Two are better than one (Ecclesiastes 4:9-12)
Since the introduction of non-invasive mechanical ventilation (NIMV), the number of patients who receive this treatment in both acute and home settings has increased exponentially.1,2 This is explained by the growing number of diseases in which NIMV has been shown to be effective, and by technological advances in the development of high-performance portable ventilators.
However, despite its recognized efficacy, we frequently witness mediocre outcomes in home NIMV. The rate of failure is variable, and has been estimated at between 5% and 66%.3,4 Detection and correction of these events is essential, since it has been shown that optimizing NIMV has an impact on prognosis.5,6
Correct patient-ventilator synchrony is the key to effective ventilatory assistance. In spontaneous ventilation, all the work of breathing is performed by the respiratory muscles. In mechanical ventilation, 2 systems are involved: the ventilator pump and the patient's own respiratory muscles. When these systems work in harmony, the gas supplied by the ventilator matches the demand of the patient. However, this “collaboration” is often problematic, leading to asynchrony between the patient's neural activity and the insufflation provided by the ventilator. Patient-ventilator asynchrony is quite common, especially in NIMV, where it is due to the non-hermetic nature of the system (non intentiona leaks) and the presence of intervening upper airway (UA) resistance.7,8 In critical patients receiving invasive ventilation, patient-ventilator synchrony can be evaluated from curves recorded on the ventilator screens, which provide a real-time analysis of flow and pressure. In the case of patients receiving nocturnal ventilation, data can be obtained from the monitoring systems that are a feature of some portable ventilators.4,9 However, the contribution, namely, the intensity and duration of inspiratory effort, is difficult to assess in the ventilators. The treating clinician often makes the mistake of trying to deduce the contribution of the patient exclusively from the graphs obtained from the ventilator. The use of an external polygraph offers the possibility of including an analysis of thoracoabdominal movements and, occasionally, muscle effort. The analysis of thoracoabdominal expansion by inductance plethysmography (the best technology available), in combination with simultaneous recording of flow and pressure, is crucial for assessing the synchronicity between the patient's neural drive and the work of the ventilator.10
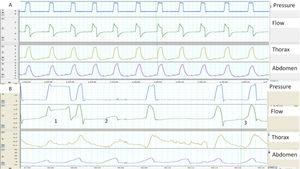
When synchrony is good, the start of the inspiration and the inspiration-expiration transition in the flow and pressure tracings correspond with the beginning and end of thoracoabdominal insufflation. In contrast, a thoracoabdominal expansion without ventilator pressurization is a good marker of unrewarded inspiratory efforts (Fig. 1). Plethysmograph effort belts also provide a qualitative estimate of the ventilatory pattern (not to mention a semi-quantitative estimate, when the signals are properly calibrated). Finally, the simultaneous analysis of both belts can be used to assess thoracoabdominal synchronicity. In physiological conditions, both belts must have the same polarity and synchronized start and end times. The degree of synchrony is measured from the phase angle, which is normally close to 0 degrees. An increase in this angle is a marker of thoracoabdominal asynchrony11 (Fig. 2). This may reflect either a phenomenon of struggle against an obstructed UA (abdominal band expanding/thoracic belt retracting during inspiration), or diaphragmatic fatigue with use of accessory muscles (thoracic belt expanding/abdominal belt retracting). The main limitation of the belts is that they express not only inspiratory effort, but may also reflect movement during passive insufflation.
(A and B) Examples of normal and pathological patient-ventilator synchrony, determined with the help of thoracoabdominal belts. (A) The image shows perfect synchrony between the patient's thoracoabdominal movements and the ventilator cycles (continuous line). (B) The image of cycle 1 shows a long cycle, presumably due to leakage (the patient has finished exhaling while the ventilator continues in inspiration) and cycle 2 displays an ineffective respiratory effort (movement of belts without pressurization). Finally, in cycle 3, the beginning of the ventilator cycle is delayed with respect to the start of the movement of the belts (trigger delay, more apparent in the abdominal belt).
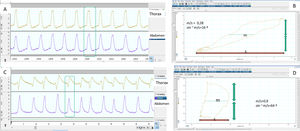
Determination of normal and pathological synchrony measured from thoracoabdominal belts by calculating the phase angle. (A) The respiratory movements of the patient are synchronous, with the belts showing symmetrical movement with the same polarity. (B) The corresponding phase angle is calculated by comparing thoracic movement (vertical axis) to abdominal shift (horizontal axis) on the same plane, using a Lissajous curve. For quantification, the horizontal width of the loop delimited by the representation on the plane of both movements is taken at the midpoint (m) of the thoracic movement and compared with the total abdominal movement (s). The smaller the m/s ratio, the closer to zero the phase angle (which is the inverse of the sine of the proportion), and the better the thoracoabdominal synchrony (16° in this case). (C) In contrast, the abdominal belt starts to move first and the thoracic belt ends first, so that the thoracoabdominal synchrony is rather worse than in the first example, reflected by a higher m/s ratio and a greater phase angle (D), in this case 64°. It must be borne in mind that in situations of complete mismatch or phase opposition (see text), the phase angle can be as high as 180°.
