Two previous national epidemiological studies, IBERPOC in 1997 and EPISCAN in 2007, determined the COPD burden in Spain. Changes in demographics and exposure to risk factors demand the periodic update of COPD prevalence and its determinants.
MethodsEPISCAN II aimed to estimate the prevalence of COPD in the general population aged 40 years or older in all 17 regions of Spain. A random population screening sample, requiring 600 participants per region performed a questionnaire plus post-bronchodilator (post-BD) spirometry.
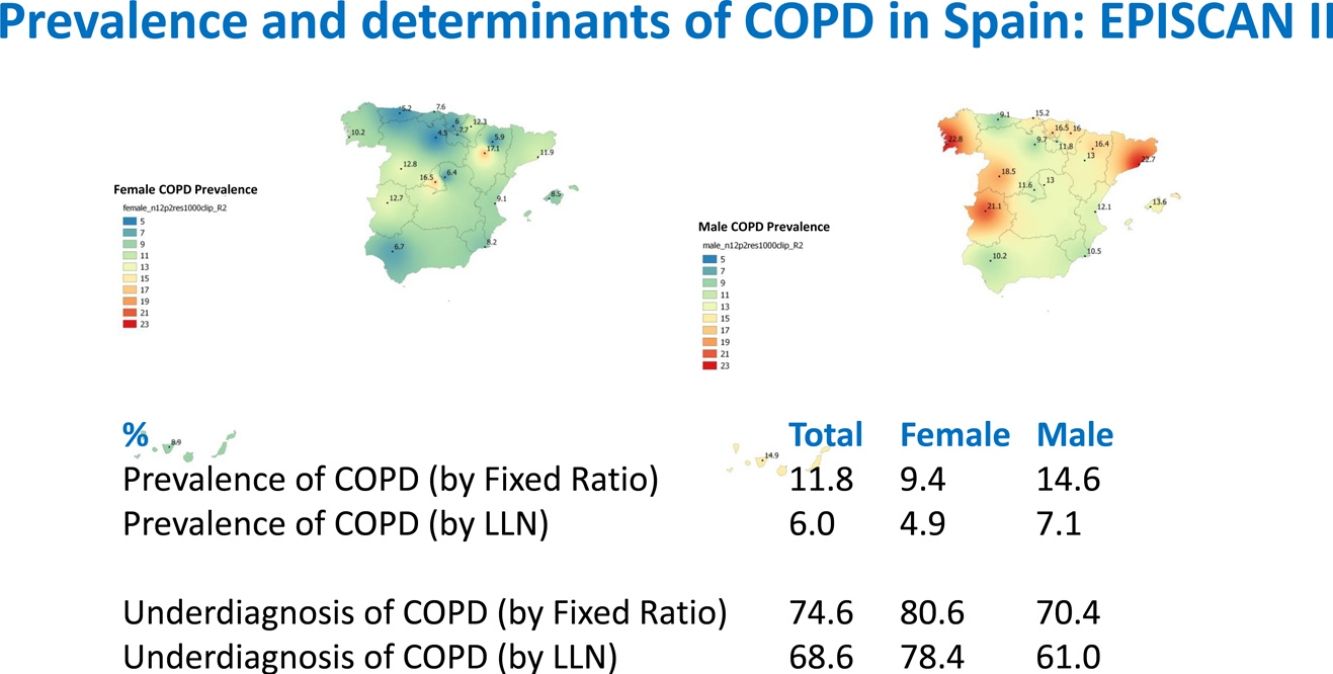
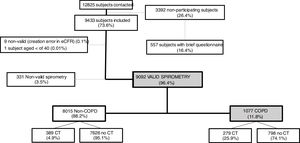
ResultsA total of 12,825 subjects were initially contacted, and 9433 (73.6%) agreed to participate, of whom 9092 performed a valid spirometry. Baseline characteristics were: 52.6% women, mean±SD age 60±11 years, 19.8% current- and 34.2% former-smokers. The prevalence of COPD measured by post-BD fixed ratio FEV1/FVC<0.7 was 11.8% (95% C.I. 11.2–12.5) with a high variability by region (2.4-fold). Prevalence was 14.6% (95% C.I. 13.5–15.7) in males and 9.4% (95% C.I. 8.6–10.2) in females; according to the lower limit of normal (LLN) was 6.0% (95% C.I. 5.5–6.5) overall, by sex being 7.1% (95% C.I. 6.4–8.0) in males and 4.9% (95% C.I. 4.3–5.6) in females. Underdiagnosis of COPD was 74.7%. Cases with COPD were a mean of seven years older, more frequently male, of lower attained education, and with more smokers than the non-COPD population (p<0.001). However, the number of cigarettes and pack-years in non-COPD participants was substantial, as it was the reported use of e-cigarettes (7.0% vs. 5.5%) (p=0.045). There were also significant social and clinical differences including living alone, previous respiratory diagnoses, more comorbidities measured with the Charlson index, greater BODE and COTE scores, cognitive impairment, and depression (all p<0.001).
ConclusionsCOPD remains prevalent in Spain and frequently underdiagnosed.
Dos estudios epidemiológicos nacionales anteriores, IBERPOC en 1997 y EPISCAN en 2007, determinaron la carga de EPOC en España. Los cambios en la demografía y la exposición a factores de riesgo exigen una actualización periódica de la prevalencia de EPOC y sus determinantes.
MétodosEPISCAN II tuvo como objetivo estimar la prevalencia de EPOC en la población general de 40 años o más en las 17 Comunidades Autónomas de España. Una muestra aleatoria de población para cribado, que requirió 600 participantes por región, realizó un cuestionario y una espirometría tras la administración de un broncodilatador (post-BD).
ResultadosUn total de 12.825 sujetos fueron contactados inicialmente, y 9.433 (73,6%) aceptaron participar, de los cuales 9.092 realizaron una espirometría válida. Las características sociodemográficas basales fueron: un 52,6% eran mujeres, la edad media ±DE era de 60±11 años, un 19,8% eran fumadores activos y un 34,2% eran exfumadores. La prevalencia de EPOC medida por el criterio de cociente fijo post-BD FEV1/FVC<0,7 fue del 11,8% (IC 95%: 11,2-12,5) con una alta variabilidad por región (2,4 veces). La prevalencia fue del 14,6% (IC 95%: 13,5-15,7) en varones y del 9,4% (IC 95%: 8,6-10,2) en mujeres; considerando el límite inferior de la normalidad (LIN), fue del 6,0% (IC 95%: 5,5-6,5) en la muestra global y, por sexos, del 7,1% (IC 95%: 6,4-8,0) en varones y del 4,9% (IC 95%: 4,3-5,6) en mujeres. El infradiagnóstico de la EPOC fue del 74,7%. Los casos con EPOC tenían de media 7 años más, eran con mayor frecuencia varones, tenían menor nivel educativo y había más fumadores que en la población sin EPOC (p<0,001). Sin embargo, el número de cigarrillos y paquetes/año en los participantes que no tenían EPOC fue sustancial, como también fue elevado el uso de cigarrillos electrónicos (7,0 vs. 5,5%) (p=0,045). También hubo diferencias sociales y clínicas significativas que incluyeron: vivir solo, diagnósticos previos de enfermedad respiratoria, más comorbilidades medidas con el índice de Charlson, puntuaciones más altas en el índice BODE y la escala COTE, deterioro cognitivo y depresión (todos p<0,001).
