53
Data on morbidity and mortality in bacterial/viral co-infection are contradictory. Hong et al.54 consider that these co-infections are no more severe than purely bacterial infections, and affect older patients and those with chronic lung diseases. In contrast, Johansson et al.55 and Seki et al.56 found that mixed etiology pneumonia was associated with higher severity scale scores and poorer progress.
Respiratory Virus Outbreaks: Experience with H1N1 Influenza Virus in 2009, Avian Influenza (H5N1 and H7N9), SARS and Middle East Coronavirus (MERS-CoV)Some very well-known families of respiratory viruses have produced new species and some very virulent serotypes, which in recent years have caused epidemics with significant associated morbidity and mortality.57
The great H1N1 IV-A epidemic of 2009 was unusual: transmission was enhanced by the lack of prior immunity, causing a serious medical situation worldwide.42 Studies performed subsequently showed that the younger population and certain risk groups were more affected, resulting in the loss of many more life-years.58 However, from an inter-generational perspective, the burden of death and major complications was no higher than that caused by normal seasonal influenza.59,60
In 2003, an outbreak of coronavirus in the Far East caused a severe acute respiratory syndrome. In the Middle East in 2012, another outbreak of a new coronavirus occurred, called Middle East respiratory syndrome coronavirus (MERS-CoV).61 In both epidemics, the incidence was reduced to a few hundred cases, partly due to the protective measures implemented,62 but mortality was high due to high virulence.63 Clinical symptoms were not only limited to rapidly developing bilateral pneumonia, but also included acute renal failure and severe hematological disturbances.63,64
Recombinations in animals of different IV subtypes are a global concern, as they are potentially capable of passing the species barrier, and occasionally propagating among humans.65 The serious cases of H5N1 avian influenza recorded in 2005 in Southeast Asia,66 or more recently the H3N2-porcine variant in the United States (2012),67 and H7N9 avian influence in China (2013),68 are examples of this new threat. Fortunately, surveillance systems for monitoring animal reserves and activating a rapid medical response are improving.69
Microbiological DiagnosisThe detection of virus or viral antigens in upper (nasopharyngeal aspirates) and lower (bronchoalveolar lavage and induced sputum) respiratory tract samples is usually based on culture and immunofluorescence microscopy. The detection of antibodies generated during the process of viral infection has also been used for years: seroconversion in 2 samples obtained over a period of time is suggestive of a new exposure to the pathogen. The introduction of polymerase chain reaction (PCR) techniques has improved the detection of respiratory viruses, particularly with the use of real-time techniques.3
The preferred specimen for upper respiratory samples, particularly in children, is nasopharyngeal aspirate, which combines a mixture of nasal and retropharyngeal secretions.70 The use of sterile cotton wool swabs yields a similar sensitivity for most viruses, with the exception of SRV.71,72 Devices that combine a nylon fiber swab and a container of universal transport medium provide a high diagnostic yield at all ages, comparable to nasopharyngeal lavage.30,73
Samples from the lower respiratory tract, being obtained at the site of the infection, have obvious advantages when determining the cause of pneumonia. However, contamination may occur during sampling, as the specimen passes through the upper airways. Induced sputum is often obtained from children, but bronchoalveolar lavage is preferred in adults.3 In contrast, the invasive character of other forms of diagnosis, such as transthoracic puncture, has led to their disuse.70
In sample processing, PCR-based systems are 2–5 times more sensitive than conventional methods for the detection of respiratory viruses.30 This technique is particularly useful in the elderly, who often have a lower nasopharyngeal viral burden than children.74,75 Moreover, some viruses (such as bocavirus) are hard to culture, and antigen testing may be equivocal (e.g. parainfluenza or adenovirus), so they can only be satisfactorily identified using PCR.76–78 A standard identification test has been developed that uses a PCR platform combined with microarrays to simultaneously determine more than 15 different respiratory viruses.79 These platforms are the techniques most commonly used in our hospitals at the moment.80
Etiological TreatmentSince the end of the last century, empirical antibiotic treatment of CAP has been initiated as soon as possible, as this has been shown to reduce morbidity and mortality.81
However, no prospective studies have examined the benefits of antibiotics in pneumonias with a high suspicion of viral origin. The possibility of bacterial superinfection or a mixed viral-bacterial etiology, progressive improvements in antibiotic tolerability, and the medical-legal requirement to “use of all available measures”, mean that physicians are unlikely to resist prescribing antibiotics in a patient presenting with pneumonia. This dilemma has only been prospectively studied in children, in the context of SRV bronchiolitis epidemics; the results, while not definitive, do not support the generalized administration of antibiotics.82
Experience in the use of antiviral drugs in respiratory viruses can be divided into 3 areas: (a) treatment for influenza virus, which is relatively effective and well documented, thanks mainly to studies performed during the 2009 pandemic83; (b) treatment for SRV, that has been mostly explored in children and immunosuppressed populations, although results remain unclear84; and (c) treatment of other respiratory viruses, which has not been studied in depth and remains largely unevaluated.85
