Nontuberculous mycobacteria (NTM), also called atypical or environmental mycobacteria, are widely distributed in water and soil,1 but their pathogenic potential has long been underestimated.
With the HIV/AIDS pandemic and longer survival of patients with chronic respiratory disease and other debilitating conditions such as cancer, diabetes, autoimmune diseases, transplants and immunosuppressive treatments, interest in NTM has grown, with an increasing number of cases and new species recognized.1,2
Mycobacterium peregrinum is a rare species of NTM, with very few cases reported worldwide, so its clinical significance and optimal treatment are not well established.3
In this context, case reports are of great value to increase awareness of the issue.
We report the case of a 40-year-old woman who attended the respiratory medicine clinic for a 2-month history of cough with a small amount of mucous expectoration and no other associated symptoms. She had received empirical treatment with amoxicillin and, given the persistent cough, had been prescribed salbutamol, but with no improvement.
Her history was significant for autoimmune uveitis, for which she had been receiving mycophenolate 2 g/day for 3 years prior to the visit, and she worked as a phlebotomist in our hospital laboratory.
No relevant findings were evident on physical examination.
Further tests noted: hematocrit 32%, hemoglobin 9.9 g/dL, and discrete anischromia with hypochromia. Leukocyte count 10 670/mm3 (neutrophils 64%, lymphocytes 22%, monocytes 11%, eosinophils 2%, basophils 0.5%), and erythrocyte sedimentation rate (ESR) 54 mm/h; liver and renal function tests, blood glucose and serum protein values were within reference ranges. Tuberculin skin test: 2 mm.
Spirometry showed normal values (FVC 4.05 L [122% of predicted], FEV1 3.27 L [119% of predicted], FEV1/FVC 81%) with no significant bronchodilator response.
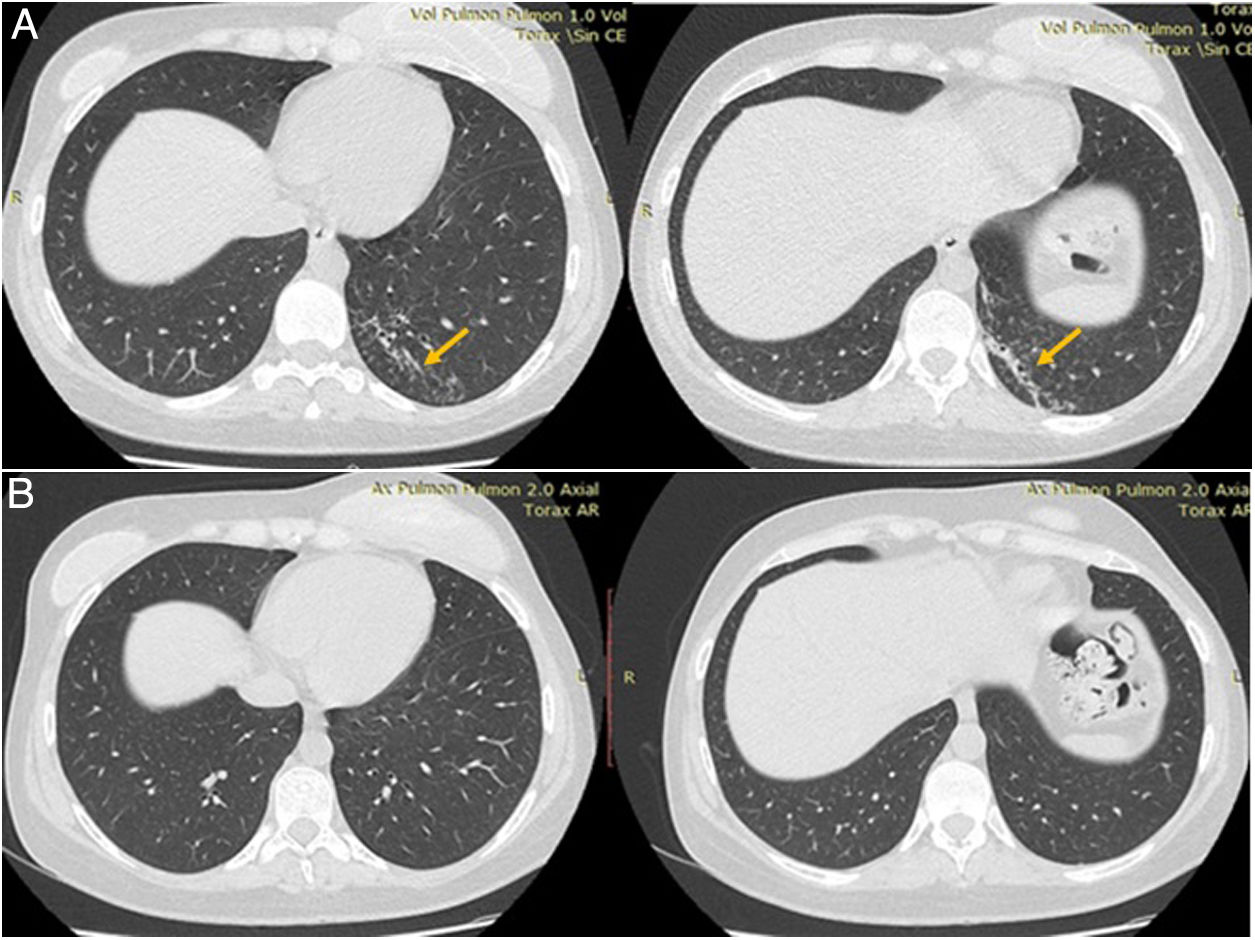
Plain chest computed tomography (CT) showed a hyperdense infiltrate with bronchiectasis in the posterior segment of the left lower lobe (Fig. 1).