ConclusionesLa EPOC sigue siendo prevalente en España y con frecuencia está infradiagnosticada.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality in the World and in Spain. Its economic impact is high, due in part to underdiagnosis that results in most patients going undetected and reaching advanced disease stages while receiving inappropriate treatment.1–3 In clinical practice, the diagnosis of COPD is based on the assessment of exposure to tobacco smoke and other noxious gases, the presence of respiratory symptoms and chronic airflow limitation, documented with post-bronchodilator (post-BD) spirometry. Airflow limitation, measured by the ratio of post-BD FEV1/FVC and other spirometric indices, provides important information for optimizing disease management and establishing severity.1,2 COPD screening should be considered in any individual who presents respiratory symptoms and who has been exposed to risk factors, the most important of which is smoking.4
Two studies conducted ten years apart in Spain, IBERPOC and EPISCAN, determined a 9.1% prevalence of COPD in the general Spanish population aged 40–69 years in 1997,5 and a 10.2% in the 40–80-year age range in 2007.6 Other studies, such as PLATINO, found an even higher prevalence (14.3%) in various Latin American capitals, also in individuals aged over 40 years.7,8 Despite these figures, COPD is still a disease with high rates of underdiagnosis: estimated rates in Spain were 78% in 1997 and 73% in 2007. The consequence is that diagnosis is made at more advanced disease stages, when the risk of exacerbations and mortality is higher.9
Global mortality estimates indicate that COPD was the fifth cause of death in 1990, and by 2010 it had already become the third cause of death,10–12 so an early diagnosis is of vital importance.
A new epidemiological study was conducted to update data on the prevalence and determinants of COPD in all 17 regions in Spain.13 The primary objective of EPISCAN II was to estimate the prevalence and determinants of COPD, and their distribution in the general population of Spain aged 40 years or older.
MethodsThe full protocol of EPISCAN II is based upon the EPISCAN study, and has been summarized elsewhere.12 Briefly, EPISCAN II is a national, multicentre, cross-sectional, population-based epidemiological study. Study subjects were selected from the general population of Spain who were resident in the postal code areas nearest to the participating hospitals. The 20 participating hospitals were selected from all 17 autonomous communities (regions). The inclusion criteria were as follows: men or women aged 40 years or more, resident in Spain, with no physical or cognitive difficulties that would prevent them from completing spirometry or any of the study procedures. Participants attended either a short- or long-visit in the hospital centre. The study population was divided into two cohorts, depending on the results of the post-BD spirometry: patients with COPD (FEV1/FVC<0.7) and non-COPD individuals (FEV1/FVC≥0.7).
Field work was undertaken from April 2017 to February 2019. The study was approved by the ethics committee (EC) of each of the participating centres, with the EC of the Hospital Universitario La Princesa acting as the reference committee. All participants signed an informed consent. The EPISCAN II protocol is registered at https://clinicaltrials.gov with the No. NCT03028207 and at www.gsk-clinicalstudyregister.com/study/205932.
Selection of participantsStudy sampling was conducted using a pre-selected list of the post codes closest to each hospital. A list of random telephone numbers was obtained, stratified according to these post codes and quotas for sex and age groups, all according to the latest EU directives on data protection and privacy.14,15
Variables and proceduresDuring the first telephone call, the subject was informed about confidentiality and data protection, and if they agreed to respond, they were asked questions about their cohabitants, confirmation of the post code for assignment of the nearest hospital, previous diagnoses of respiratory disease (chronic bronchitis, emphysema, COPD, or asthma), smoking habit (smoker and number of cigarettes, never-smoker, former smoker and number of cigarettes) and presence of cough or expectoration. During the second telephone call, conducted by the investigator from the hospital, a survey was administered with questions on the previous diagnosis of respiratory diseases, smoking habit, and the presence of other symptoms associated with COPD. The variables collected during the visit with the healthcare professional provided a comprehensive profile of both non-COPD individuals who were selected for the study visit, and, in particular, participants in the COPD group.
Information were collected on age, sex, level of education, family conditions, weight and height, and conventional use of tobacco (cigarettes, pipe, cigar) or use of other modes of delivery (electronic cigarette, chewing tobacco, etc.).
Other questionnaires included: COPD Assessment Test (CAT); Hospital anxiety and depression scale (HADS); Yale Physical Activity Questionnaire (YPAS); European Coal and Steel Community Questionnaire on respiratory symptoms; Questions on exposure to dust and fumes in the workplace; Mini-Mental State Questionnaire.(administered only to participants 60 years of age or older); Fagerström test; and Prochaska's Stages of Change.
Lung Function and Clinical Tests conducted for the entire sample included: Baseline pulse oximetry; Forced spirometry; and assessment of multicomponent indices and comorbidities.
Lung function and clinical tests (for the entire sample)Baseline pulse oximetry was determined using a Pulsox 300i (Konica-Minolta, Japan) pulsioxymeter and the fraction of carbon monoxide (CO) in exhaled air was determined with aco-oxymeter (MicroCO, Carefusion, UK).
Forced spirometry was performed using a pneumotachograph (Vyntus Spiro, Carefusion, Germany), according to standardized procedures as indicated by SEPAR guidance16 and Global Lung Function Initiative (GLI) equations were used as reference value.17 Bronchodilator test was conducted with the inhalation of 400μg salbutamol. According to the ATS/ERS guidelines,18 criteria for bronchodilation were an increase in FVC or FEV1>200ml and greater than 12% compared to the baseline value. Only spirometries with quality grades A-C were accepted for analysis.15 Airflow limitation was defined as a postbronchodilator FEV1/FVC ratio<0.7 (or alternatively also expressed as an FEV1/FVC<lower limit of normal (LLN)). Underdiagnosis of COPD by age, sex and area was defined as the percentage of those not reporting a previous diagnosis of COPD, chronic bronchitis or emphysema among those with airflow limitation.
Multicomponent indices and comorbiditiesIn order to have a better characterization of the population, comorbidities were quantified by means of: the Charlson index (19 comorbidities)19; the COTE index (12 comorbidities)20; and previous diagnosis of other respiratory diseases. As multicomponent indices, BODE and BODEx were calculated.21
Statistical analysisAccording to data from the 2011 Census of Population and Dwellings, published by the National Institute of Statistics,22 the population of Spain aged 40 years or more comprises 23,957,645 individuals. Taking into account the 10.2% prevalence of COPD found in the EPISCAN study,6 an a priori sample size calculation estimated with an accuracy of ±0.6% and a 10% dropout rate, that approximately 10,200 eligible individuals were needed to be included in the study. Therefore, between 300 and 600 participants (150–300 men and 150–300 women) were included in each site. The geographic information system inverse distance weighted (IDW) interpolation technique was used for mapping the spatial distribution of epidemiological variables.23 The statistical and analysis plan are available elsewhere.13 A level of significance of 0.05 was used for all statistical tests performed on the study variables.
