Introduction
Tobacco dependence is now considered to be a chronic addictive disease capable of causing premature death in over half of the people who suffer from it.1,2 The most recent data for Spain indicates that tobacco kills 56 000 people every year in our country.3 The same study identifies smoking dependence as one of the principal causes of respiratory disease in Spain, responsible for 87% of lung cancer cases and 93% of chronic obstructive pulmonary disease cases.3
All doctors should be very conscious of two important characteristics of tobacco dependence. Firstly, it is the leading cause of premature death in developed countries, and secondly, it is an addiction that carries with it a vulnerability to relapse that persists over a long period, a characteristic that supports the recognition of smoking dependence as a chronic disease.1,3-5 Because it is a chronic disease, doctors are always obliged to intervene in the smoking dependence of their patients and to provide them with the best advice, support, and pharmacological treatment possible to help them to stop smoking and remain tobacco free. Pulmonologists, the health professionals who attend patients who are, in many cases, affected by the diseases caused directly by tobacco use, have an even greater responsibility in this respect.
The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), through its Assembly on Smoking Addiction, has published a series of guidelines and recommendations on how and where to carry out these interventions.6-8 Other Spanish scientific associations have participated in these recommendations or have even produced their own guidelines.8-10 A large number of evidence-based guidelines and recommendations for the clinical treatment of tobacco addiction have been published in other countries.1,11-15 All of these documents propose different kinds of interventions, depending on the greater or lesser motivation of the patient to make a serious attempt to stop smoking, and report on the efficacy and efficiency of different approaches using evidence-based medical criteria.
Since recent years have seen the publication of a large number of new studies and meta-analyses that provide additional information about the different kinds of treatments for smoking addiction currently available and which of them should be used, the SEPAR Assembly on Smoking Addiction has drawn up these new recommendations on the treatment of tobacco dependence in people who are willing to make a serious attempt to quit. The main aim of these recommendations is to provide all Spanish doctors, and in particular pulmonologists, with safe, useful, evidence-based information on the different kinds of therapy available for smoking addiction in order to make them more effective in the help they provide their patients in tobacco cessation. In this document, the reader will find information on the treatments that should be offered to all smokers who want to quit. Information is given about their efficacy and efficiency as well as on the advantages and disadvantages of each type of therapy. The document ends with two additional sections. The first of these establishes the criteria that should be taken into account before choosing and starting a particular course of treatment. The second considers the use of pharmacotherapy as an aid to smoking cessation in certain special circumstances (pregnancy, smokers of under 10 cigarettes per day, harm reduction, etc.).
The Treatment of Tobacco Dependence in Smokers Willing to Make a Serious Attempt to Quit
The most recent guidelines on the treatment of tobacco use and dependence describe 4 types of patients: nonsmokers who never smoked, nonsmokers who gave up recently, smokers who do not want to quit, and smokers who are willing to make a serious attempt to quit.1 The type of intervention the doctor makes will be determined by the group to which the patient belongs. All smokers who want to make a serious attempt to quit smoking should receive two types of intervention: behavioral support directed at combating any psychological dependence the patient may have, and pharmacological therapy to alleviate physical dependence on nicotine.1,6,8-15 We will now discuss both of these interventions.
Behavioral Interventions
Smokers who are willing to make a serious attempt to quit should receive behavioral support. This can consist of anything from a simple recommendation to quit smoking to more complex behavioral therapies. The following are some of these interventions.
