In this article, we review the utility of the most common lung function tests (spirometry, reversibility test, peak expiratory flow, lung volumes, maximal respiratory pressure, carbon monoxide transference, arterial blood gas, 6-min walk test and desaturation with exercise and ergospirometry) related to the most frequent pathologies (dyspnea of undetermined origin, chronic cough, asthma, COPD, neuromuscular diseases, interstitial diseases, pulmonary vascular diseases, pre-operative evaluation and disability evaluation). Our analysis has been developed from the perspective of decision-making, clinical interpretation or aspects that the physician should take into account with their use. Consequently, the paper does not deal with aspects of quality, technique or equipment, with the exception of when regarding costs as we believe that this is an important element in the decision-making process. The document is extensively supported by references from the literature.
En este artículo revisamos primero por pruebas (espirometría, prueba de reversibilidad, flujo espiratorio pico, volúmenes pulmonares, presiones respiratorias máximas, transferencia de monóxido de carbono, gasometría arterial, prueba de marcha de 6min y desaturación con ejercicio y ergoespirometría), y luego por patologías más frecuentes (disnea no aclarada, tos crónica, asma, EPOC, enfermedades neuromusculares, enfermedades intersticiales, enfermedades vasculares pulmonares, valoración preoperatoria y valoración de la discapacidad), la utilidad de las pruebas funcionales respiratorias más habituales desde la perspectiva de la toma de decisiones, de la interpretación clínica o de aspectos que el clínico debe tener en cuenta a la hora de utilizarlas. En consecuencia, no se incide en aspectos de calidad, de la técnica ni de los equipos, salvo referencia a los costos, porque pensamos que es un elemento importante en la toma de decisiones. El documento está ampliamente fundamentado con referencias a la literatura.
Lung function studies are a key part of the diagnostic evaluation and follow-up of patients with respiratory diseases. In addition, they have other very important clinical applications, such as evaluating surgical risk, disability, and prognosis.1 The information they provide is objective, precise, reproducible, and reliable.
There are several different lung function tests (LFT), and each has its indications. Those that are referred to as basic LFT are baseline spirometry and flow–volume curve, bronchodilator test, and arterial blood gasses. Other important tests in clinical practice are the carbon monoxide transference test (DLCO), lung volume determination, bronchial provocation tests, exercise tests and the determination of maximal pressures. In this review, we will present their most relevant aspects.
In order to carry out all these tests, different equipment is needed, which must meet the technical requirements established by current guidelines.1,2 Likewise, it is essential to check the calibration of the devices prior to their use, as well as to follow the established regulations for hygiene and control of infection.2 The personnel who administer the tests should be familiarized with the equipment and have sufficient experience in their duties to obtain quality results. Patients should follow the previous preparation instructions, and they should be explained how to correctly perform the tests.2
