Lymphangioleiomyomatosis (LAM) is a rare disease characterized by abnormal proliferation of immature smooth muscle cells and cystic lung destruction, which determines the prognosis of the disease. The kidney angiomyolipomas are usually very common in this disease and are usually asymptomatic unless complications arise. In the absence of a curative treatment, recent publications show promising results in molecular therapy to prevent functional decline and to control the size of the angiomyolipomas. These therapies include mTOR complex inhibitors, especially sirolimus.
We report a case of a patient diagnosed with LAM who underwent lung transplantation with reduction of renal angiomyolipoma size after treatment with the mTOR inhibitor everolimus.
La linfangioleiomiomatosis (LAM) es una enfermedad rara caracterizada por la proliferación anormal de células musculares lisas inmaduras y una destrucción quística del pulmón, que condiciona el pronóstico de la enfermedad. Los angiomiolipomas renales suelen ser muy frecuentes en esta enfermedad, generalmente de curso asintomático, salvo complicaciones.
Ante la ausencia de un tratamiento curativo, las últimas publicaciones reflejan resultados esperanzadores en la terapia molecular para evitar el deterioro funcional y el control del tamaño de los angiomiolipomas. Entre estas terapias destacan los inhibidores del complejo mTOR, sobre todo sirolimus.
Presentamos un caso clínico de una paciente diagnosticada de LAM sometida a trasplante pulmonar con reducción del tamaño del angiomiolipoma renal tras el tratamiento con el inhibidor mTOR everolimus.
Lymphangioleiomyomatosis (LAM) is a rare disease that almost exclusively affects women of childbearing age and is characterized by the abnormal proliferation of immature smooth muscle cells and cystic destruction of the lungs. It is a disease with a genetic base involving the TSC1 and 2 (tuberous sclerosis complex) genes. These genes, through the mTOR (mammalian target of rapamycin) enzyme complex, are in charge of controlling cell survival and proliferation.1–3
Pulmonary affectation is usually responsible for the symptoms, while the extrapulmonary affectation, fundamentally renal angiomyolipomas, is usually asymptomatic. Currently, there is no curative treatment. In patients with poor clinical evolution and progression towards respiratory failure, lung transplantation (LTx) may be an alternative. Recent publications have shown hopeful results in molecular therapy,1–6 among these mTOR complex inhibitors, sirolimus and everolimus. However, these drugs are not free from potential adverse effects, and their application has not been generalized outside clinical assays.
Almost all the publications dealing with LAM and mTOR inhibitor drugs refer to rapamycin (sirolimus). In this publication, we present a clinical case of a patient diagnosed with LAM who underwent LTx with reduction of the size of the renal angiomyolipoma after treatment with the mTOR inhibitor everolimus.
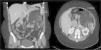
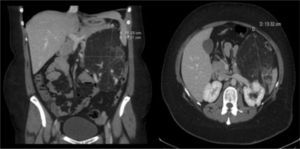
Clinical ObservationA 33-year-old woman was referred to us as a candidate for LTx due to persistent right pneumothorax in spite of endothoracic drainage. The patient was an ex-smoker whose accumulated consumption was not very significant. She had had right pneumothorax during the year prior to the consultation and early relapse after the endothoracic drain had been withdrawn. Initially, she did not present respiratory failure and spirometry detected a severe obstruction with air trapping and hyperinflation. Radiological studies demonstrated bilateral interstitial pattern with presence of multiple small-sized, thin-walled cysts that affected the entire pulmonary parenchyma, with a preserved architecture. With the clinical suspicion for LAM, the patient presented left pneumothorax and collapsed lung. Mechanical pleurodesis was carried out and open lung biopsy confirmed the diagnosis. Given the deterioration of the lung function and the presence of a new right pneumothorax with persistent loss, the patient was referred to our Lung Transplantation Unit. During the evaluation, an abdominal CT detected a mass with heterogeneous density in the left kidney compatible with a renal angiomyolipoma (Fig. 1) and cystic lesions in the liver. She was included on the preferential national waiting list for LTx and, days later, underwent right single-lung transplantation with no immediate complications.