Minimal Intervention. All health professionals should identify smokers, counsel them about their smoking addiction, and advise them to stop smoking. It is essential that an assessment of every patient's tobacco use status be included in any clinical evaluation and recorded as a vital sign on his or her medical record. Smokers who consult any health professional should always be advised in a serious, clear, brief and personalized manner to stop smoking.1,6,8-15 This kind of intervention, when carried out by physicians, produces a small but significant increase in the abstinence rate (odds ratio [OR] = 1.69; 95% confidence interval [CI], 1.45-1.98). This represents an absolute difference in this rate of 2.5% for the group of people who received the advice as compared to those who did not.16,17 Moreover a meta-analysis of studies in which the intervention was carried out by professional nursing personnel also showed a significant increase (OR = 1.50; 95% CI, 1.29-1.73).18,19
Minimal intervention on the part of physicians and other health personnel, whether in the primary care setting, in specialized clinics, or in hospitals, has been shown to be efficacious and effective, and this should therefore be routine clinical practice.16-20
Individual and Group Support. All smokers who wish to make a serious attempt to quit should receive the behavioral therapy they need to help them deal with the psychological, social, and gestural dependence associated with tobacco addiction. This behavioral therapy can be provided either individually or as group therapy. To date, no difference has been observed in the efficacy of one method over another (OR = 1.17; 95% CI, 0.59-2.34, and OR = 1.33; 95% CI, 0.83-2.13).21-24
Individual support should be provided in the form of face-to-face sessions with the patient lasting between 5 and 10 minutes, although the form and the number of these interventions can vary between different groups. It is effective to combine this type of contact with telephone counseling. Scheduling a series of phone calls to take place during the time the patient is quitting slightly increases the efficacy of individual support.25,26 The following is a proposal for following up on this type of individual support: at the baseline visit, during which the patient's tobacco use status is assessed and their treatment established, the patient chooses a quit date (Q-day). Follow up takes the form of two types of intervention: face-to-face contact (8 visits distributed as follows: first, second, fourth, sixth, eighth, and twelfth week after Q-Day; and the sixth and twelfth month after Q-Day) and telephone contact (6 calls distributed as follows: third, fifth, seventh, and tenth weeks after Q-Day; and the fourth and fifth months after Q-Day).7,8,25,26
The optimal number of sessions for group therapy has not been established, nor is the most appropriate duration for each session known. However, when behavioral therapy is provided using this format, 4 to 8 weekly sessions are suggested, lasting between 10 and 30 minutes each.25,26
During minimal interventions, and even in the context of individual and group therapy, health professionals should use any kind of aid that might increase the efficacy of these interventions. The following are some of the aids that have been described: written self-help materials, carbon monoxide (CO)-oximetry, an appeal for social support, physical exercise, etc. The indiscriminate use of written self-help material has not demonstrated any efficacy or any increase in the effectiveness of these interventions. The use of self-help materials should, therefore, be restricted to the use that health professionals make of them during behavioral interventions.27,28 The use of CO-oximetry as a biological support for the recommendation to stop smoking, and individual or group therapy have been shown to be effective only in smokers with low physical dependence.20 Furthermore, it has been shown that interventions designed to improve the social support of smokers who are trying to quit (the help and support of their partner for example) do not increase the abstinence rate in these patients.29 Neither has it been found that the practice of physical exercise during a tobacco cessation program increases the efficacy of the program.30
Pharmacological Therapy
Recommendation number 2 in the clinical practice guidelines on treating tobacco use and dependence issued by the Public Health Service of the USA establishes that all patients who are willing to try to quit smoking should be provided with pharmacotherapy that has been shown to be effective by the meta-analyses carried out.1,15 These guidelines define two groups of treatment options: the first-line pharmacotherapies, which have proven efficacy and give rise to only slight side effects; and the second-line medications, which are less effective and cause more adverse events.1 The first-line therapies include various forms of nicotine replacement therapy (NRT), such as nicotine gum, patches, nasal spray, and oral inhalers, in addition to bupropion. The second-line medications are clonidine and nortriptiline.1 For practical reasons, only the first-line therapies currently available in Spain are discussed in this document.
Nicotine Replacement Therapy. NRT is defined as the administration of nicotine to a smoker who wishes to quit by a means of delivery other than tobacco consumption and in quantities sufficient to alleviate craving and withdrawal symptoms, but not large enough to cause dependence.31 Various types of NRT have been described: chewing-gum, transdermal patches, nasal spray, inhalers, sublingual tablets, and lozenges. All of these produce mean plasma nicotine values exceeding 7 to 10 ng/mL. This figure is considered to be the minimum required to produce a reduction in the withdrawal symptoms experienced by the abstinent smoker.32<
Various meta-analyses have been carried out that clearly show the efficacy of NRT as a tobacco cessation treatment. The OR for abstinence of different types of NRT versus placebo was 1.73 (95% CI, 1.62-1.85) in one meta-analysis, and 1.71 (95% CI,1.60-18.2) in another.33,34 This efficacy does not only appear when NRT is provided and controlled by health professionals in the context of a tobacco cessation program, but is also maintained when it is supplied over-the-counter without any kind of psychological support.33-36
The following is a description of the most important characteristics of each of the types of NRT currently available on the Spanish market.