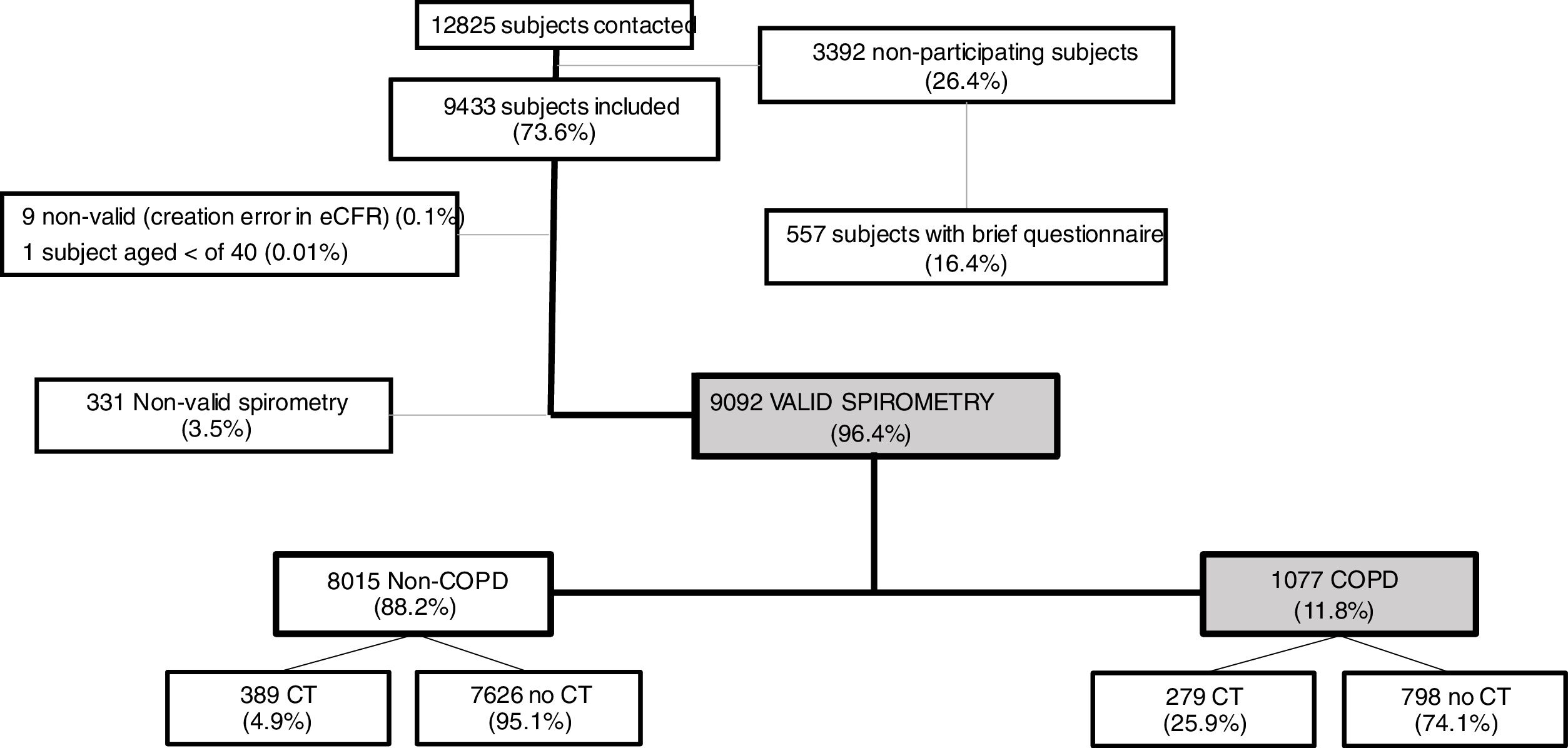
ResultsOverall, a total of 12,825 subjects were initially contacted by phone, and 9433 (73.6%) agreed to be seen in the hospital, as per Fig. 1. The final sample of 9092 participants, compared to non-participants was on average two years younger, less frequently women, less frequently diagnosed of COPD, chronic bronchitis or emphysema, but more frequently diagnosed of asthma (all p<0.001); on smoking exposure, the percentage of current smokers was similar, although with more ex-smokers, and overall consumed more pack-years (p=all p<0.001) (data not shown).
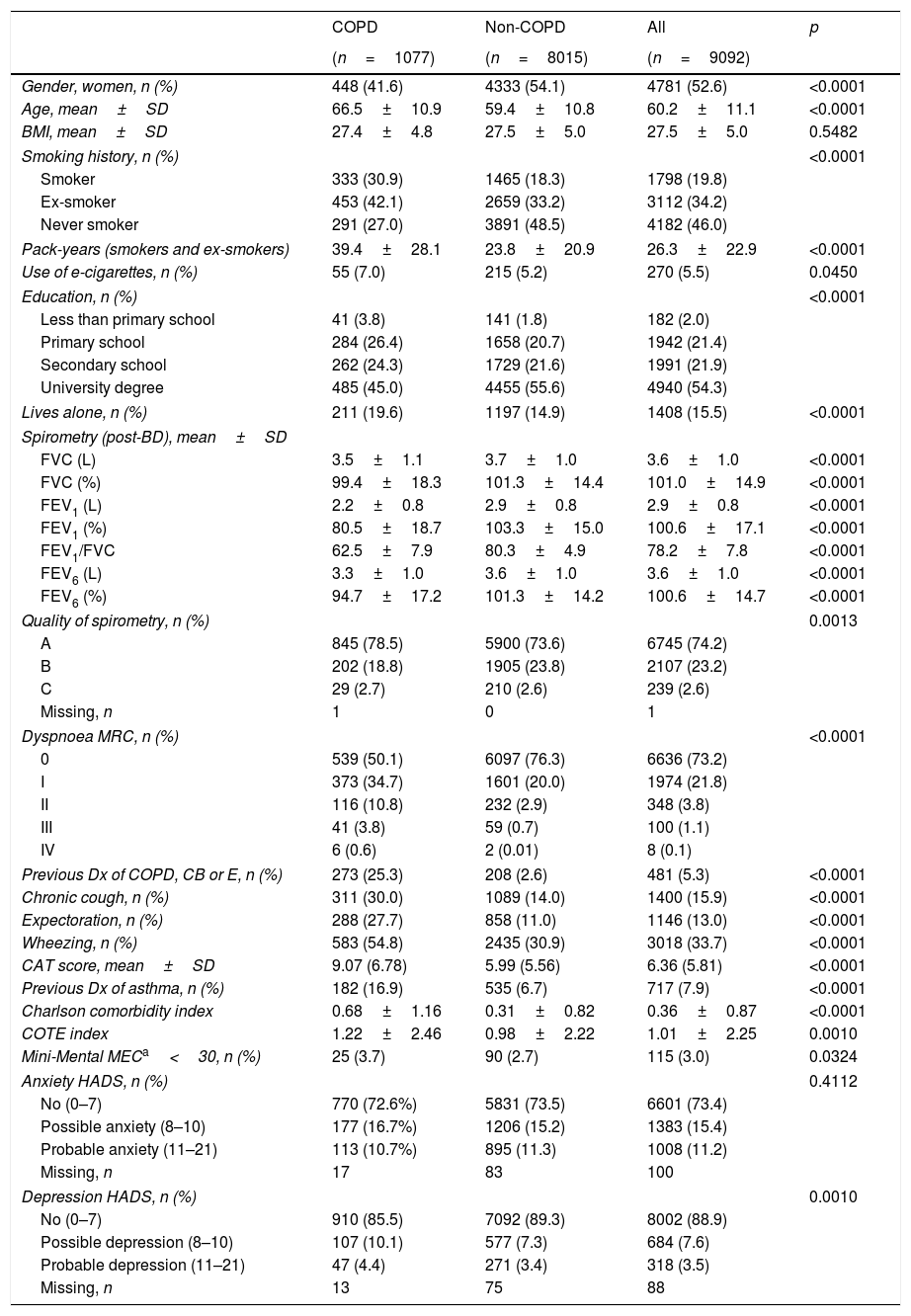
Out of 9433 subjects included and seen in hospital, 9092 (96.4%) performed valid spirometry (A, B or C quality), and represent an overall response rate of 70.9%. Baseline characteristics of participants were: 52.6% women, mean±SD age 60.2±11.1 years, 19.8% current smokers and 34.2% former smokers (Table 1).