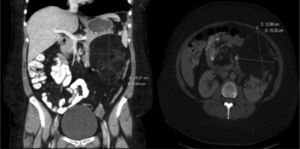
The initial immunosuppression was triple therapy with calcineurin inhibitors (cyclosporine), antiproliferative agents (mofetil mycophenolate) and steroids. Six months later, the immunosuppression regimen was modified by substituting mofetil mycophenolate with everolimus with the two-fold objective of controlling the mild post-transplantation renal dysfunction and assessing the response of the angiomyolipoma to the pharmacological treatment. The dosage of the immunosuppressants was individualized in order to reach plasma levels close to 5ng/ml of everolimus and 100ng/ml of cyclosporine. During follow-up, the renal and pulmonary function remained stable, at stage 0 of bronchiolitis obliterans syndrome. After 6 months of treatment with everolimus, a new abdominal CT demonstrated, after reconstruction, a significant reduction in the size of the renal angiomyolipoma (Fig. 2).
DiscussionLAM is a pathology that is grouped within the rare diseases and whose clinical manifestations are fundamentally respiratory. It may appear sporadically (S-LAM) or associated with tuberous sclerosis (TSC-LAM), a dominant autosomal neurocutaneous syndrome with the formation of hamartomas in the central nervous system, skin, eyes, abdominal organs (especially kidneys) and the lungs.
In these pathologies, the most frequent abdominal alteration is the presence of angiomyolipomas, which are generally asymptomatic and located predominantly in the kidneys. They affect 40% of the patients with S-LAM and up to 80% in TSC-LAM.7 In the first case, the angiomyolipomas are usually unilateral, small, solitary and restricted to the kidneys; while in patients with TSC-LAM they are larger, bilateral, multiple, multi-organic and with a greater tendency to hemorrhage.8 In our case, the patient presented with a large, uncomplicated renal angiomyolipoma in the context of S-LAM.
Both diseases have a genetic base that involve the TSC1 and 2 genes, which encode the protein products hamartin and tuberin. These genes have an important tumor suppressor role. The main role of the hamartin–tuberin complex is to inhibit the mTOR complex, a central regulatory element of cell growth through protein synthesis.9
Supported by new knowledge in physiopathology, specific inhibitor agents are being assayed in the different pathogenic pathways. One of these pathways, the inhibition of mTOR, has brought about new hope for the use of drugs that impede the progression of the disease. Among these are rapamycin (sirolimus) and everolimus, immunosuppressant agents used to prevent rejection in transplant recipients given its powerful inhibitory effect on lymphocytic activation. The preliminary results reflected in case studies and in a clinical assay published in 2008 are hopeful,4–6 both in the reduction of the volume of renal tumors as well as in the declining lung function.
In spite of the limited experience in LTx due to LAM (1% of the total number of cases according to the registry of the International Society for Heart and Lung Transplantation [ISHLT]10), there are specific criteria for the selection of candidates for LTx in this pathology.11 Furthermore, the results in survival and post-transplant quality of life of these patients are either comparable or even better than in the rest of indications for LTx.12,13 Due to the limited number of patients, the best immunosuppression regime in this subgroup cannot be inferred. Nowadays, the tendency in the majority of transplant groups is to individualize immunosuppression regimes according to risk factors.14 This concept is generally known as tailoring immunosuppression. Given the high incidence of abdominal tumors in LAM and the published results for reducing their size after treatment with mTOR inhibitors4 (I-mTOR), it is possible that, in the future, these drugs will be part of the immunosuppression protocol recommended in patients with LAM.
To our knowledge, in LAM all the publications refer to I-mTOR sirolimus. Similarly, in the international registry of the ISHLT, all the immunosuppression regimes that include I-mTOR refer to sirolimus.10 Everolimus is a drug in the same therapeutic group but with a greater oral bioavailability and pharmacokinetic profile than sirolimus.15 Recently, clinical experience is increasing with this drug in solid organ transplantation, including lungs,16–18 but its place in the immunosuppression therapy of transplanted patients has still not been clearly defined. The growing experience with these drugs10 is due to their usefulness in repeated acute rejection, in the loss of graft function as a reflection of chronic rejection, for the control of chronic renal dysfunction due to calcineurin inhibitors, in the control of repetitive infections due to cytomegalovirus and in the management of post-transplant neoplasms.
The anti-tumor or cell-growth control role of these drugs makes their use very hopeful in cases of renal angiomyolipoma in the context of LAM. The pharmacological advantages that everolimus provides lead us to believe that the use of this drug is growing among transplantation groups, particularly in the subgroups of patients with a greater expected benefit. In this regard, we report the positive results in renal and lung function and reduction of the size of the angiomyolipoma in our patient with LAM and lung transplantation.
Please cite this article as: Bujalance-Cabrera C, et al. Reducción de tamaño del angiomiolipoma renal tras el tratamiento con everolimus en trasplante pulmonar por linfangioleiomiomatosis. Arch Bronconeumol. 2012;48:479–81.