1. Nicotine Gum. This takes the form of a piece of chewing gum containing 2 or 4 mg of nicotine. The nicotine in the gum is bound to an ion exchange resin and is released inside the buccal cavity when the gum is chewed and the resin combines with the sodium and potassium ions in the saliva. The nicotine is then absorbed by the genial mucus, reaches the bloodstream, and from there stimulates the nicotine receptors of the smoker´s dopaminergic mesolimbic system.
In 2 meta-analyses, the OR for tobacco cessation of nicotine gum versus placebo was 1.66 (95% CI,1.62-1.85) in one and 1.63 (95% CI, 1.60-1.82) in the other.33,34 These figures are independent of the duration of therapy, the intensity of the psychological help the patient received, and the location where the tobacco cessation program took place.33,34 Sufficient evidence exists to recommend the use of 4 mg nicotine gum over 2 mg gum in highly dependent smokers scoring 7 points or higher on the Fagerström test (OR = 2.67; 95% CI, 1.69-4.22).33,34
Nicotine gum must be used correctly in order to attain maximum efficacy. Once in the mouth, the piece of gum should be chewed slowly until a strong flavor is noticeable. At this point, the user should stop chewing and park the wad between the cheek and gum until this flavor has disappeared. Uncontrolled chewing of nicotine gum causes the nicotine to be released too fast, in which case it cannot be absorbed through the oropharyngeal mucosa and, rather than producing the desired effect, it is likely to be swallowed and may cause gastric disturbances. Moreover, since it is metabolized in the liver, the ingested nicotine has no therapeutic effect. It is therefore advisable that, before nicotine gum is prescribed, patients should try it out in the presence of the clinician so that they can be instructed on the correct method.31
It is advisable to insist with patients not only on the correct use of the gum, but also on the need to follow the recommended dose schedule. In general, smokers with low dependency (3 points on the Fagerström test) should be prescribed 2 mg gum at a dosage of 1 piece every 1 to 2 hours during waking hours; patients with a higher level of dependency should follow the same regimen with 4 mg gum.1,6,8-15,31,33,34 Duration of treatment should range from 8 to 12 weeks. The use of gum is recommended for 8 to 10 weeks in less dependent smokers, but in highly dependent smokers it is advisable to prolong treatment to 3 months, although it is true that in these patients the use of gum can last from 6 to 12 months. The dose should be gradually reduced after 4 to 8 months of treatment.1,6,8-15
Table 1 shows a summary of the principal advantages and disadvantages of this type of NRT, together with a proposal for its use, and the cost per day.1,6,8,37,38
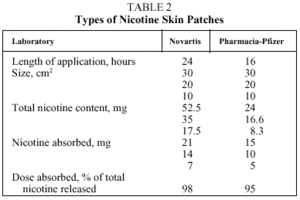
2. Nicotine Skin Patch. The patch is impregnated with nicotine, and it releases the substance through the skin to which it is adhered. There are two types of patches: those which release nicotine for 24 hours, for use during the entire day and night; and those which release nicotine for 16 hours, for use during the waking hours. Table 2 shows the different kinds of nicotine patches available on the Spanish market.6,8,9
Two meta-analyses showed that the OR for abstinence with nicotine patches versus placebo was 1.76 (95% CI, 1.6-1.85) in one and 1.73 (95% CI, 1.60-1.82) in the other.33,34 These figures are independent of the intensity of the psychological help the patient received and the location where the tobacco cessation program took place.33,34 Moreover it has been demonstrated that 8 weeks of treatment with a patch is as effective as longer treatments. Sufficient evidence has not been found to recommend a progressive decrease of the dose over abrupt withdrawal.33,34,39 The 24-hour patches are as effective as the 16-hour patches.33,34
The patch must be applied to a clean, hairless area of the skin on the upper limbs or torso. The patch should be placed every day when the patient gets up and should be removed on the same day when the patient goes to bed in the case of the 16-hour patch, or on the following day when the patient gets up in the case of the 24-hour release patch.31,37 The site used for the patch should be changed every day in order to prevent the appearance of erythema and pruriginous exanthema.