Suspecting infectious disease, sputum bacilloscopy was requested. The result was positive, with development in culture of M. peregrinum (in 2 different sputum samples), identified using the molecular method of restriction fragment analysis of a PCR-amplified 439-bp amplicon of the hsp65 gene. Susceptibility to clarithromycin, ciprofloxacin, amikacin and linezolid was demonstrated using the microdilution method for determining the minimum inhibitory concentration, according to CLSI standards.
After verifying normal hearing function and electrocardiogram, the patient began treatment with ciprofloxacin 500 mg/12 h and clarithromycin 500 mg/12 h. Thirty days after starting antibiotic treatment, the cough and expectoration had subsided, so new sputum samples could not be obtained for microbiological examination, and her blood count and ESR had returned to normal.
After 6 months of treatment, which was well tolerated with no significant adverse events, repeat chest CT was normal, and the patient was discharged.
NTM are classified according to their growth characteristics and the pigments they produce.4 The slow-growing group (more than 7 days) includes M. kansasii and M. avium complex, while the rapidly-growing group includes M. fortuitum, M. chelonae and M. abscessus.
These species show great variability in their geographical distribution, pathological potential and presentation, even within the same region.1,4,5 The vast majority of clinically detectable diseases are due to slow-growing mycobacteria (about 82% of cases), with reporting of rapidly-growing mycobacteria being much less frequent.
M. peregrinum falls within the category of rapidly-growing mycobacteria, in the Mycobacterium fortuitum group, which includes M. fortuitum (the most common of these, known to cause opportunistic infections), and another 8 species that share characteristics such as lack of pigmentation and susceptibility to polymyxin B, sulfonamides and the new fluoroquinolones.3,6
In an environmental study conducted on various water sources in Bahia Blanca, Buenos Aires, Argentina, NTM were isolated in 51.6% of reservoir and tap water samples (64/124), 11% of which corresponded to M. peregrinum.7
Despite its wide distribution, M. peregrinum is very rare as a pathogen for humans,8 and is estimated to account for 1% to 2% of rapidly-growing mycobacteria infections.3 The prevalence of respiratory infections caused by this germ is unknown.
The few reported cases are associated with diseases similar to those produced by other members of the M. fortuitum group, such as tonsillar abscess,8 surgical site infection with cutaneous fistula,9 skin infection in a patient with psoriasis,10 endocarditis in a patient with a prosthetic aortic valve,11 and lung infections (we found only three cases published so far).12–14
NTMs are generally resistant to antituberculosis agents and sensitive to traditional antibiotics such as clarithromycin, amikacin, ciprofloxacin, tigecycline and sulfonamides, but discrepancy is usually found between in vitro susceptibility tests and the clinical response.4 The recommended treatment is with two drugs that have shown in vitro susceptibility.5
After studying the in vitro susceptibility of M. peregrinum, it was found that the new fluoroquinolones exhibit great activity against the bacterium, especially moxifloxacin. It is therefore recommended to use regimens similar to those indicated for M. fortuitum, combining a fluoroquinolone with another drug.15 However, since there are few case reports, the activity of these antibiotics should be tested in vivo before making a recommendation.
In conclusion, to our knowledge, this is only the fourth report of lung infection by M. peregrinum worldwide. The antibiotic regimen applied differs from that used in the previous 3 cases, but was safe and effective.
Reporting of diagnostic and therapeutic strategies to deal with this pathogen helps to better understand its clinical significance and is needed to help guide its management in the future.
Please cite this article as: Rolan N, Limongi L, Putruele AM. Mycobacterium peregrinum: micobacteria atípica e infrecuente. Reporte de un caso. Arch Bronconeumol. 2020;56:331–332.