Demographic and clinical characteristics of EPISCAN II COPD and non-COPD participants.
| COPD | Non-COPD | All | p | |
|---|---|---|---|---|
| (n=1077) | (n=8015) | (n=9092) | ||
| Gender, women, n (%) | 448 (41.6) | 4333 (54.1) | 4781 (52.6) | <0.0001 |
| Age, mean±SD | 66.5±10.9 | 59.4±10.8 | 60.2±11.1 | <0.0001 |
| BMI, mean±SD | 27.4±4.8 | 27.5±5.0 | 27.5±5.0 | 0.5482 |
| Smoking history, n (%) | <0.0001 | |||
| Smoker | 333 (30.9) | 1465 (18.3) | 1798 (19.8) | |
| Ex-smoker | 453 (42.1) | 2659 (33.2) | 3112 (34.2) | |
| Never smoker | 291 (27.0) | 3891 (48.5) | 4182 (46.0) | |
| Pack-years (smokers and ex-smokers) | 39.4±28.1 | 23.8±20.9 | 26.3±22.9 | <0.0001 |
| Use of e-cigarettes, n (%) | 55 (7.0) | 215 (5.2) | 270 (5.5) | 0.0450 |
| Education, n (%) | <0.0001 | |||
| Less than primary school | 41 (3.8) | 141 (1.8) | 182 (2.0) | |
| Primary school | 284 (26.4) | 1658 (20.7) | 1942 (21.4) | |
| Secondary school | 262 (24.3) | 1729 (21.6) | 1991 (21.9) | |
| University degree | 485 (45.0) | 4455 (55.6) | 4940 (54.3) | |
| Lives alone, n (%) | 211 (19.6) | 1197 (14.9) | 1408 (15.5) | <0.0001 |
| Spirometry (post-BD), mean±SD | ||||
| FVC (L) | 3.5±1.1 | 3.7±1.0 | 3.6±1.0 | <0.0001 |
| FVC (%) | 99.4±18.3 | 101.3±14.4 | 101.0±14.9 | <0.0001 |
| FEV1 (L) | 2.2±0.8 | 2.9±0.8 | 2.9±0.8 | <0.0001 |
| FEV1 (%) | 80.5±18.7 | 103.3±15.0 | 100.6±17.1 | <0.0001 |
| FEV1/FVC | 62.5±7.9 | 80.3±4.9 | 78.2±7.8 | <0.0001 |
| FEV6 (L) | 3.3±1.0 | 3.6±1.0 | 3.6±1.0 | <0.0001 |
| FEV6 (%) | 94.7±17.2 | 101.3±14.2 | 100.6±14.7 | <0.0001 |
| Quality of spirometry, n (%) | 0.0013 | |||
| A | 845 (78.5) | 5900 (73.6) | 6745 (74.2) | |
| B | 202 (18.8) | 1905 (23.8) | 2107 (23.2) | |
| C | 29 (2.7) | 210 (2.6) | 239 (2.6) | |
| Missing, n | 1 | 0 | 1 | |
| Dyspnoea MRC, n (%) | <0.0001 | |||
| 0 | 539 (50.1) | 6097 (76.3) | 6636 (73.2) | |
| I | 373 (34.7) | 1601 (20.0) | 1974 (21.8) | |
| II | 116 (10.8) | 232 (2.9) | 348 (3.8) | |
| III | 41 (3.8) | 59 (0.7) | 100 (1.1) | |
| IV | 6 (0.6) | 2 (0.01) | 8 (0.1) | |
| Previous Dx of COPD, CB or E, n (%) | 273 (25.3) | 208 (2.6) | 481 (5.3) | <0.0001 |
| Chronic cough, n (%) | 311 (30.0) | 1089 (14.0) | 1400 (15.9) | <0.0001 |
| Expectoration, n (%) | 288 (27.7) | 858 (11.0) | 1146 (13.0) | <0.0001 |
| Wheezing, n (%) | 583 (54.8) | 2435 (30.9) | 3018 (33.7) | <0.0001 |
| CAT score, mean±SD | 9.07 (6.78) | 5.99 (5.56) | 6.36 (5.81) | <0.0001 |
| Previous Dx of asthma, n (%) | 182 (16.9) | 535 (6.7) | 717 (7.9) | <0.0001 |
| Charlson comorbidity index | 0.68±1.16 | 0.31±0.82 | 0.36±0.87 | <0.0001 |
| COTE index | 1.22±2.46 | 0.98±2.22 | 1.01±2.25 | 0.0010 |
| Mini-Mental MECa<30, n (%) | 25 (3.7) | 90 (2.7) | 115 (3.0) | 0.0324 |
| Anxiety HADS, n (%) | 0.4112 | |||
| No (0–7) | 770 (72.6%) | 5831 (73.5) | 6601 (73.4) | |
| Possible anxiety (8–10) | 177 (16.7%) | 1206 (15.2) | 1383 (15.4) | |
| Probable anxiety (11–21) | 113 (10.7%) | 895 (11.3) | 1008 (11.2) | |
| Missing, n | 17 | 83 | 100 | |
| Depression HADS, n (%) | 0.0010 | |||
| No (0–7) | 910 (85.5) | 7092 (89.3) | 8002 (88.9) | |
| Possible depression (8–10) | 107 (10.1) | 577 (7.3) | 684 (7.6) | |
| Probable depression (11–21) | 47 (4.4) | 271 (3.4) | 318 (3.5) | |
| Missing, n | 13 | 75 | 88 | |
CB (chronic bronchitis) or E (emphysema).
As per the primary analysis, 1077 out of 9092 participants were considered COPD, while 8015 were not, with a prevalence of 11.8% (95% C.I. 11.2–12.5). By sex, prevalence was 14.6% (95% C.I. 13.5–15.7) in males and 9.4% (95% C.I. 8.6–10.2) in females.
Cases with COPD were a mean of seven years older, less frequently female (41.6% vs. 54.1%), of lower attained education, and were more frequently current- and former-smokers than controls (p<0.001), although the number of cigarettes and pack-years in non-COPD participants was substantial (Table 1); reported use of e-cigarettes, (7.0% vs. 5.5%) was significantly different (0.0450). There were also significant differences versus participants without COPD in all respiratory and other clinical variables, including living alone, previous respiratory diagnoses, respiratory symptoms, more comorbidities with the Charlson index, greater BODE and COTE scores, cognitive impairment, and depression HADS (all p<0.001), except for BMI (p=0.5482) and Anxiety HADS (p=0.4112).