Lung Function TestsSpirometry and Flow–Volume CurveSpirometry measures the forced expiratory volume in 1s (FEV1) and the forced vital capacity (FVC), and it is the most accessible and useful lung function test. It takes 10–15min to perform and the equipment costs from 2000 to 6000€. The test entails practically no risk, and each test costs approximately 40€. If the spirometer is able to collect data for more than 30s, slow vital capacity (SVC) can also be measured. SVC increases the sensitivity of spirometry for detecting obstruction,1–3 but it does require the test to be longer. Other parameters are mesoexpiratory flow (MEF 25%–75%) and instantaneous maximum flow at 75%, 50%, or 25% of FVC (MEF 75%, 50%, 25%). These parameters are considered indicators of the state of the small airway and a statistical correlation has been observed in subject groups. They are not, however, very sensitive due to their variability, and the cut-points (5% percentile) are nearly 50%4 in subjects >50 years of age.5
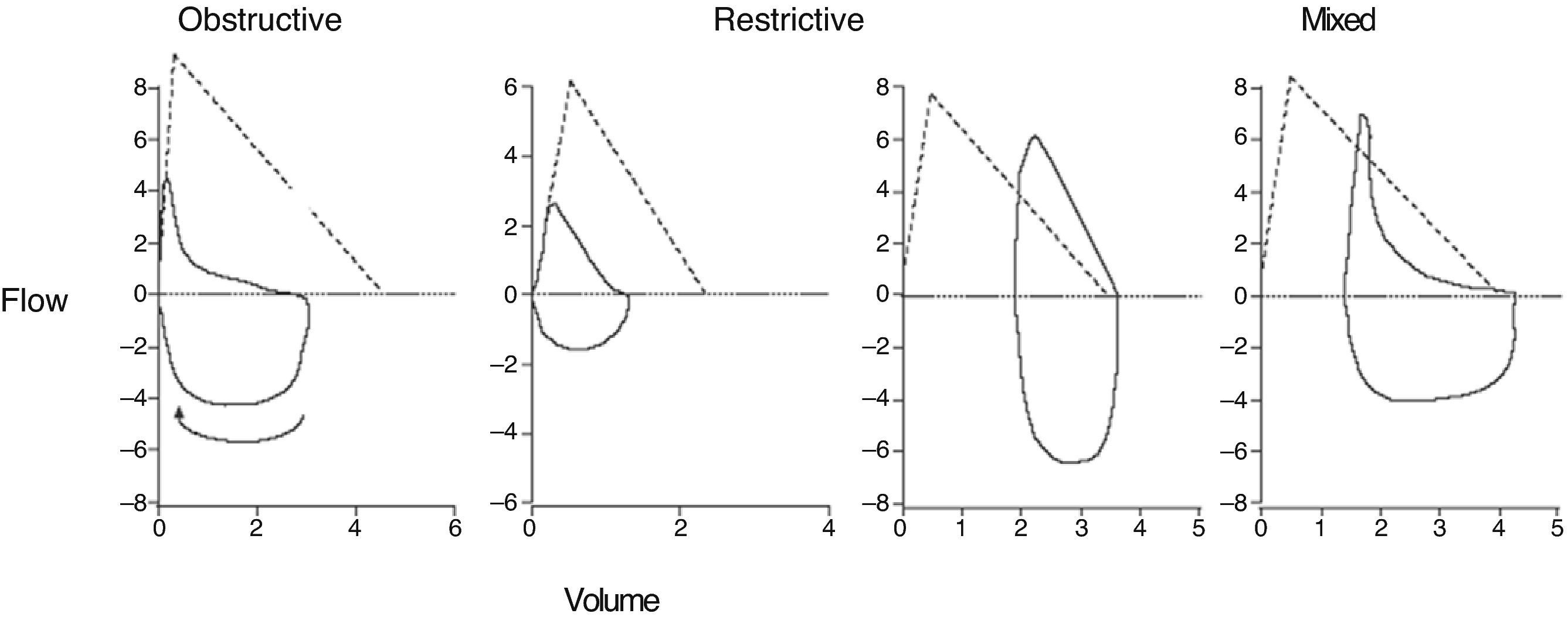
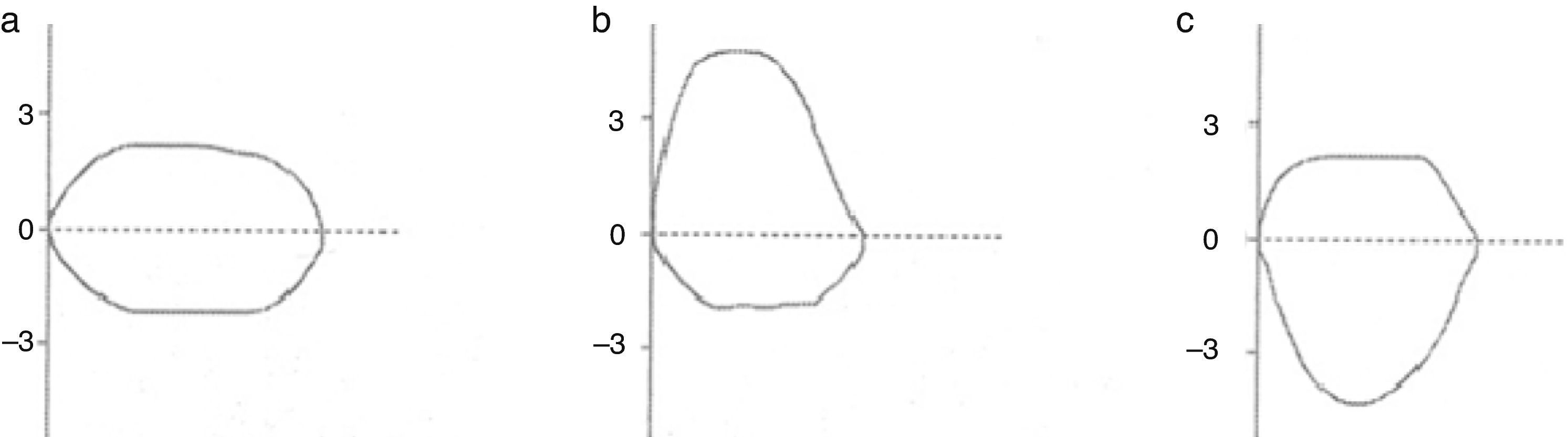
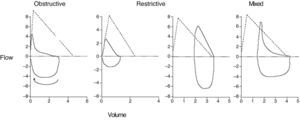
The morphology of the flow–volume curve is very useful for detecting the characteristic concavity of the slowing of expiration at low volumes in obstructive patterns6 and the convexity in restrictive patterns6 (Fig. 1); moreover, it has a distinctive shape in upper airway obstruction, as shown in Fig. 2. It must be noted, however, that the sensitivity is low in tracheal stenoses measuring more than 1cm in diameter.7
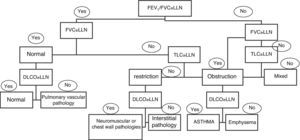
Correctly interpreting spirometry requires it being analyzed within the context of the patient's clinical data, but we can refer to general respiratory function patterns: obstructive, restrictive, and mixed (Fig. 3). The most important parameter for identifying an obstruction is an FEV1/VC ratio <70%.2,6 This cut-point can lead to a significant number of false positives in males over the age of 40 and in women over the age of 50, as well as the to the overdiagnosis of obstruction in seniors, asymptomatic subjects and non-smokers.6,8 It has therefore been recommended to use the lower limit of the confidence interval (LLN)=mean predicted value−(standard error of the residuals×1645),2,6 but tradition and the fact that this percentile is not available in the majority of the devices mean that it is not often used.
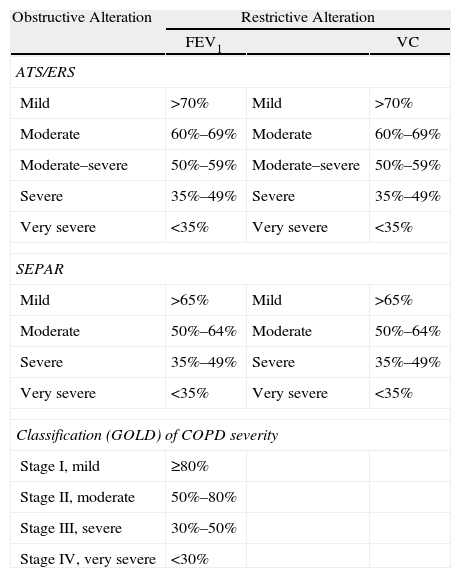
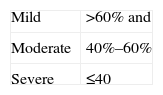
Spirometry also is able to evaluate the severity of ventilatory alterations.2,6 To do so, categories have been defined as a simple conceptual framework depending on post-bronchodilator FEV1 for obstructive defects and according to vital capacity (VC) or total lung capacity (TLC) for restrictive defects.2,6 The cut-points of the different guidelines are shown in Table 1. These cut-points are related with the ability to perform daily activities, morbidity and mortality in COPD,9–13 but these are less relevant in asthma, where hyperreactivity, variability in function (and symptoms), and the response to treatment are more relevant factors than momentary FEV1.14 The cut-points are also not relevant in the obstruction of the upper airway.6
Gradation of The Severity According to Different Guidelines.
| Obstructive Alteration | Restrictive Alteration | ||
| FEV1 | VC | ||
| ATS/ERS | |||
| Mild | >70% | Mild | >70% |
| Moderate | 60%–69% | Moderate | 60%–69% |
| Moderate–severe | 50%–59% | Moderate–severe | 50%–59% |
| Severe | 35%–49% | Severe | 35%–49% |
| Very severe | <35% | Very severe | <35% |
| SEPAR | |||
| Mild | >65% | Mild | >65% |
| Moderate | 50%–64% | Moderate | 50%–64% |
| Severe | 35%–49% | Severe | 35%–49% |
| Very severe | <35% | Very severe | <35% |
| Classification (GOLD) of COPD severity | |||
| Stage I, mild | ≥80% | ||
| Stage II, moderate | 50%–80% | ||
| Stage III, severe | 30%–50% | ||
| Stage IV, very severe | <30% | ||
ATS: American Thoracic Society; COPD: chronic obstructive pulmonary disease; ERS: European Respiratory Society; FEV1: forced expiratory volume in 1s; GOLD: Global Initiative for Chronic Obstructive Lung Disease; SEPAR: Spanish Society of Pulmonology and Thoracic Surgery; VC: vital capacity.