It is advisable to follow the recommendations for use included in the guidelines drawn up by various scientific institutions and those specified by the different meta analyses carried out.1,6,8-12,14,15,33,34,37,39 Some of the most important of these are as follows:
1. Nicotine patches should be used by smokers with slight to moderate physical dependence on nicotine. When used alone in highly dependent smokers (≥7 in the Fagerström test), no increase in the success rate has been demonstrated.
2. Nicotine patches should be used during a period of not less than 6 to 8 weeks and no longer than 12 weeks. Early withdrawal of nicotine patch therapy, before 6-8 weeks, tends to facilitate relapse. Prolonging treatment with nicotine patches for more than 12 weeks does not increase the likelihood of success.
3. The patches should be used at a high dose during the first 6 to 8 weeks. In the case of 16-hour patches, the high dose recommendation is 25 mg/day, which can be achieved by using a 15 mg patch together with a 10 mg patch. In the case of the 24-hour nicotine patch, the high dose is 21 mg/day, attained by using a patch with a 30 cm² surface area.
Table 3 shows the principal advantages and disadvantages of this type of NRT.1,6,8-12,14,15,33,34,37-39
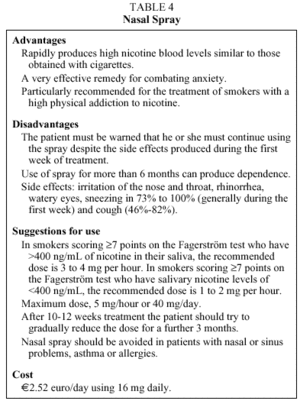
3. Nicotine Nasal Spray. The nasal spray consists of a small pocket-sized bottle containing a watery isotonic solution with a neutral pH, which contains nicotine at a concentration of 10 mg/mL. Fourteen minutes8 after delivery of 1 mg of nicotine nasal spray, a maximum concentration of 8.93,4 ng/mL is obtained in blood.40 This means that the nicotine concentrations obtained with the nasal spray are similar to those achieved with the gum in half the time. This characteristic is unique to this kind of NRT. The nasal spray is the fastest way of obtaining high levels of nicotine in the blood.
Two meta-analyses show that the OR for abstinence with nicotine nasal spray versus placebo was 2.27 (95% CI, 1.62-1.85) in one, and 2.27 (95% CI, 1.60-1.82) in the other.33,34 These figures are independent of the intensity of the psychological help the patient received and of the location where the tobacco cessation program took place.33,34
A dose of nicotine nasal spray should be delivered into each nostril. Before using the spray, the subject should try to ensure that the nostrils are clean and permeable. The patient should be instructed to apply the spray so that the longitudinal axis of the bottle is held parallel to the nose bones. A dose of nicotine nasal spray consists of one 0.5 mg delivery to each nostril (1 mg total).40
Table 4 shows the principal advantages and disadvantages of this type of NRT.1,6,8-12,14,15,33,34,37,38,40-42
4. Nicotine Lozenges. Nicotine lozenges contain 1 mg of nicotine. The absorption mechanism and pharmacokinetic profile are similar to those of the 2 mg nicotine gum. The efficacy of this type of NRT has been confirmed by a double-blind, placebo-controlled trial, and it has been shown that smokers who use nicotine lozenges are twice as likely to stop smoking as those who use a placebo.43
The recommended dosage is 1 to 2 lozenges every hour while the subject is awake for 6 to 8 weeks, followed by a gradual reduction in the dose until 12 weeks.
The principal advantages of this kind of NRT are its ease-of-use and the comparative lack of side effects.
Bupropion
Bupropion is the first nicotine-free medication that has proven effective in the treatment of tobacco addiction.1 Bupropion is a bitter white powder supplied in the form of sustained release tablets containing 150 mg of the active ingredient.
Although the exact mechanism by which this medication aids smoking cessation is not understood, it is known that it acts on the nucleus accumbens inhibiting the neuronal re-uptake of dopamine. This effect would explain the reduction in anxiety experienced by smokers when they use bupropion.44 It also inhibits the neuronal re-uptake of noradrenaline in the nucleus locus caeruleus, thereby achieving a significant reduction in the intensity of the nicotine withdrawal symptoms.44 Recent in vitro studies have detected that bupropion is a non-competitive functional inhibitor of the nicotinic acetylcholine receptors. This anti-nicotinic action may contribute to its efficacy in the treatment of nicotine dependence.