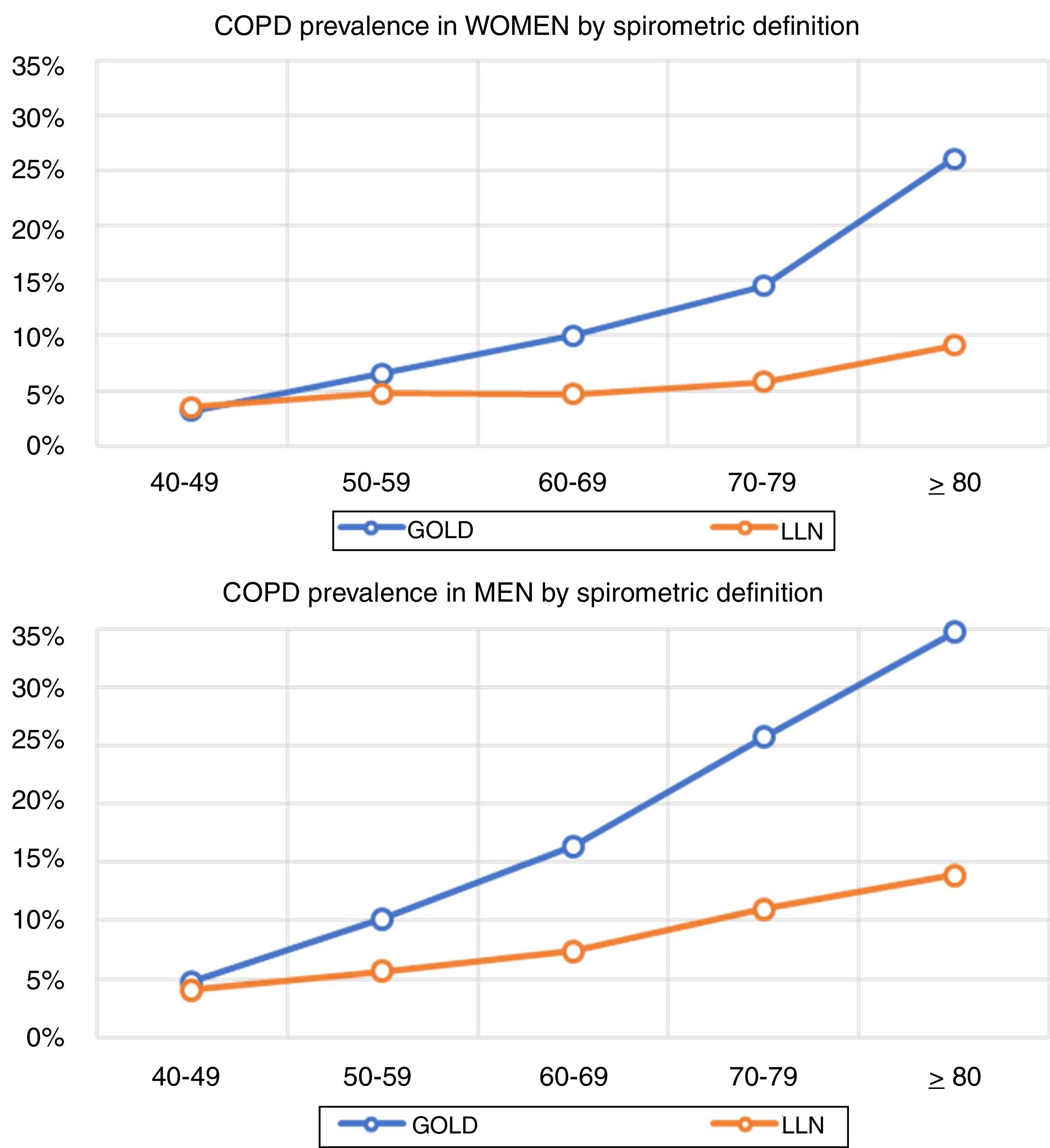
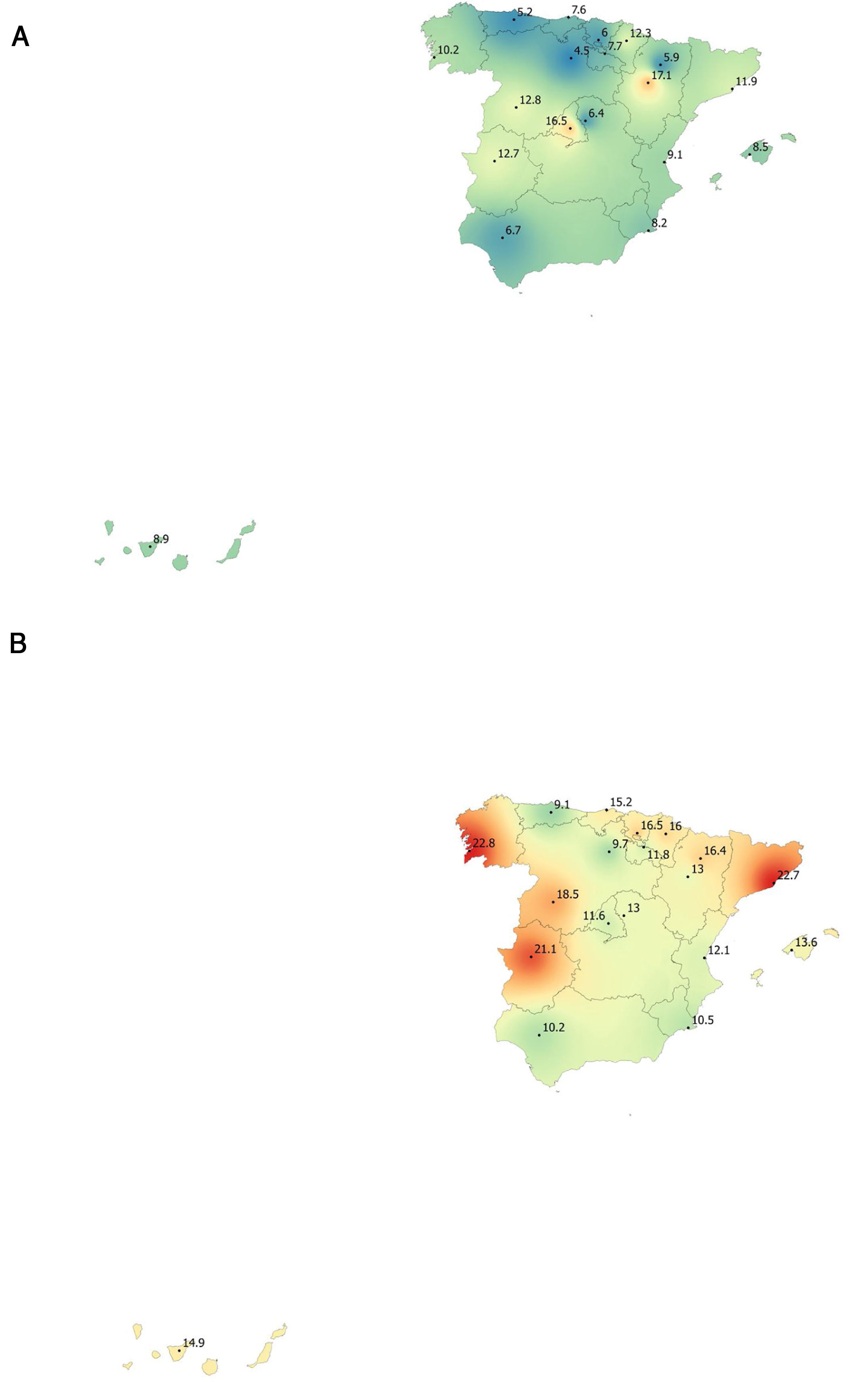
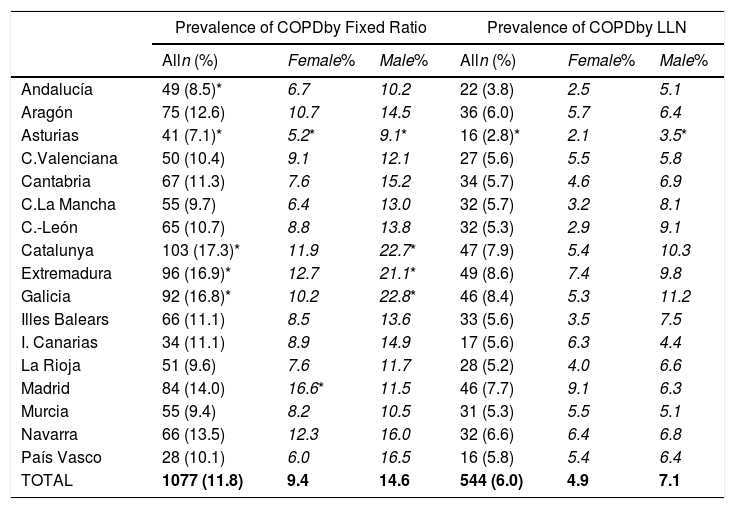
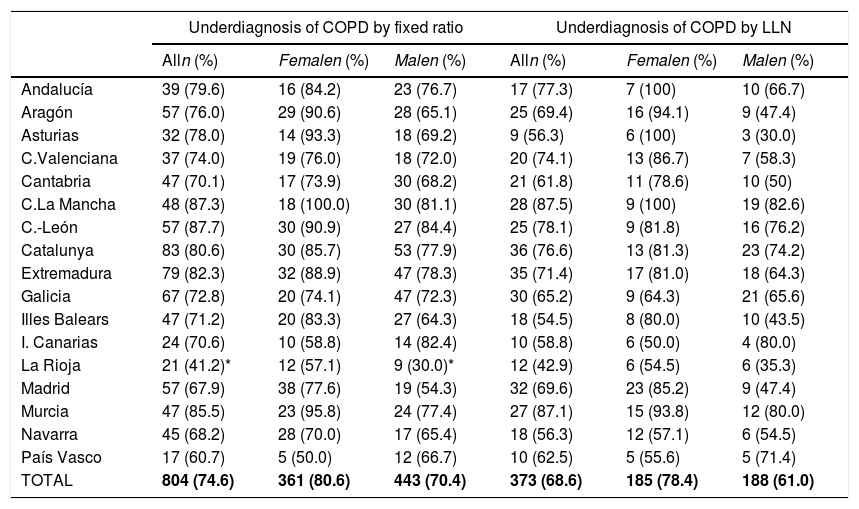
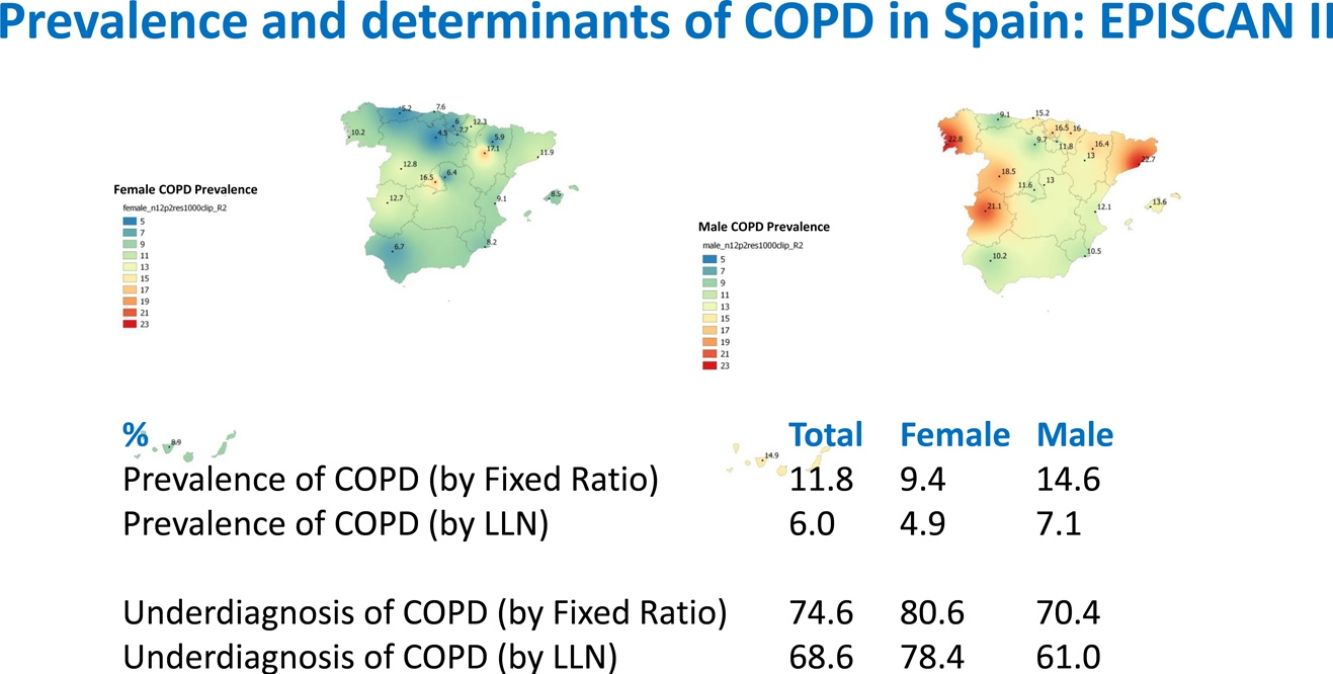
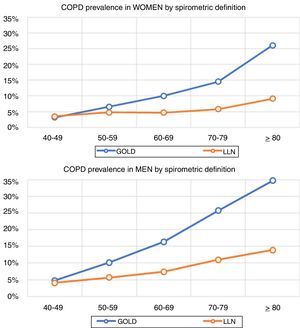
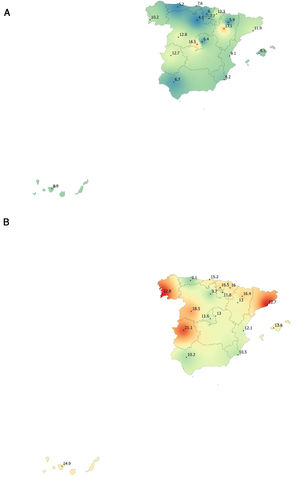
Lung function and COPD underdiagnosisAs expected, COPD prevalence increased by age in both men and women, and the greatest prevalence was observed in those 80 years and older, with a prevalence of 26.1% (95% C.I. 20.9–31.9) in women and of 34.7% (95% C.I. 28.1–41.6) in men (Fig. 2). There was a high variability (2.4-fold) among the 17 regions, with a minimum prevalence of 7.1% in Asturias and a maximum of 17.3% in Catalonia (Table 2 and Fig. 3). Estimates of the prevalence of COPD according to the LLN were 6.0% (95% C.I. 5.5–6.5) overall, by sex being 7.1% (95% C.I 6.4–8.0) in males and 4.9% (95% C.I. 4.3–5.6) in females (Table 2). Underdiagnosis of COPD was 74.7%, higher in women than in men (80.6% vs. 70.4%, p<0.001), yet again with a high variability by region (Table 3). However, COPD overdiagnosis was overall low (2.6%). Underdiagnosis of COPD measured by the LLN was 61.0%, again higher in women than in men (78.4% vs. 68.6%, p<0.001), yet again with a high variability by region. However, according to the LLN there were no statistically significant differences in the autonomous communities compared to the total (Table 3).
Variability of the prevalence of airflow limitation by Autonomous Community (region), measured as a fixed ratio and as LLN, total and by sex.
