Mucormycosis is an infection caused by filamentous fungi that presents in different forms: rhinocerebral, pulmonary, renal, cutaneous, and gastrointestinal. The species Rhizopus oryzae, responsible for 70% of cases, is the most frequently isolated organism.1 Risk factors for developing mucormycosis include blood diseases, diabetes mellitus with poor metabolic control, solid organ or hematopoietic transplantation, neutropenia, injury, iron overload, and severe burns. It is unclear whether the chronic use of corticosteroids predisposes patients to developing mucormycosis. In recent years, we have witnessed an increase in the incidence of this entity due to population aging, which goes hand in hand with an increase in the above-mentioned risk factors.2,3
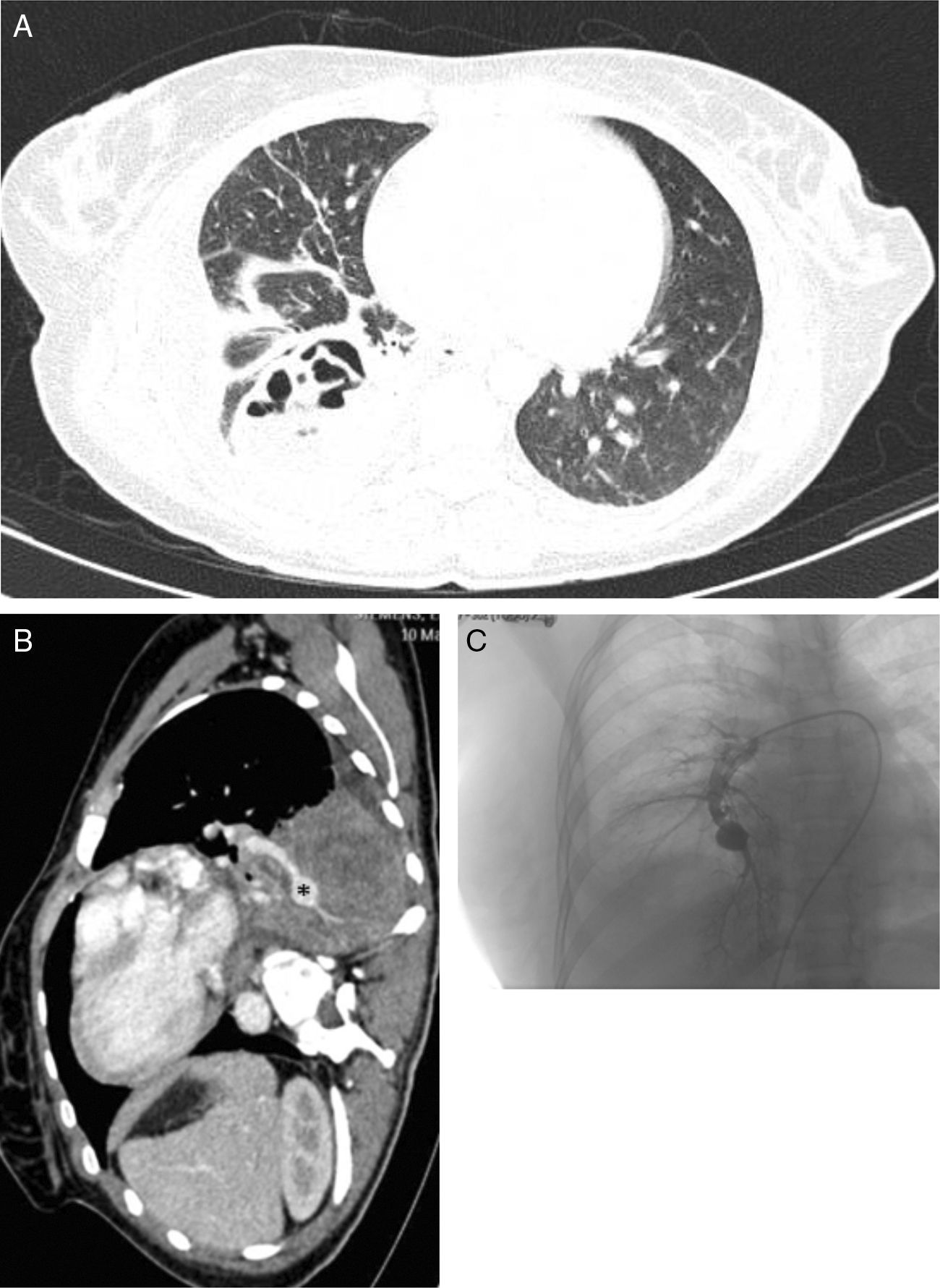
We report the case of a 29-year-old woman, smoker of 10 pack-years, with recent onset of diabetes mellitus type 1 (ketoacidosis the week before presentation of this clinical episode). She consulted due to a few hours history of dyspnea, fever 38°C, pain in the right flank, cough and rust-colored expectoration. Auscultation revealed crackles in the right lung base. Clinical laboratory tests showed significant leukocytosis (30100/μl) and elevated CRP (224mg/l). Consolidation of the right lower lobe was observed on chest radiograph. The patient was diagnosed with community-acquired pneumonia, and empirical antibiotic therapy was started. The chest computed tomography (CT) scan showed consolidation of the pulmonary parenchyma in the right lower lobe, with the formation of a thick-walled hypodense lesion containing air bubbles, with axial diameters measuring 6.1cm×4.2cm, consistent with an abscess (Fig. 1A). During hospitalization in the general ward, the patient had several episodes of hemoptysis, so fiberoptic bronchoscopy was performed, revealing total stenosis of the right anterior basal bronchus (B8) and partial stenosis of the right basal-lateral bronchus (B9) with necrotic tissue on solid tissue. Given the lack of clinical improvement, the chest CT was repeated, showing, in addition to the necrotizing consolidation, aneurysmal dilation of the exit of the segmental artery of right bronchial segment 10, consistent with mycotic aneurysm, measuring 1.4cm in length and 5cm in diameter (Fig. 1B). Arteriography (Fig. 1C) confirmed the diagnosis. The aneurysm was successfully embolized with a 14-mm type 2 Amplatzer® plug and hemoptysis was controlled. The source of the aneurysm and the right lower truncal branch were subsequently embolized with 10-mm coils and an 8-mm Amplatzer® plug. Bronchial biopsy obtained during the bronchoscopy showed the presence of hyphae consistent with mucormycosis, so treatment began with liposomal amphotericin and caspofungin. Following this, the patient showed slow but clear clinical improvement. Lower right lobectomy was performed by posterolateral thoracotomy, revealing pleural adhesions throughout the lung surface, particularly between the lower right lobe and the diaphragm. The postoperative period was free of complications, recovery was favorable, and cure was achieved.