Although in most restrictive disorders worsened symptoms are accompanied by a drop in VC, VC may only be moderately reduced in diffuse interstitial lung diseases (DILD) with a marked loss in diffusion capacity and severe blood gas alterations.6,15–17 Severe respiratory failure may occur in patients with rapidly progressive neuromuscular diseases who had had normal or slightly reduced VC shortly before.6
Another aspect of FEV1 and VC is that they are general indicators of health related with life expectancy, even in non-smoker patients18 (Fig. 4).
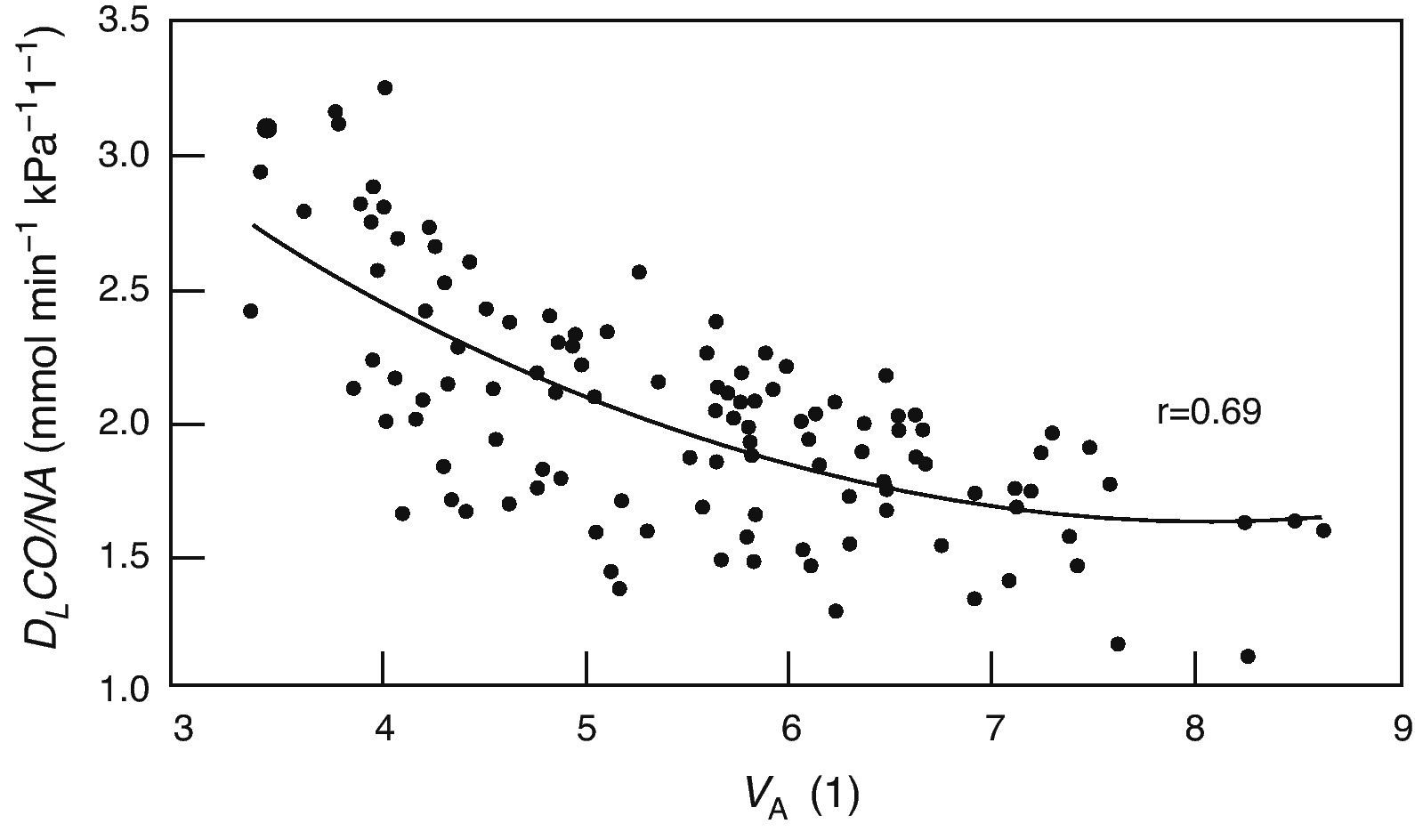
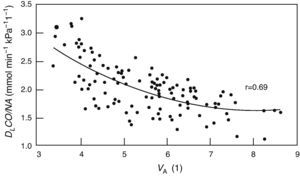
Alveolar diffusion–volume ratio. DLCO: diffusing capacity for carbon monoxide in one single breath; VA: alveolar volume. From Frans et al.52
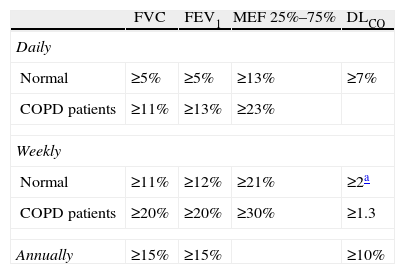
Spirometry is very useful for following evolution and monitoring functional changes over time. Table 2 describes the cut-points in order to consider a change significant in accordance with what was published in the literature.6
Significant Changes in the Spirometric and Carbon Monoxide Diffusing Capacity Variables.
| FVC | FEV1 | MEF 25%–75% | DLCO | |
| Daily | ||||
| Normal | ≥5% | ≥5% | ≥13% | ≥7% |
| COPD patients | ≥11% | ≥13% | ≥23% | |
| Weekly | ||||
| Normal | ≥11% | ≥12% | ≥21% | ≥2a |
| COPD patients | ≥20% | ≥20% | ≥30% | ≥1.3 |
| Annually | ≥15% | ≥15% | ≥10% | |
FVC: forced vital capacity; FEV1: forced expiratory volume in 1s; MEF 25%–75%: mesoexpiratory flow at 25%–75%; DLCO: single-breath transference of carbon monoxide
In mmolmin−1kPa−1; in order to transform into the units most frequently used in the United States (mLmin−1mmHg−1), multiply by ∼3 (3.013). Taken from Pellegrino et al.6
The reversibility test costs 40€ more than spirometry (app. 80€ in total). It is indicated in all asthmatics at the time of diagnosis19 and, if there is still obstruction, at follow-up. As in many laboratories the medicine is routinely withdrawn, we should warn the patient and the laboratory not to withdraw it if our objective is to verify the response to treatment (individual therapeutic assay).