In a patient receiving long-term ventilation, this configuration (pressure, flow, and bands, plus SaO2) has recently been proposed by a group of experts as the most appropriate for the analysis of polygraphic tracings during ventilation.10
However, in complex cases, this configuration may be insufficient for accurately analyzing patient-ventilator interaction, and additional signals may be needed.
Electromyography (EMG) can measure the electrical activity of the respiratory muscles. It has been used for assessing asynchronies12 and for titrating pressures to optimize muscle unloading.13 Two main methods are available:
- –
Transesophageal diaphragmatic EMG: This is the gold standard of respiratory EMG, as it is not susceptible to contamination by nearby muscles. Disadvantages are that it is invasive, and moreover, it primarily records the costal activity of the diaphragm, which is not always equivalent to crural activity, and it can be affected by esophageal peristalsis and by the electrocardiographic signal.14
- –
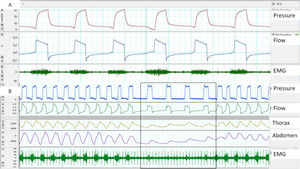
Surface EMG: Advantages of this approach are accessibility and the possibility of assessing different muscle groups (accessories, expiratory muscles, etc.) using surface electrodes. The main disadvantages are the poor signal/noise ratio (proper preparation of the skin is essential) and contamination. Parasternal EMG is the most widely used, given its accessibility and good correlation with diaphragmatic activity. With respect to sampling frequency, according to the Nyquist theorem, the minimum sampling frequency should be 1000Hz (Fig. 3).
Fig. 3.(A) Trigger delay, measured using parasternal EMG; note the delay between the start of muscle activation and the start of the ventilator cycle. (B) Lack of respiratory effort measured by parasternal EMG at the start of the controlled cycles provided by the ventilator, although it should be noted that the bands show a shift.
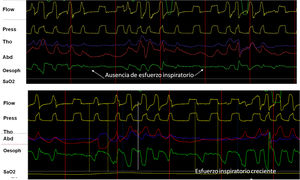
The contraction of respiratory muscles generates negative intrapleural pressure (IPP) producing an atmospheric-alveolar pressure gradient, allowing inspiration. The placement of a catheter in the middle third of the esophagus provides a reliable assessment by measuring esophageal pressure (esP). This parameter has shown good correlation with IPP, and in assisted ventilation it can be used to assess the contribution of the respiratory muscles.15 Its main advantage is that it isolates respiratory effort from the “shift” effect characteristic of the bands, so when combined with pressure and flow, it is the gold standard for evaluating patient-ventilator synchrony and the detection of asynchrony. In addition, when the signal is calibrated, it is useful for evaluating the work of breathing that corresponds to the area under the esP curve and the patient's intrinsic PEEP (Fig. 4). Its disadvantages include its invasive nature, risk of shift, and contamination by cardiac contraction.
(A) Utility and behavior of esophageal pressure recording in a UA event without respiratory effort (glottal closure). Note the absence of negative deflection on the esophageal pressure tracing (arrows). (B) Behavior in event with respiratory effort (oropharyngeal apnea). Note the progressive increase of inspiratory effort measured by esophageal pressure.
In conclusion, in clinical practice, auxiliary signals provide an overview of the patient's ventilatory pattern (muscle effort, thoracoabdominal shift) during NIMV. In certain situations, the use and correlation of these parameters with ventilator graphics may be the key to correct diagnosis of patient-ventilator interactions. In patients with obstructive UA events in particular, we believe that they are indispensable for determining if these events are accompanied or not with respiratory effort, and for the differential diagnosis of complex asynchronies.
Conflict of interestsClaudio Rabec: Consulting activities and collaboration (in the last 5 years) with: Resmed, Philips, Breas, Lowenstein, Air Liquide Medical System.
Manuel Luján: Member of the Breas Clinical Advisory Board. Collaborative activities with Resmed and Philips.
Javier Sayas has received honoraria for educational activities from ResMed, Philips Respironics, Oximesa-Praxair, Chiesi, and Menarini.
Please cite this article as: Rabec C, Sayas J, Luján M. Uno más uno pueden no ser dos: interacción paciente-ventilador en ventilación no invasiva. ¿Quién hace qué? Arch Bronconeumol. 2019;55:403–406.