Treatment of Influenza VirusNeuraminidase, an enzyme of the IV capsid, is essential for intercellular viral propagation. Selective inhibitors of this enzyme (oseltamivir, zanamivir and peramivir) have been seen to contain infection during the peak of viral replication – the first 24–72hours – improving clinical symptoms and reducing morbidity and mortality.86,87 Retrospective studies of patients with a diagnosis of influenza have shown reductions in the incidence of pneumonia in patients who received early treatment.88 Although these drugs are active against both subtypes, seasonal epidemics of oseltamivir-resistant H1N1 IV-A have been described, so some guidelines recommend zanamivir as a first-line approach.83 The pandemic H1N1 strain of 2009 did not generally show this resistance profile, and was widely treated with oseltamivir, with no detriment to outcomes.89 In children, the clinical effect is less clear, but administration appears to be safe.90 Laninamivir octanoate, a new neuraminidase inhibitor for inhaled administration only, has been seen to be effective in the treatment of IV infection, including oseltamivir-resistant strains.91
Adamantanes (amantadine and rimantadine) are drugs that are conventionally used in IV infection. They are exclusively specific to influenza virus type A. This, along with their potential for side effects and rapid development of resistances, has meant that they have fallen into disuse in clinical practice.92 Drugs that can block RNA production and others that limit viral integration are under investigation, but no clinical trials have been started.93 Studies are also being conducted with immunomodulators, with the aim of reducing viral-mediated inflammation and alleviating its effect on the host.94
Treatment of Syncytial Respiratory VirusNone of the treatments tested has been significantly useful in the management of acute episodes of pneumonia or for improving respiratory parameters in follow-up. According to the largest meta-analysis performed in children to date, inhaled ribavirin during pneumonia can reduce hospital stay and time on mechanical ventilation, but does not significantly improve overall mortality.95 Intravenous or oral administration of ribavirin has been used almost exclusively in severely immunocompromised patients (bone marrow or lung transplant recipients). Outcomes have been positive, but the results were not applicable to other patients.96 The use of immunoglobulins in children has not shown any benefits over supportive treatment alone.97 Generalized use of bronchodilators, corticosteroids or antibiotics is not recommended in the American pediatric guidelines for SRV bronchiolitis.98 Trials are currently underway on new products such as catelicidin LL-37 (a vitamin D derivate)99 and dingchuan decoction, a Chinese medicinal product,100 and results are promising.
Treatment of Other Respiratory VirusesUntil recently, only supportive treatment has been available. However, some antiviral medications are currently under investigation. The cytidine analog, cidofovir, originally developed as treatment for CMV, has shown success in vitro against adenoviruses, although the response in immunocompromised patients with several forms of severe pneumonia was poor.101 Intravenous ribavirin has been used with success in lung transplant recipients with respiratory infections caused by metapneumovirus.102 Pleconaril, which incorporates itself into the capsid of Rhinovirus and Enterovirus, has been successfully used in limited case series; it is not yet on the market and is limited to compassionate use.103 There is little doubt that intravenous acyclovir is beneficial in the rare cases of varicella zoster pneumonia in immunocompromised patients.85,104 Lastly, treatment with high-dose corticosteroids can improve the clinical course of viral CAP,85 although this application is still controversial.
Prevention MeasuresIn infectious and contagious diseases, particularly of the respiratory tract, barrier methods are essential for preventing infection. Use of masks and gloves and hand-washing have been shown to be effective in reducing transmission rates in the healthcare setting.105 Social isolation of patients during the clinical phase of the disease is also strongly recommended and reduces overall incidence.106 However, it is difficult to implement these measures properly.106
Immunization plays a very important role in prevention, but is only available for a few viruses. Anti-influenza A and B vaccines have been shown to reduce transmission during seasonal influenza epidemics in the general population,107 but their effect on the course of pneumonia or on mortality is not so clear108; nor is their efficiency in children younger than 2 years, although they continue to be administered in many countries.109 In contrast, they appear to be very effective in elderly institutionalized subjects.110 They are currently recommended in Spain for patients with respiratory comorbidities or immunosuppression, individuals over 65 years of age, and healthcare workers.111
In addition to vaccines, chemoprophylaxis with neuroaminidase inhibitors has been successfully tested during seasonal influenza epidemics.112 As yet, no effective vaccine is available for SRV, but palivizumab has been used as chemoprophylaxis. This is a humanized monoclonal antibody that has shown a reduction of up to 50% in the incidence of pneumonia and associated hospital admissions in neonates with a high risk of infection.113
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Galván JM, Rajas O, Aspa J. Revisión sobre las infecciones no bacterianas del aparato respiratorio: neumonías víricas. Arch Bronconeumol. 2015;51:590–597.