The definition of COPD itself indicates that this disease is characterized by “airflow limitation that is not reversible”20 and “there are no marked changes in the function over the course of several months”.21 This means that the condition should be met that, if there is reversibility, it is not total, and therefore the measured FEV1/VC ratio should be <70% after bronchodilators. In fact, epidemiological studies have shown that the prevalence of COPD reduces between 10% and 40% using post-bronchodilator FEV1/VC, although the socioeconomic impact of said difference is not known.21
A positive result to bronchodilators cannot distinguish between COPD and asthma,20,21 although improvements of more than 400ml after bronchodilators or glucocorticoids suggest the second possibility21 or at least a mixed phenotype.22
One concept that has changes in recent years, based on large clinical assays, is that the presence or absence of bronchodilation, except when very pronounced (>400ml), does not seem to precisely predict symptom alleviation, changes in exercise capacity, long-term response or response to either corticosteroids or bronchodilators.23,24 Therefore, the bronchodilator test is not a valid guideline for treatment.
As for the prognosis, there is abundant evidence that identifies FEV1 as a risk factor in COPD,9–13 and, in this case as well as in the diagnosis, the optimal parameter is post-bronchodilator FEV1. Reversibility has been found to be associated with an accelerated decrease in FEV1, but not all studies find this association.25
The percentage of COPD patients who respond to bronchodilators is variable. In the UPLIFT study,26 which administered ipratropium bromide and salbutamol with spirometries repeated 30min afterwards, 52% of the patients responded to bronchodilators. Nevertheless, these levels were 64%, 48%, and 18% when the patients were classified into GOLD stages II, III, or IV. It has been seen that up to 35% of patients with an initial negative bronchodilator test, the result may be positive in later tests. However, two tests can detect the majority of patients (88%) who occasionally may respond to bronchodilators, and therefore its routine use would not be justified in patients with COPD who already have one or at most two previous bronchodilation tests.26
Any drug may be used, but due to questions of effectiveness, an inhaled, fast-acting β2-agonist is almost always used (salbutamol is most often used at a dose of 400μg or 4 puffs taken 30s apart and with a proper inhalation technique). The effect begins 5min after the inhalation and reaches its maximum after 20min. If ipratropium is used, the recommended dose is 160μg (8 puffs) and the “post” spirometry is done 45min later. Another way to evaluate reversibility is to administer a test treatment (individual therapeutic assay) and to evaluate the patient 30 days later, making the laboratory and the patient aware that the medication prescribed should not be withdrawn before doing the effectiveness evaluation test.
Peak Expiratory FlowPeak expiratory flow (PEF) is the maximal flow that a person can exhale during a short maximal expiratory effort after complete inspiration. In patients with asthma, PEF correlates with FEV1, but it should not be used as its substitute. Peak expiratory flow is a measurement that is easy to do with a device that costs 30€, but it is not very popular. It has the following uses:
DiagnosisVariations of more than 20% are diagnostic for asthma in the proper context.27 PEF can also allow us to observe the variability in certain situations, such as an improvement during vacation or worsening when exposed to certain environments, which, if there are economic or legal implications, should be checked religiously.
Monitoring DiseaseIn order to do so, we should establish the initial value by taking measurements for 15 days in conditions of clinical stability and maximum treatment28 and use this reference in order to establish plans of action28; however, it is not easy to maintain long-term adherence,29 which limits its use.
Non-Specific Provocation TestsThere are different non-specific provocation tests (ATP, mannitol, isocapnic hyperventilation, exercise, food coloring) that are useful in specific contexts. These tests take time and cost 200€.
The test may be indicated when the asthma diagnosis is in question (atypical symptoms, normal spirometry), when a patient is suspicious of having occupational asthma or asthma induced by irritants, and when a test is required to rule out asthma in divers, athletes, military personnel or other individuals in whom bronchospasm would be an unacceptable risk for themselves or for other people, or it is required by regulations in order to use anti-asthma medication,30 and it is not contraindicated. In the cases in which asthma is triggered by exercise, exertional asthma has professional implications; given the persistence of symptoms with exercise in a correctly treated asthmatic, exercise provocation tests or isocapnic hyperventilation tests may be indicated.30
Although non-specific bronchial provocation tests can evaluate asthma severity and monitor its treatment, they are not used for this objective in clinical practice.30
Interpretation of a Negative TestIn general, a negative test rules out asthma, except in certain cases of allergic asthma or asthma induced by irritants in which the test was done a time after the exposure and the symptoms.30,31
Interpretation of a Positive TestApproximately 1%–7% of the general asymptomatic population has bronchial hyperreactivity (up to 26% if smokers or atopic patients are included),32 although there are those who believe that these patients are mild asthmatics who do not perceive their symptoms.30,33 Therefore, the diagnosis should not be based on a positive hyperreactivity test, and it should be confirmed that the symptoms of the patient disappear with the treatment.
Determination of Static Volumes and Lung ResistancesThe determination of pulmonary volumes and capacities cannot be measured with spirometry, including: residual volume (RV), functional residual capacity (FRC), and total lung capacity (TLC). It costs about 150€. The most widely used methods are the helium dilution technique and nitrogen washout (FRC), which can usually be measured with the same device as carbon monoxide diffusion and body plethysmography (FRCpleth), which requires a booth that costs approximately 36000€. Plethysmography gives results that are somewhat higher than the other two methods, but it is the fastest, most precise and reproducible method.6 The TLC measured during the determination of DLCO should never be used as a measurement of actual TLC because it is underestimated, and the greater the obstruction the greater the underestimation.6,34
When studying lung volumes, we can find two pathological patterns: restriction, defined by a TLC less than 80% of the reference value, and hyperinflation, defined by either an FRC% or an RV/TLC ratio above 120%. There are no data to document the use of FRC or RV categories in airflow obstruction or TLC in pulmonary restriction in order to classify severity, as done in spirometry.
Cases of low TLC and normal VC are exceptional3,6,35–37; therefore these measurements are generally not very useful in subjects with normal VC. Its use has not been demonstrated in the differential diagnosis between emphysema and chronic bronchitis, or between COPD and asthma.36,38 There is a general correlation between the reduction in FEV1 and the increase in RV,36,38 although the agreement is not good in up to 15%,36,38 thus it could help to interpret some cases of unjustified dyspnea in obstructive patients when unexpected hyperinflation is detected. In patients with COPD with or without normal VC, these measurements are useful for selecting patients for volume reduction, requiring more than 100% TLC and 135% FRC.39
The measurements of volumes can be useful in the study of subjects with low VC. In cases of mixed pattern, only approximately 10% have low TLC (the majority of them have an FEV1/VC >60% and an FEV1 >40%),3,36 so this would be the target population for measuring lung volumes.