| Prevalence of COPDby Fixed Ratio | Prevalence of COPDby LLN | |||||
|---|---|---|---|---|---|---|
| Alln (%) | Female% | Male% | Alln (%) | Female% | Male% | |
| Andalucía | 49 (8.5)* | 6.7 | 10.2 | 22 (3.8) | 2.5 | 5.1 |
| Aragón | 75 (12.6) | 10.7 | 14.5 | 36 (6.0) | 5.7 | 6.4 |
| Asturias | 41 (7.1)* | 5.2* | 9.1* | 16 (2.8)* | 2.1 | 3.5* |
| C.Valenciana | 50 (10.4) | 9.1 | 12.1 | 27 (5.6) | 5.5 | 5.8 |
| Cantabria | 67 (11.3) | 7.6 | 15.2 | 34 (5.7) | 4.6 | 6.9 |
| C.La Mancha | 55 (9.7) | 6.4 | 13.0 | 32 (5.7) | 3.2 | 8.1 |
| C.-León | 65 (10.7) | 8.8 | 13.8 | 32 (5.3) | 2.9 | 9.1 |
| Catalunya | 103 (17.3)* | 11.9 | 22.7* | 47 (7.9) | 5.4 | 10.3 |
| Extremadura | 96 (16.9)* | 12.7 | 21.1* | 49 (8.6) | 7.4 | 9.8 |
| Galicia | 92 (16.8)* | 10.2 | 22.8* | 46 (8.4) | 5.3 | 11.2 |
| Illes Balears | 66 (11.1) | 8.5 | 13.6 | 33 (5.6) | 3.5 | 7.5 |
| I. Canarias | 34 (11.1) | 8.9 | 14.9 | 17 (5.6) | 6.3 | 4.4 |
| La Rioja | 51 (9.6) | 7.6 | 11.7 | 28 (5.2) | 4.0 | 6.6 |
| Madrid | 84 (14.0) | 16.6* | 11.5 | 46 (7.7) | 9.1 | 6.3 |
| Murcia | 55 (9.4) | 8.2 | 10.5 | 31 (5.3) | 5.5 | 5.1 |
| Navarra | 66 (13.5) | 12.3 | 16.0 | 32 (6.6) | 6.4 | 6.8 |
| País Vasco | 28 (10.1) | 6.0 | 16.5 | 16 (5.8) | 5.4 | 6.4 |
| TOTAL | 1077 (11.8) | 9.4 | 14.6 | 544 (6.0) | 4.9 | 7.1 |
Variability of the under diagnosis of COPD by Autonomous Community (region), measured as a fixed ratio and as LLN, total and by sex.
| Underdiagnosis of COPD by fixed ratio | Underdiagnosis of COPD by LLN | |||||
|---|---|---|---|---|---|---|
| Alln (%) | Femalen (%) | Malen (%) | Alln (%) | Femalen (%) | Malen (%) | |
| Andalucía | 39 (79.6) | 16 (84.2) | 23 (76.7) | 17 (77.3) | 7 (100) | 10 (66.7) |
| Aragón | 57 (76.0) | 29 (90.6) | 28 (65.1) | 25 (69.4) | 16 (94.1) | 9 (47.4) |
| Asturias | 32 (78.0) | 14 (93.3) | 18 (69.2) | 9 (56.3) | 6 (100) | 3 (30.0) |
| C.Valenciana | 37 (74.0) | 19 (76.0) | 18 (72.0) | 20 (74.1) | 13 (86.7) | 7 (58.3) |
| Cantabria | 47 (70.1) | 17 (73.9) | 30 (68.2) | 21 (61.8) | 11 (78.6) | 10 (50) |
| C.La Mancha | 48 (87.3) | 18 (100.0) | 30 (81.1) | 28 (87.5) | 9 (100) | 19 (82.6) |
| C.-León | 57 (87.7) | 30 (90.9) | 27 (84.4) | 25 (78.1) | 9 (81.8) | 16 (76.2) |
| Catalunya | 83 (80.6) | 30 (85.7) | 53 (77.9) | 36 (76.6) | 13 (81.3) | 23 (74.2) |
| Extremadura | 79 (82.3) | 32 (88.9) | 47 (78.3) | 35 (71.4) | 17 (81.0) | 18 (64.3) |
| Galicia | 67 (72.8) | 20 (74.1) | 47 (72.3) | 30 (65.2) | 9 (64.3) | 21 (65.6) |
| Illes Balears | 47 (71.2) | 20 (83.3) | 27 (64.3) | 18 (54.5) | 8 (80.0) | 10 (43.5) |
| I. Canarias | 24 (70.6) | 10 (58.8) | 14 (82.4) | 10 (58.8) | 6 (50.0) | 4 (80.0) |
| La Rioja | 21 (41.2)* | 12 (57.1) | 9 (30.0)* | 12 (42.9) | 6 (54.5) | 6 (35.3) |
| Madrid | 57 (67.9) | 38 (77.6) | 19 (54.3) | 32 (69.6) | 23 (85.2) | 9 (47.4) |
| Murcia | 47 (85.5) | 23 (95.8) | 24 (77.4) | 27 (87.1) | 15 (93.8) | 12 (80.0) |
| Navarra | 45 (68.2) | 28 (70.0) | 17 (65.4) | 18 (56.3) | 12 (57.1) | 6 (54.5) |
| País Vasco | 17 (60.7) | 5 (50.0) | 12 (66.7) | 10 (62.5) | 5 (55.6) | 5 (71.4) |
| TOTAL | 804 (74.6) | 361 (80.6) | 443 (70.4) | 373 (68.6) | 185 (78.4) | 188 (61.0) |
Adhering to the compromise in global health agendas of an expanded emphasis on non-communicable diseases, sound evidence on trends and determinants of COPD at the national and international levels are essential. In here, EPISCAN II updates previous findings and includes new estimates and trends on COPD prevalence in Spain. The confirmation that COPD is one of the most prevalent conditions (more than one in ten) in the general population, found in all ages from young adults to the very elderly, and that is more frequent in men but with an increasing burden in women, yet with a regional variability (2.4-fold) of a similar magnitude than asthma,24 will set ground for further Public Health interventions. Note that a previous diagnosis of asthma was two-fold higher in those with COPD (16.9%) than without (6.7%) in this study. Regrettably our efforts to reduce COPD underdiagnosis, appear not enough, as three out of four individuals with objective airflow limitation compatible with COPD reported no medical diagnosis previously, therefore suffering unnecessary individual and population burden, as COPD is considered a preventable and treatable disease.2
Previous literatureThe so-called shoe-leather epidemiology25 is essential to monitor trends of chronic diseases, as populations change, but diseases and risk factors also change.26 Direct comparison with previous estimates of spirometry in Spain will require of further analyses,27 as age thresholds differ, spirometers and guidelines have changed, and even interpretation of respiratory manoeuvres has evolved.28 However, compared with the first EPISCAN, an increase of COPD in women is seen in the overlapping age strata from age 50 years and onwards (3.2% vs 3.2% in 40–49 yrs., 4.5% vs 6.6% in 50–59 yrs., 7.6% vs 10.0% in 60–69 yrs., and 10.8% vs 14.5% in 70–79 yrs.), which is a new reminder of the growing toll of women catching up with men in cigarette smoking.29 Of note, Spain is considered to be in phase IIIb of the tobacco epidemic,30 but new forms of smoking, including the health effects of vaping and heat-not-burn products such as IQOS,31 should be actively assessed. Our finding of reported use of e-cigarettes, in 7.0% of COPD cases but also in 5.5% of non-COPD participants is worrying.32 Exploring the asthma and COPD overlap,33 or those with preserved ratio impaired spirometry (PRISm), alternatively known as restrictive, unclassified spirometry,34 are just a few of the pre-established analyses scheduled with EPISCAN II,12 all beyond the scope of this manuscript.
Our finding of a 74.7% underdiagnosis of COPD in Spain should be put into perspective, as it is no better than the 73% in 1997,5 and only marginally different than the 78% seen in 20076; these are unwelcome news in our global effort to reduce COPD unmet burden,4,35 where new strategies might be field tested and then implemented.36 The 80.6% underdiagnosis in Spanish women, ten points higher than the 74.6% observed in men, confirms previous findings of higher female underdiagnosis in Spain, which is at odds with observations elsewhere, where male COPD underdiagnosis prevails, and to date its reasons appear elusive.37 There were substantial differences in the variability of COPD underdiagnosis by Autonomous Community, total, and by sex, with percentages ranging from the 35% only in men in La Rioja and up to 100% in women in Castilla-La Mancha (Table 3). Given that a common protocol was identical in all sites, and that these findings might be outlier values but not errors, the determinants of these differences will be specifically explored in already planned analyses of EPISCAN II. However, for the record indeed La Rioja has an advanced system of COPD screening according to the National Strategy,38 and they apply a near systematic population case-finding and screening.