A recent meta-analysis has demonstrated that bupropion at a dosage of 300 mg/day for a period of 7 weeks is associated with a significant increase in continuous abstinence after 7 weeks of treatment (OR = 2.71; 95% CI, 1.88-4.07) and on follow up at 12 months (OR = 2.10; 95% CI, 1.62-2.73).45 Furthermore, it has been determined that the efficacy of this medication is related with dose, and with mean plasma concentrations of the drug and its metabolites.46 Smokers who use bupropion at doses of 100, 150 or 300 mg/day are respectively 1.42, 1.69 and 2.84 times more likely to stop smoking than those who receive placebo.46 Moreover, it has been established that the likelihood of the subject stopping smoking increases 1.01 times for every 1 µg/L increase in the plasma concentrations of the metabolites of bupropion.46 Finally, in a meta-analysis of 10 randomized controlled trials involving 3800 smokers carried out by the National Institute for Clinical Excellence, the OR for successful smoking cessation of bupropion versus placebo was 2.16 (95% CI, 1.51-3.10).13 In other words, 19% of smokers who received bupropion were completely abstinent on follow up at 1 year, as compared to only 9% of those who received placebo.13
Bupropion should be used for a period of 7 to 9 weeks at a dosage of 300 mg/day taken as 150 mg twice daily Treatment should be started 7 to 15 days before the subject definitively stops smoking. During the first week, the subject should take only one 150 mg tablet per day, and subsequently the dosage should be increased to two 150 mg tablets per day. One tablet should be taken in the morning on waking, and the second 8 hours later.47 Occasionally, treatment can be prolonged for up to 12 weeks.1,8,9,13,14,45,46
Table 5 shows the principal advantages and disadvantages of this medication, and a proposal for its use.1,8,9,12-15,38,44-50
Choice of Treatment
We will now discuss some considerations that will help in the choice of the most appropriate pharmacological treatment for each smoker. Before choosing a particular treatment, the following factors should be evaluated.
Medical History. Nicotine gum is not recommended for smokers with a history of dental disease or chewing problems. Patches should be avoided in patients with dermal atopies or chronic or acute dermatological disease. Nicotine spray should not be used by smokers who suffer from nasal polyps or other diseases of the nostrils. Bupropion should be avoided in patients with a history of seizures or when the seizure threshold of the patient is low. NRT is not contraindicated in smokers with cardiovascular or pulmonary disease.48-53
Some trials have demonstrated that smokers with a history of chronic alcoholism are less likely to quit than smokers who do not suffer from this condition.54,55 However, a recent study showed that the probability of quitting with bupropion is identical in all patients, irrespective of whether or not they have a history of alcoholism.56
Psychiatric History. Some studies have shown that smokers who have a history of depression are less likely to quit than smokers who do not.57 However, analysis of the trials carried out with buproprion has shown that, when they use bupropion to support their attempts, patients with a history of depression are just as likely to quit as any other smoker.49,58
The use of NRT is recommended in smokers diagnosed with bipolar disorder, bulimia, or anorexia and in patients being treated with haloperidol or other psychopharmaceuticals.
Sex. Various studies have shown that women stop smoking less often than men.49,59,60 However, the analysis of the female subpopulation in a clinical trial showed that female sex was a predictor of a lower abstinence rate when female smokers were treated with placebo or nicotine patches, but not when they received bupropion.49,58,61 These data suggest that bupropion may be the preferred treatment for female smokers, although more research is required to confirm this hypothesis.
Weight Gain. Tobacco cessation is followed by weight gain. Some studies have determined that this weight gain can be a cause for relapse.49,62 In fact, weight gain has been cited as the cause of relapse in over 40% of women smokers who wish to quit.1,6,8,49 The use of 4 mg nicotine gum reduces the weight gain that occurs after the smoker quits. It has been established that the higher the dose of nicotine taken in the form of gum, the greater the reduction in the weight gain.33,34
Furthermore, it has been detected that the use of bupropion delays the weight gain caused by tobacco cessation. When bupropion is used for 7 to 9 weeks, weight gain is noticed after treatment is stopped, but when treatment is prolonged for over a year, no weight gain is detected.49,61,63
Bupropion or 4 mg nicotine gum may be indicated in smokers who wish to stop smoking but who are concerned about the possible weight gain this might cause.