In restrictive diseases, TLC has prognostic value,16 but VC is almost always used as it is easier to measure. In cases with typical restrictive spirometries (meaning when VC is lower, the FEV1/VC is higher [85%–90%] and the flow–volume curve has the characteristic convex pattern [Fig. 1]6), and if the symptoms are compatible, it is probably not necessary to confirm the restriction by measuring TLC. If the lower VC on spirometry is not accompanied by a normal or slightly increased FEV1/VC, this is frequently due to the fact that the inspiration or expiration has not been maximal and in up to 50% of these patients the repetition of the spirometry demonstrates that the subject is normal.3,6,40 In these cases, it would also be indicated to measure volumes if the VC continues to be low after repeating spirometry.
Airflow resistance can be measured by plethysmography, but this is rarely used in clinical practice due to its variability and because even the most sensitive parameter, specific resistance, generally reflects more of the obstruction of the large airways than of the more peripheral areas.6,34,41 It may be useful in patients who are incapable of correctly doing spirometry, as it requires less patient collaboration.
Maximal Respiratory PressuresMaximal inspiratory pressure (PIMAX) is the maximal pressure that the patient can produce when trying to inhale through a blocked mouthpiece after maximum expiration (from RV). PIMAX pressure can be measured in the nose by inserting a nasal plug and sniffing with the other open nostril. This procedure is called sniff nasal-inspiratory pressure (SNIP), and it has the same indications as PIMAX. Its advantage is that it can measure pressure in patients with neuromuscular diseases who are unable to close their mouths properly because sniffing is a natural maneuver that is more easily understood by patients and which they are sometime able to perform better. Usually, both are measured (PIMAX and SNIP), and the better of the two is considered more representative, which is later used in the follow-up. Maximal expiratory pressure PEMAX is the maximal pressure exerted into a blocked mouthpiece, measured during forced expiration after complete inhalation (from TLC), with inflated cheeks. It is easy to measure with a simple pressure manometer connected to a mouthpiece that costs between 1500 and 2000€. The cost of the test is 60€, plus an additional 80€ if combined with sitting and standing spirometry. PIMAX and PEMAX are measurements of the capacity for generating effort of the inspiratory and expiratory muscles, and therefore they can be affected by the configuration of the thorax, particularly the diaphragm, without any alterations that are muscular, as occurs in hyperinflated COPD. PIMAX (SNIP) and average PEMAX for adult men are −100cmH2O (−98hPa) and 170cmH2O (167hPa), respectively, meanwhile the corresponding values for adult women are approximately −70cmH2O (−69hPa) and 110cmH2O (108hPa), respectively.42,43 The lower limit of the normal range is about two-thirds of these values.6
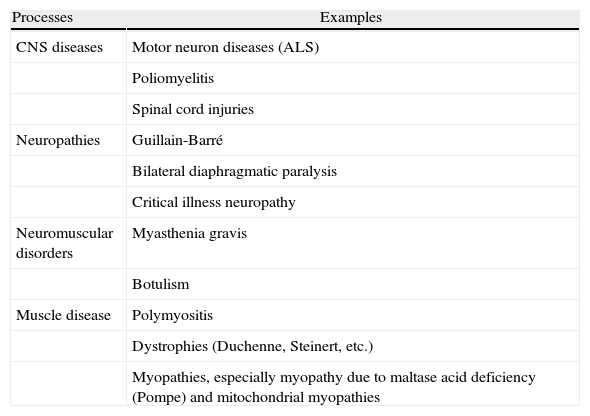
Whenever there is an inexplicable reduction in vital capacity or there is clinical suspicion of respiratory muscle weakness. Monitoring PIMAX (SNIP) and PEMAX is useful, together with VC, for following the evolution of patients with neuromuscular disorders (Table 3). However, they are only able to verify the situation at the time of the measurement and their prognostic value is limited, as some of them evolve with flare-ups and the muscular function may unpredictably worsen at any time.
Processes in Which It May Be Useful to Measure Respiratory Pressures.
| Processes | Examples |
| CNS diseases | Motor neuron diseases (ALS) |
| Poliomyelitis | |
| Spinal cord injuries | |
| Neuropathies | Guillain-Barré |
| Bilateral diaphragmatic paralysis | |
| Critical illness neuropathy | |
| Neuromuscular disorders | Myasthenia gravis |
| Botulism | |
| Muscle disease | Polymyositis |
| Dystrophies (Duchenne, Steinert, etc.) | |
| Myopathies, especially myopathy due to maltase acid deficiency (Pompe) and mitochondrial myopathies |
ALS: amyotrophic lateral sclerosis; CNS: central nervous system.
The diffusing capacity of the lung for carbon monoxide in a single breath (DLCO), also known as carbon monoxide transference factor (TLCO), is useful in the evaluation of both restrictive and obstructive disease.44 It requires a device that costs between 18000 and 24000€. The cost of a test is about 100€. DLCO should always be given as corrected for hemoglobin, which in the majority of devices appears as DLCOc, and, if possible, for carboxyhemoglobin.
A reduction in DLCOc with normal spirometry suggests pulmonary vascular disorders,6 but it can also be seen in incipient DILD or emphysema.6A reduced DLCO in the presence of restriction suggests DILD,15,16 although sometimes restriction is seen in pulmonary vascular diseases.45
A reduced DLCO in the presence of obstruction suggests emphysema46 and also with other much rarer diseases such as histiocytosis X, lymphangioleiomyomatosis, and tuberous sclerosis with lung affectation.47,48
In heart failure due to left ventricular failure, a low DLCO can be observed that is directly related with severity and is a powerful prognostic factor of the disease.15
A high DLCO can be seen in asthma,49 obesity,50 and in intrapulmonary hemorrhage.51
DLCO can also be used to categorize disease severity.6
The DLCO/VA, also known as KCO diffusion constant, is theoretically able to differentiate between the processes that reduce alveolar volume (VA) that limit the normal expansion of the chest (that behave as a loss in VA) or that, as in COPD, the effective VA is reduced in such a way in that the gases used to measure DLCO are not completely diluted by the entire alveolar space (reduced in proportion with the VA) from other diseases that reduce the DLCO because they affect diffusely the exchange surface either due to thickening of the alveoli or due to the loss in capillarization. However, the comparison with the predicted value obtained in subjects with normal VA can lead to errors when the VA is low52 as the TLCO/VA does not vary linearly with VA (Fig. 1).53 Therefore, there is a great debate about their clinical use.6,54,55 Our experience is that, knowing the clinical symptoms of the patient, DLCO/VA usually provides little information (Table 4).