There are few studies that “medicalize” new COPD diagnoses (or airflow limitation) after a population spirometric study.39,40 Perhaps the most relevant is the one by Llordés et al.41 They conducted spirometry in 1738 population smokers aged 45 years or older. All those newly diagnosed with COPD, defined as post-BD FEV1/FVC<0.7, were tested with a 4-week treatment with formoterol and budesonide. The prevalence of COPD was 24.3% (95%, CI 22.3–26.4), with an overall underdiagnosis of 56.7%. After 4 weeks of treatment, 16% of initially obstructed patients had normal spirometry; in addition, 15.6% of individuals with a diagnosis of COPD did not sustain airflow obstruction, while 84.4% did.
Strengths and limitationsEPISCAN II has several strengths, as it is consistent and even surpasses previous studies in Spain (IBERPOC and EPISCAN),5,6 namely: By assessing all 17 regions in Spain, we were able to produce a complete map of the distribution of COPD and its determinants for the first time. We did not establish an upper age limit for surveying, as population growth and ageing, particularly affecting women, is a global trend42; high quality spirometry was applied to nearly 10,000 individuals, that was centrally monitored for quality, and adhered to the strictest international guidance and protocols, as with all other measurements. Indeed, the oldest participant in EPISCAN II was a woman aged 98 years old from Extremadura, with spirometry quality graded B. Given the large sample size and high response rate, it can be considered that the final sample of participants is representative of the Spanish population older than 40 years.
However, a number of limitations are worth considering: Most areas surveyed were urban, and although 90%+ of the Spanish population live in cities, the unmet burden in rural areas is expected to be high but under-represented in here.2 Like all COPD epidemiological studies, diagnosis is based solely on spirometry (airflow limitation) and this may cause other obstructive diseases than COPD itself to be included here; same token, there is a high proportion of participants (7.9%) with a self-reported diagnosis of asthma. At the population level there are no studies that quantify the so-called ‘clinical COPD’, as the thresholds to determine which symptoms and which exposures define it are even more diffuse than those of airflow limitation. Therefore, for consistency with the design of other epidemiological studies on COPD internationally (BOLD,8 etc.) and the previous ones in Spain (IBERPOC,5 EPISCAN6) the same design is maintained.
Future perspectiveBetter defining and grading obstructive airway diseases, and COPD in particular, is a long quest.43 The GOLD initiative has been modifying definitions, thresholds of spirometry and staging classifications in its several iterations.2 New, recent evidence from COPDGene proposed the combination of four COPD domains: environmental exposure (cigarette smoking), clinical symptoms (dyspnoea and/or chronic bronchitis), chest CT imaging abnormalities, and abnormal spirometry; this new staging was strongly associated with spirometric disease progression (FEV1>350ml loss over 5 years), and all-cause mortality.44 We strongly believe that the COPDGene proposal of possible, probable, and definite COPD will help to advance COPD research and medicine, just as a similar proposal with symptoms, EKG, enzymes helped ischaemic heart disease previously.
We conclude that COPD remains a major, common cause of disease in the general population, yet with substantial variability by age, sex, and geography, among other determinants. There is significant unmet need of airflow limitation at the individual and population levels. Last but not least, efforts to reduce COPD underdiagnosis, and the toll of cigarettes and of other old and new ways of smoking, should be streamlined.
FundingEPISCAN II is a GSK sponsored study.
Conflict of interestIndividual ICJME forms are appended to this submission.
| CC. AA. | CENTRE | INVESTIGATOR TEAM |
|---|---|---|
| MADRID | H. La Princesa | Julio Ancochea Bermudez (IP) / Elena García Castillo / Claudia Valenzuela / Joan B Soriano |
| CASTILLA LEÓN | H. U. de Burgos | Ana Pueyo Bastida (IP) / Lourdes Lázaro Asegurado / Luis Rodríguez Pascual / Mª José Mora |
| ARAGÓN | H. Gral. San Jorge | Luis Borderias Clau (IP) / Lourdes Arizón Mendoza / Sandra García |
| EXTREMADURA | H. San Pedro de Alcántara | Juan Antonio Riesco Miranda (IP) / Julián Grande Gutiérrez / Jesús Agustín Manzano / Manuel Agustín Sojo González |
| CASTILLA LEÓN | H. Clínico U. de Salamanca | Miguel Barrueco Ferrero (IP) / Milagros Rosales |
| GALICIA | H. Álvaro Cunqueiro | José Alberto Fernández Villar (IP) / Cristina Represas / Ana Priegue / Isabel Portela Ferreño / Cecilia Mouronte Roibás / Sara Fernández García |
| I. BALEARES | H. Son Espases | Borja G Cosío (IP) / Rocío Cordova Díaz / Nuria Toledo Pons / Margalida Llabrés |
| ARAGÓN | H. U. Miguel Servet | José María Marín Trigo (IP) / Marta Forner / Begoña Gallego / Pablo Cubero / Elisabet Vera |
| C. VALENCIANA | H. Arnau de Vilanova (Valencia) | Juan José Soler Cataluña (IP) / Mª Begoña Picurelli Albero / Noelia González García |
| ANDALUCÍA | H. Virgen de la Macarena | Agustín Valido Morales (IP) / Carolina Panadero / Cristina Benito Bernáldez/ Laura Martín -Bejarano y Maria Velarde (enfermeras sin acceso web) |
| MURCIA | H. Gral. U. Santa Lucía (Cartagena) | Antonio Santa Cruz Siminiani (IP) / Carlos Castillo Quintanilla / Rocío Ibáñez Meléndez / José Javier Martínez Garcerán / Desirée Lozano Vicente / Pedro García Torres / Maria del Mar Valdivia |
| NAVARRA | Clínica Universidad de Navarra | Juan Pablo de Torres Tajes (IP) / Montserrat Cizur Girones / Carmen Labiano Turrillas |
| LA RIOJA | H. de San Pedro (Logroño) | Carlos Ruiz Martínez (IP)/ Elena Hernando / Elvira Alfaro / José Manuel García / Jorge Lázaro |
| PAÍS VASCO | H. Santiago Apóstol (H. Txagorritxu) | David Bravo (IP) /Laura Hidalgo / Silvia Francisco Terreros / Iñaki Zorrilla / Ainara Alonso Colmenero / |
| ASTURIAS | H. Central de Asturias | Cristina Martínez González (IP) /Susana Margon/ Rosirys Guzman Taveras/ Ramón Fernández / Alicia Álvarez |
| CANTABRIA | H. de Valdecilla (Servicio de Neumología en el H. Santa Cruz de Liencres) | José Ramón Agüero Balbín (IP) / Juan Agüero Calvo |
| CATALUÑA | H. U. Vall d’Hebron | Jaume Joan Ferrer Sancho (IP) / Esther Rodríguez González/ Eduardo Loeb |
| CASTILLA LA MANCHA | H. U. de Guadalajara | José Luis Izquierdo Alonso (IP) / Mª Antonia Rodríguez García |
| I. CANARIAS | H. U. de Tenerife | Juan Abreu González (IP)/ Candelaria Martín García/ Rebeca Muñoz/ Haydée Martín García |
| ASTURIAS | H. U. de San Agustín (Avilés): | Miguel Angel Martínez Muñiz (IP) / Andrés Avelino Sánchez Antuña / Jesús Allende González / Jose Antonio Gullón Blanco / Fernando José Alvarez Navascues / Manuel Angel Villanueva Montes / María Rodríguez Pericacho / Concepción Rodríguez García / Juan Diego Alvarez Mavárez |