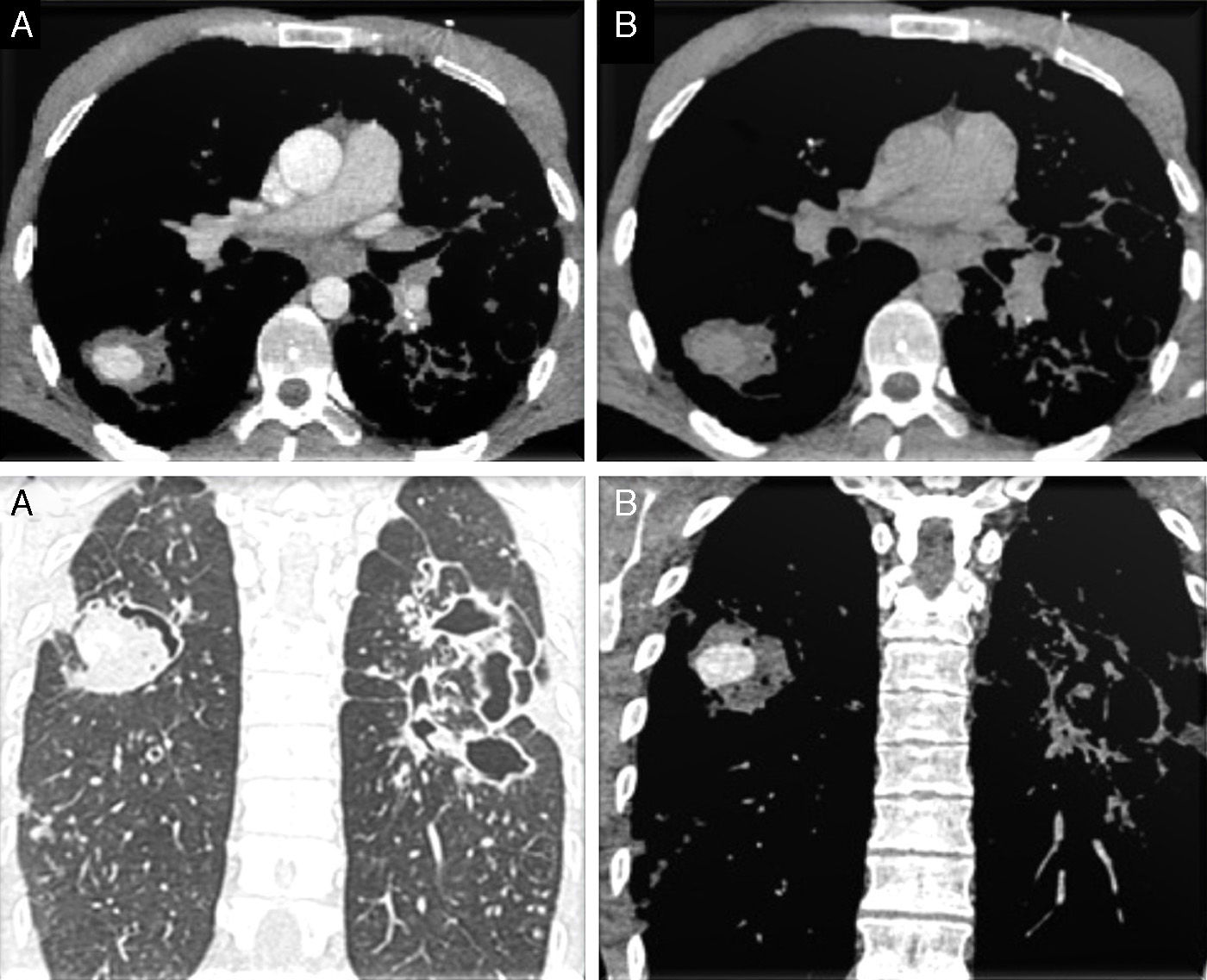
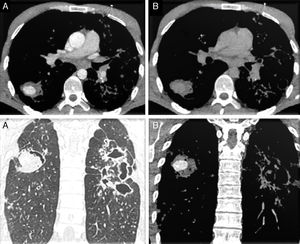
A 33-year old Romanian man was seen in the emergency room of our hospital for cough with hemoptysis. He reported fever lasting 48h and night sweats lasting 2 weeks. He mentioned a history of pulmonary tuberculosis (TB) treated 5 years previously. The patient also showed signs of respiratory distress. Based on this information, we performed a chest X-ray, which showed bilateral interstitial and alveolar opacities. Subsequent contrast-enhanced multidetector computed tomography (MDCT) of the chest showed several consolidations (some cavitary), extensive bronchiectasis and a well-defined round lesion measuring 3cm in the apical segment of the right lower lobe (RLL), with contrast uptake in the arterial phase and washout in the venous phase (Fig. 1). These findings were suggestive of Rasmussen's pseudoaneurysm secondary to tuberculous infection.
Mediastinal window CT scan axial slices: lesion in the RLL, with well-defined borders, showing contrast uptake in the arterial phase (A top) and washout in the venous phase (B top). Parenchymal window (A bottom) and mediastinal window (B bottom) CT scan coronal slices in the arterial phase: contrast-enhanced nodular lesion in the RLL and images of bronchiectasis and extensive cavitation in the left hemithorax.
The patient was admitted to the intensive care unit and given tuberculostatic therapy after the initial diagnosis was confirmed by Ziehl-Neelsen staining. Because of his hemodynamic instability, embolization as a secondary prevention measure was ruled out, as was resection of the lesion. Two days after admission, the patient presented massive hemoptysis, probably due to rupture of the lesion, and died.
Up to one third of patients with active TB will present massive hemoptysis over the course of the disease, with asphyxia, not the hemorrhage per se, as the principal cause of death.1 In TB, the arterial damage is caused by replacement of the adventitia with granulation tissue, which is then replaced with fibrin, resulting in dilatation of the arterial wall. However, most hemoptyses will be caused by vascular erosion, without the formation of pseudoaneurysms.
These pseudoaneurysms, which were first described in 1868 by Fritz Valdemar Rasmussen, can originate in the bronchial vasculature (most frequently, in up to 90% of cases)2 non-bronchial systemic arteries, or pulmonary artery branches. Hemoptysis, when secondary to TB, should alert clinicians to this diagnosis, which is best confirmed with a CT scan.
Hemoptysis appears in the pulmonary parenchyma as areas of ground-glass attenuation and areas of obstructive atelectasis due to blood in the bronchi, although these signs are non-specific.3 The identification of a nodular image with intense contrast uptake during the arterial phase followed by washout in the venous phase is indicative of this type of vascular lesion.
A multidisciplinary4 therapeutic approach is needed, aimed at maintaining airway permeability, optimizing oxygenation, and achieving hemodynamic stability.4 Due to the considerable risk of complications, the final treatment of choice is percutaneous embolization (which can also be preventive) of the systemic arteries feeding the lesion, or even lobectomy in cases of serious, refractory disease.5 Our protocol includes MDCT in order to locate the source of bleeding. This is followed by selective embolization of bronchial or pulmonary systemic arteries guided by the vascular map obtained with MDCT. If embolization is not effective, lobectomy can be considered.
Please cite this article as: Peghini Gavilanes E, López Yepes LA, Peñalver Paolini CL, Morales Ruiz R. Seudoaneurisma de Rasmussen en un paciente con antecedente de tuberculosis pulmonar. Arch Bronconeumol. 2015;51:96–97.