Arterial Blood Gas AnalysisIn stable ambulatory patients, baseline arterial blood gas analysis (without oxygen supplementation), or ABG, can be a useful complement for lung function tests in specific patients. It costs about 30€. It is used to confirm hypoventilation when it is suspected based on the clinical history (e.g. neuromuscular disease or advanced COPD). ABG is also used to confirm chronic hypoxemia and to provide a more detailed evaluation of the severity. It is a painful test, so its use should not be routine and it is only indicated in patients who have low SatO2 on pulse-oximetry.
Measurement of Exhaled Nitric OxideIn recent years, there has been growing interest in the determination of the nitric oxide fraction in exhaled air (FENO), which has become a routine test after standardized guidelines were published.56 The cost of the test is about 50€. It is a quantitative, simple, non-invasive, safe method for measuring the inflammation of the airways, and it is a tool that complements other LFT for the evaluation of bronchial diseases like asthma.
Even though its role is still not free from controversy due to the fact that the evidence on which the recommendations are based are not from clinical assays, measuring FENO can be used for57:
- •
Detecting eosinophilic inflammation of the respiratory tract (FENO >35–50ppb),19,57 which in the presence of compatible symptoms or airflow obstruction (FEV1/VC <70%) can establish a diagnosis of presumed asthma (or at least presumed response to treatment with inhaled corticosteroids similar to asthma) that obligatorily should be confirmed with the demonstration of acute reversibility or an “individual therapeutic assay” with inhaled or oral corticosteroids (see reversibility test) that improves the function or at least symptoms.19
- •
Determining the probability of response to corticosteroids in patients with chronic respiratory symptoms. The recommendation is:
- –
To use a cut-point of ≤25ppb to consider a subject as unlikely to respond.
- –
To use a cut-point of more than 50ppb to consider a subject as likely to respond.
- –
In the intermediate levels (>25 and ≤50ppb), evaluate depending on the symptoms.
- –
- •
Monitoring the inflammation of the respiratory tract in order to determine the dose of corticosteroids. It is recommended to first establish whether the patient is still exposed to the allergens suspicious of being the cause of the airway inflammation. Based on the opinion of experts, the recommendation is to consider significantly those increases (lack of response) or decreases (response) in FENO that are greater than 20% for values of more than 50ppb or greater than 10ppb for values lower than 50ppb from one visit to the next.
- •
Detecting the lack of compliance with corticosteroid treatment.
The 6-min walk test (6MWT) is a good index for physical function,58–61 and it also has prognostic value in many chronic respiratory diseases.58,62–64 It costs about 50€. In general, healthy people can walk 400–700m, depending on age, height, stature, and sex.59,61
Desaturation during exercise, generally measured with a walk test, is an index with prognostic value in pulmonary vascular diseases, interstitial diseases, and COPD.65 A fall in SpO2 greater than 4% (with a total under 93%) suggests important desaturation and it is used to evaluate the need for oxygen and its titration in patients with chronic pulmonary diseases.65–67
ErgospirometryThere are multiple applications for cardiopulmonary exercise tests in pulmonology (Table 5). The equipment costs about 24000€, plus 9000€ for the cycle-ergometer. The cost per test is 150€. It is beyond the purpose of this article to review in detail such tests, and interested readers are recommended to read more advanced papers.66–75 Maximal oxygen uptake has prognostic value in respiratory diseases.65,68,70–75
Indications for the Exertion Test in Pulmonology.
| Evaluation of the tolerance to exercise and its limiting factors |
| Observing the limitation of capacity for exertion |
| Analysis of the factors that limit exercise capacity |
| Distinction between dyspnea of respiratory or cardiac origin |
| Study of unexplained dyspnea with tests at rest |
| Functional assessment and prognosis and detection of alterations that are caused by or noticeably worsen with exercise in chronic pulmonary diseases |
| Evaluation of disability in respiratory diseases |
| Exercise prescription in rehabilitation |
| Diagnosis of exercise-induced bronchospasm |
| Evaluation of the effects of therapeutic interventions |
| Pre-surgery evaluation in lung resection surgery |
LFT are useful for evaluating all types of pulmonary disease and as a screening for the presence of disease in personas with risk factors, such as smoking. Other indications for lung function tests are:
- •
Evaluating symptoms such as persistent chronic cough, wheezing, dyspnea, and cough at resting conditions or with exercise.
- •
Objectively evaluating bronchodilator treatment.
- •
Evaluating the effects of exposure to agents that are noxious for the lungs (dust or chemical products).
- •
Assessing patient risk before thoracic surgery and the prevention of any type of surgery in patients in whom unknown treatable lung disease is suspected.
- •
Objectively evaluating dysfunction or disability.