Treatment Received During Prior Quit Attempts. It is very likely that a smoker who quits permanently will have failed in at least 6 prior attempts.1 It is therefore important to consider what treatments were used in prior attempts in order to improve the indication of a new therapy in a new attempt. The repeat use of NRT in a new attempt to stop smoking has been shown to be only moderately effective, with abstinence rates of between 0% and 6% on follow up at 6 months.64,65
Bupropion has been shown to be effective in the retreatment of smokers who have received this medication before or who have received NRT. Gonzales et al66 have found that smokers who received a second course of bupropion treatment for 12 weeks despite failure in the prior attempt had an abstinence rate of 28% as compared to 9% in a placebo group on follow up at 12 weeks, and of 9% compared to 2% at 12 months. Another study has found that in a group of smokers with a history of failed attempts using NRT, bupropion at a dosage of 300 mg/day taken as 150 mg twice daily weeks resulted in 14 % abstinence, as compared to 8% in the placebo group.67
Before prescribing treatment for a new quit attempt, the therapy received by the subject during prior attempts should be taken into account. The unsuccessful use of NRT should counsel consideration of the use of bupropion. Prior unsuccessful use of bupropion should not be considered to be an obstacle to its re-use. However, more studies are needed to confirm this indication.
Characteristics of the Smoker's Tobacco Addiction. The first step in the elaboration of an appropriate treatment plan is the correct diagnosis of the type and degree of the smoker's dependence on tobacco. The differential characteristics of each smoker that should be taken into account before specifying one type of treatment or another are discussed below.
The degree of the smoker's physical dependence on nicotine and the type of cravings and withdrawal symptoms he or she suffers from must be carefully taken into account to achieve a better treatment prescription. The Fagerström test is the most useful tool for determining the degree of dependence.68 However, since there are more restrictions every day on smoking in public places, when determining the smoker's degree of dependence, it is more useful to consider only the parameter "time elapsed after waking before first cigarette of the day" than the parameter "number of cigarettes smoked per day." On the other hand, an excellent correlation has been established between the degree of dependence as measured by the Fagerström test and the parameter "time elapsed after waking before first cigarette of the day."69 Furthermore, in a survey carried out in Britain, it was found that only 25% of smokers smoke more than 20 cigarettes per day, while 67% smoke their first cigarette within 30 minutes of waking.70
It is important to assess each smoker's withdrawal symptoms and to try to differentiate between the symptoms caused by the lack of nicotine in the blood (anxiety, irritability, nervousness, sleep disturbances, etc) and those caused by the absence of nicotine peaks in the blood (anxiety) since the treatment differs in each case.71
The 2 mg nicotine gum should be used only for smokers with low dependence who smoke their first cigarette more than 30 minutes after waking. The patches have been shown to be less effective in highly dependent smokers than in those with low or moderate dependence.33,34,39 The 4 mg gum is highly recommended for smokers with a moderate to high degree of physical dependence on nicotine, and in these cases it is helpful long the treatment for 3 to 6 months, or even 1 year.33,34,39 Nasal spray is highly indicated for smokers with a high degree of physical dependence on nicotine.
Studies carried out with bupropion have demonstrated its effectiveness in all types of smokers.1,8,9,12-15,38,44-50 However, the dose and length of treatment should be adjusted according to the degree of dependence. Smokers who smoke their first cigarette more than 30 minutes after waking should use this medication for a period of 7 to 9 weeks, while treatment of 3 to 6 months is recommended in the case of more highly dependent smokers.1,8,9,12-15,38,44-50
When anxiety is the strongest and most intense withdrawal symptom, 4 mg nicotine gum or nicotine nasal spray is highly indicated. The patient should be advised to use these medications at precisely the moment when he or she is suffering from the symptoms, or even better, a few minutes before they appear, or in situations in which the patient foresees their occurrence.1,6,8-15 In the various trials carried out with bupropion, this medication has also been shown to be effective in controlling anxiety.72
Smokers' Preferences. Before recommending a certain course of treatment to the smoker, the physician should take into account all of the considerations mentioned above. It is helpful to take into account any preferences the patient may have since this will result in improved compliance with the recommended treatment.
Use of Pharmacological Treatment in Special Situations
Smokers of Fewer Than 10 Cigarettes a Day. The majority of studies that have been carried out to analyze the efficacy of pharmacotherapies to aid tobacco cessation have studied smokers of 15 or more cigarettes per day.1,6,8-15,33,34,44-50 There is no clear indication for the use of these products by smokers of less than 10 cigarettes per day, although the decision should always be made taking into account the patient's health status and preferences, and in such cases the decision to use a pharmacological treatment is optional.