(A) Chest CT with contrast medium (parenchymal window), showing a lung abscess associated with necrotizing pneumonia of the right lower lobe. (B) Sagittal chest CT slice (mediastinal window), showing a mycotic aneurysm in the infero-medial segmental branch (asterisk). (C) Pulmonary arteriography performed before embolization of the aneurysm.
A defining characteristic of pulmonary mucormycosis is its rapid progress and marked angioinvasive capacity. Invaded tissue becomes necrotized and occupied by hyphae, causing infarction and fostering the development of cavitating pneumonias. Mucormycosis can also invade adjacent structures such as the mediastinum, the heart, or the bloodstream (fungemia). Hemoptysis is a common complication and can be massive. Diagnosing mucormycosis is a complex process, since the presentation is similar to that of community-acquired pneumonia: the most common symptoms are fever, pleuritic pain, and cough with purulent expectoration. Imaging tests are nonspecific, since no characteristics signs exist that can be distinguished from other processes. Identification of the organism in the tissue is necessary to reach a safe diagnosis of invasive fungal infection. The species can be confirmed on culture. Bronchoalveolar lavage (BAL) can be used in immunosuppressed patients, although a positive result for fungi is only orientative. However, it is considered highly suggestive if hyphae are visualized on optical microscopy. Medical treatment of choice is intravenous liposomal amphotericin B at a dose of 5mg/kg/day, which should continue until clinical and radiological resolution of the process. Resistance to voriconazole is a significant feature of these organisms. For drug treatment to be effective, existing necrotic tissue must be removed, either by debridement or by lobectomy, so that the antifungals can penetrate well-perfused tissues.4,5 Mucormycosis is an emerging disease that must be considered in daily clinical practice, and therein lies the interest in this case: necrotizing pneumonia in a patient with predisposing factors, presence of hemoptysis, and necrotic areas in the bronchial mucosa are key data that point toward a diagnosis of invasive fungal infection.
Please cite this article as: Espíldora-Hernández J, Pérez-López C, Abarca-Costalago M, Nuño-Álvarez E. Mucormicosis pulmonar en paciente joven con inicio de diabetes mellitus. Arch Bronconeumol. 2017;53:531–533.