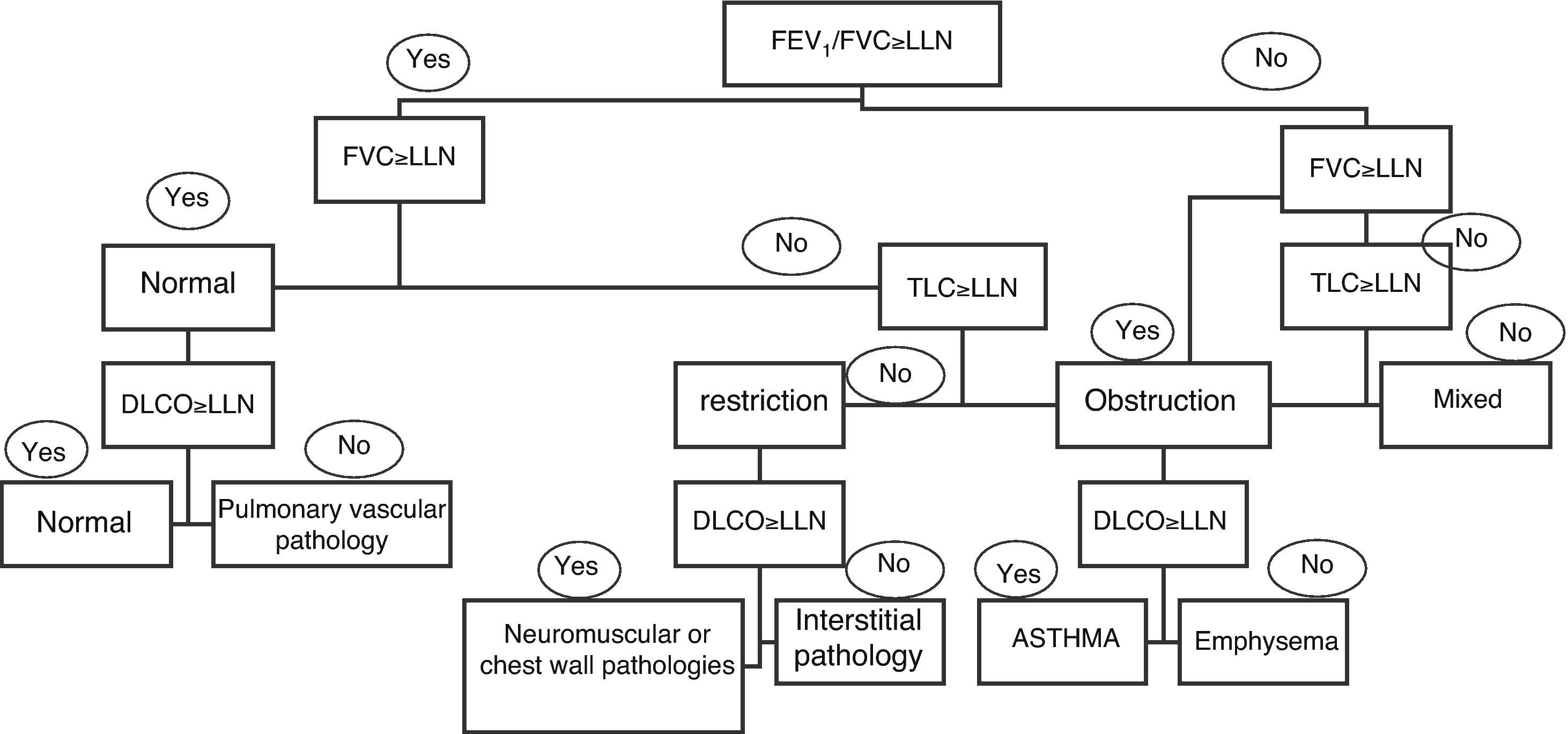
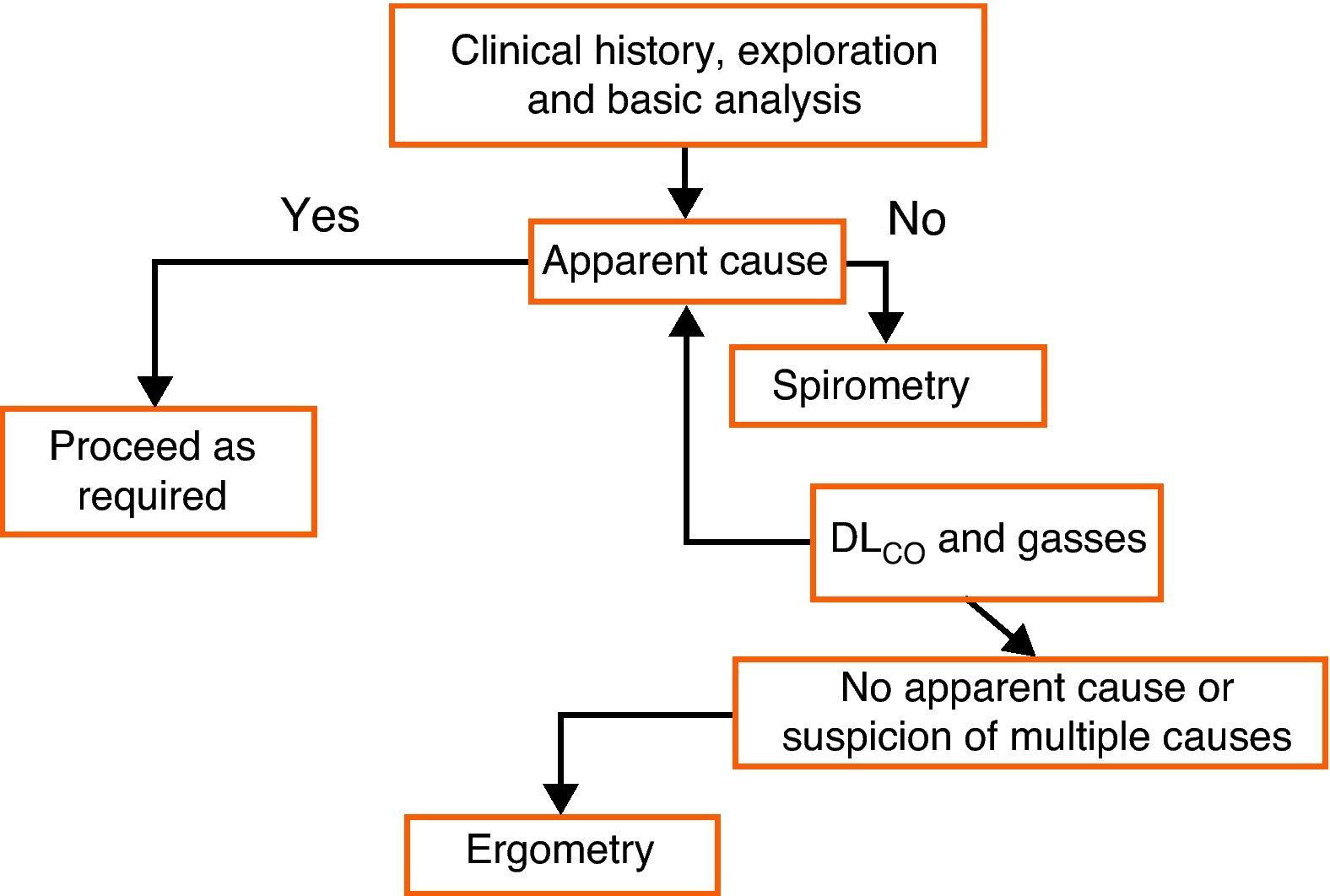
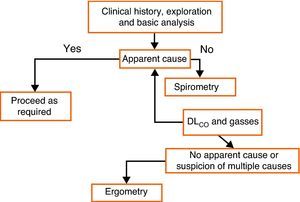
Many lung diseases start slowly and insidiously and they finally become evident with non-specific symptoms, such as exertional dyspnea. LFT are an essential part of the diagnostic routine in these patients. Spirometry should be the first test and other tests should be ordered depending on its results. If the cause of exertional dyspnea remains unclarified after bronchodilation tests, DLCO, and lung volumes, ergospirometry may be useful (Fig. 5).