Young Smokers. There is no evidence that the use of pharmacotherapy to aid tobacco cessation significantly increases the probability of success in smokers under 15 to 16 years old.1,11-15 The prescription of such therapies to this group of smokers must be evaluated on a case-by-case basis.
Pregnancy. The use of bupropion is contraindicated in pregnant women.50 However, studies have been carried out in which NRT was used to treat pregnant smokers.73-76 Both nicotine gum73,74 and nicotine patches75,76 were used in these studies, and the main conclusions reported were as follows: no form of NRT was shown to be harmful to the fetus, but it was also found that both nicotine gum and transdermal patches showed an efficacy similar to that of placebo.
Cardiovascular Disease. Neither NRT nor bupropion are contraindicated in patients suffering from cardiovascular disease. In studies carried out with healthy individuals who used the gum for 5 years, no increase in cardiovascular disease could be demonstrated.77 Neither could any evidence be found of an increased risk of abnormalities in ECG, arrhythmia, angina, or sudden death in patients with cardiovascular disease who used NRT for tobacco cessation.78 It was also noted that NRT is associated with a lower risk of causing acute myocardial infarction than smoking cigarettes.51
Furthermore, bupropion has been shown to be safe and efficacious in the treatment of smokers with cardiovascular disease.49 A study of smokers suffering from cardiovascular disease found similar efficacy for bupropion among this group and the population in general, while no particular adverse events were detected.79
Combined Pharmacological Treatment. For some patients, a combination of various types of pharmacotherapy is indicated. This combination may include 2 or more forms of NRT, or a combination of NRT and bupropion. The different meta-analyses found not very strong evidence that the combination of different types of NRT is associated with a significant increase in its efficacy.33,34 On the other hand, one study has demonstrated that the combination of bupropion and nicotine patches significantly increases the efficacy of the patches, but not that of bupropion used alone.33,34,61 There are no precise indications for the use of combined therapy. However, this option should be considered in the following cases: smokers of over 30 cartons/year, smokers scoring 7 points or higher on the Fagerström test, smokers with more than 250 ng/mL of nicotine in the blood, and smokers who have used single therapies in previous quit attempts and who, despite this, suffered a relapse because of withdrawal symptoms.1,6,8-15
High Doses of NRT. A review of the literature suggests that the use of NRT in high doses may be associated with a slight increase in the efficacy of the treatment.33,34,39 The use of an appropriate NRT in high doses should be carried out in specialized clinics with pre-treatment and post-treatment monitoring of blood nicotine levels, and with highly dependent smokers who have failed in previous attempts in which they used NRT at the usual doses.1,6,8-15,33,34,80,81
Harm Reduction. During recent years, a new form of treatment has been used to treat tobacco addiction in smokers who do not wish to or cannot completely quit smoking. This consists in achieving a significant reduction in the number of cigarettes smoked per day in order to diminish the intensity of risk associated with tobacco use, and thus to ultimately reduce the probability of the patient developing the numerous illnesses associated with tobacco use.82 To date it has been observed that the use of NRT and bupropion among these smokers who do not wish to or cannot give up smoking entirely is effective in helping them to achieve and maintain for over 12 months a significant reduction in the number of cigarettes smoked per day.65,82-84 Moreover, it has been demonstrated that a reduction in the number of cigarettes smoked per day is accompanied by a significant decrease in the various risk factors for cardiovascular and respiratory diseases.83-85 It remains to be determined whether this reduction is also associated with a decrease in the morbidity and mortality due to illnesses associated with tobacco use. Health professionals should consider treatment aimed at achieving harm reduction in the following situations: highly dependent smokers who chronically consider quitting, who have made several unsuccessful attempts to quit smoking, and who are discouraged about the possibility of a new attempt; and highly dependent smokers who chronically consider quitting but who do not wish to quit smoking yet do want to significantly reduce the number of cigarettes they smoke.82-86
Correspondence: Dr. Carlos A. Jiménez-Ruiz.
Unidad de Tabaquismo. Instituto de Salud Pública. Comunidad de Madrid.
C/. Conde de Peñalver, 96. 28006 Madrid. España.