Chronic CoughThis is a very frequent symptom and can be the cause of up to 40% of outpatient consultations in pulmonology.76 When asthma or COPD is suspected due to the clinical history and chest radiograph, spirometry, and bronchodilator tests are necessary. Before ordering a hyperreactivity test, it must be verified that the patient has persistent cough (>8 weeks), that he/she is not taking medication (angiotensin-converting-enzyme inhibitors) or has any other causes for cough (normal X-ray), and that he/she does not have a very high clinical probability for asthma, gastroesophageal reflux or rhinitis, in which case a previous individual therapeutic assay is preferible.76,77
AsthmaPre- and post-bronchodilator spirometries are indicated in the initial study of patients with suspicion of asthma, as well as during their follow-up. The demonstration of bronchodilation with compatible symptoms is very suggestive of asthma.19 Spirometry is also indicated when there are atypical asthma symptoms, such as oppression in the chest or cough during exercise or when exposed to cold air, dust, smoke or when laughing, which suggest bronchial hyperreactivity. In these cases, spirometry results may be normal when patients are asymptomatic and then not improve after bronchodilator use; therefore on a second visit a hyperreactivity test should be carried out.19 Alternatively, the FENO should be measured and, if high (>35–50ppb), either a treatment assay should be done19,57 or the variability of the PEF should be measured for 15 days.19 The flow–volume curve can be useful for detecting vocal cord dysfunction in patients with atypical or difficult-to-control asthma.
COPDFEV1/VC <70% after bronchodilators (or several weeks of treatment)20,21 is a diagnostic criteria for COPD in patients with a history of smoking, exposure to biomass smoke or industrial dust and symptoms of chronic bronchitis.20,21 The bronchodilator test cannot distinguish between COPD and asthma unless it is suggested by the clinician and the response is very notable (what has been arbitrarily defined as >400ml21); in addition, there is a mixed COPD phenotype that presents characteristics of both diseases.22 This also does not help decide on the treatment,23,24 unless it is very important and accompanied by other criteria suggestive of the mixed phenotype.22 The measurement of lung volumes is not usually useful in general, but it may be indicated in patients with a mixed pattern36 and in cases in which volume reduction is being considered.39 DLCO can be useful to differentiate emphysema from chronic bronchitis; these two phenotypes do not have different treatments, but there is evidence that their evolution may be different, with an accelerated loss of FEV1 in emphysematous patients.78 Anecdotally, DLCO can have a certain value to differentiate between COPD and asthma, as in this latter process it is never low and frequently high.49 Arterial blood gas analysis under stable conditions is painful and is only necessary when there is a suspicion of hypercapnic respiratory insufficiency or when the saturation due to pulse-oximetry is less than 92%. The exertion tests have a prognostic value,72,74,79 and the 6MWT distance has been integrated into the multifactorial BODE index64 that stratifies risk better than FEV1.21 There is, however, a lack of information about its cost-effectiveness, and although the determination of the BODE index offers additional prognostic information, the general feeling is that said information is not sufficient to justify the time and cost required to routinely do 6MWT in all patients.21 One variant of the 6MWT is used for oxygen titration when ambulatory oxygen therapy is prescribed.65–67
Spirometry is essential in the follow-up of COPD patients in order to monitor the effectiveness of treatment (Table 2) and the progression of the disease.78,80 It does not seem to make sense to do more than one per year without any other reason than just for follow-up.78,80 We should expect reductions in FEV1 between 30 and 40ml/year. Higher reductions may be related with the emphysema phenotype, the persistence of smoking, poor control of exacerbations or insufficient treatment.23,81,82 These changes are below the test variability, so in order to interpret them quality spirometry should be available and done under the same conditions (same laboratory, same pharmacological setting, stable patient) and at several points (2 or 3 years), unless the decrease (or improvement) surpasses the test variability (Table 2). In patients with FEV1 <1l, spirometry may not be particularly sensitive to change due to the variability of the test (12% or 190ml). In these patients, other determinations such as measuring symptoms, quality of life, desaturation with exercise or tolerance of exertion can be more sensitive to the interventions and to the progressions of the disease than FEV1 itself.65,83
Neuromuscular DiseasesSince the advent of non-invasive mechanical ventilation, more and more patients with severe respiratory failure are maintained with this therapy, and the demand for tests to measure muscle strength has increased. From a clinical practice standpoint, VC is a useful screening test and its reduction of more than 25% in decubitus (normal, <5%) when comparing with the patient in an erect position is indicative of neuromuscular disease. The ability to cough effectively is usually lost when the VC is less than 30ml/kg, and a VC <30% is considered to predict imminent respiratory failure in chronic neuromuscular diseases, but it should be accompanied by other measurements, like PaCO2 and, sometimes, nocturnal capnography. Respiratory pressures are more sensitive than VC and they would be indicated in the diagnosis and follow-up as a complement, especially in those cases in which the reduction of the vital capacity in decubitus is not clear.
Diffuse Interstitial Lung DiseaseLung function tests should be considered within the context of the radiology results. Spirometry is useful to detect restriction (VC), but it seldom helps to establish the cause.84 DLCO is useful to screen for interstitial lung affectation when interventions are scheduled (bone marrow transplant, chemotherapy) and in order to differentiate between intrinsic lung diseases and other causes of restriction, and it also has prognostic value.15,16,84 Desaturation during exercise also has prognostic value in these patients,65,84 and the 6MWT can be used to titrate ambulatory oxygen when needed.65,66 All these tests play a role in patient follow-up every 6–12 months, or when a change is presumed in the evolution of the disease.
Pulmonary Vascular DiseaseSpirometry is indicated in the initial evaluation of pulmonary vascular diseases for their differential diagnosis with other processes.85 Sometimes, there is an observed reduction in VC.6 DLCO can play a screening role in the detection of pulmonary hypertension, for example in rheumatic diseases,6,86 and in evaluating its pronosis,44,85 although other devices including echocardiography and exertion tests are more often used.85 Even though the 6MWT is more popular, ergospirometry can have advantages as it gives more physiological information and it is done in a safer setting, which is the lab.65 The exertion tests are useful in following up these patients and for monitoring treatment effectiveness.65,85
Pre-Surgery EvaluationLung function tests are only indicated when there is a suspicion of unknown or exacerbated respiratory disease that may be susceptible to improving with treatment and therefore reducing risk, because necessary surgery should never be contraindicated based on lung function.87 Exceptions to this rule are surgery of the abdominal aorta88 and lung resection surgery. In the latter, the combination of spirometry, DLCO, and ergospirometry can better stratify the risk.71,88
Evaluation of DisabilitySpanish legislation (Real Decreto 1971/1999, of 23 December, regarding the procedure for recognizing, declaring, and determining the degree of disability) establishes that the evaluation of disability should be done based on spirometric criteria, DLCO or maximal oxygen consumption.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Puente Maestú L, García de Pedro J. Las pruebas funcionales respiratorias en las decisiones clínicas. Arch Bronconeumol. 2012;48:161–9.