COPD is a multifactorial disease which causes considerable mortality and morbidity worldwide. Previous studies assessing the possible relationship between indoor radon exposure and COPD have shown inconclusive results.
MethodsA multicentric, hospital-based, case-control study was conducted in a Spanish radon-prone area. COPD cases were confirmed by spirometry and controls were selected due to trivial surgery or procedures not related to tobacco consumption. All participants had to have lived for at least 15 years in the same dwelling. Radon measurements were conducted individually in dwellings using alpha-track detectors. Results were obtained using multivariate logistic regression.
Results189 cases and 747 controls took part. There was no significant association between residential radon concentrations and COPD onset with a OR of 1.12 (95%CI 0.41–3.06) for individuals exposed to more than 200Bq/m3 compared to those exposed to less than 50Bq/m3. Heavy smokers seem to increase their COPD risk if exposed to higher radon concentrations vs those exposed to lower concentrations. There was a statistically significant synergy index between radon exposure and tobacco consumption, S-index 11.60 (95%CI 3.71–36.26). Indoor radon concentration was higher in never/light smokers with COPD compared to controls.
ConclusionsNo association between indoor radon and COPD has been observed. However, there might be some effect modification on the COPD risk in heavy smokers when high radon exposure is present. This is supported by the additive synergy observed. Also, a possible association between indoor radon and COPD onset in never and light smokers needs to be further studied.
La enfermedad pulmonar obstructiva crónica (EPOC) es una enfermedad multifactorial que causa una mortalidad y morbilidad considerables en todo el mundo. Los estudios previos que han evaluado la posible relación entre la exposición al radón en interiores y la EPOC no han mostrado resultados concluyentes.
MétodosSe realizó un estudio de casos y controles multicéntrico, hospitalario, en una zona española propensa al radón. Los casos de EPOC se confirmaron mediante espirometría y los controles se seleccionaron por cirugías triviales o procedimientos no relacionados con el consumo de tabaco. Todos los participantes debían haber vivido al menos 15 años en la misma vivienda. Las mediciones de radón se realizaron individualmente en las viviendas utilizando detectores de partículas alfa. Los resultados se obtuvieron mediante regresión logística multivariante.
ResultadosParticiparon 189 casos y 747 controles. No hubo una asociación significativa entre las concentraciones de radón en las residencias y la aparición de la EPOC con un OR de 1,12 (IC 95%: 0,41-3,06) para las personas expuestas a más de 200 Bq/m3 en comparación con las expuestas a menos de 50 Bq/m3. Los grandes fumadores parecen aumentar su riesgo de EPOC si se exponen a concentraciones de radón más altas en comparación con aquellos expuestos a concentraciones más bajas. Hubo un índice de sinergia estadísticamente significativo entre la exposición al radón y el consumo de tabaco: índice S=11,60 (IC 95%: 3,71 - 36,26). La concentración de radón en interiores fue mayor en los no fumadores y fumadores leves con EPOC en comparación con los controles.
ConclusionesNo se ha observado asociación entre el radón en interiores y la EPOC. Sin embargo, podría haber cierta modificación del efecto sobre el riesgo de EPOC en grandes fumadores cuando hay una alta exposición al radón. Esto está respaldado por la sinergia aditiva observada. Además, es necesario estudiar más a fondo una posible asociación entre el radón en interiores y la aparición de EPOC en fumadores leves y no fumadores.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide,1 being responsible for 5.7% of all deaths in 2017. It is also the fifth cause of DALYs (disability-adjusted life years) lost.2 COPD is a chronic disease characterized by persistent respiratory symptoms and airflow limitation that appears after a long period of exposure to airway irritants.3 Nearly 300 million people are estimated to be affected globally by COPD.4 In Spain, its prevalence has grown from 10.2% to 11.8% (40–80 years old population), according to preliminary results from EPISCAN II study.5,6 Although COPD mortality rates in Europe have shown a decreasing trend since 1994,7 mainly due to changes in smoking habits,8 they are expected to grow in future years9 because of the increasing population aging.1 Changes in tobacco smoking epidemic and the differential exposure to other risk factors have produced a modification in sex distribution of COPD in recent years.10 Thereby, it is estimated that COPD mortality rates in the EU will not differ by sex in 2031.7
COPD is a multifactorial disease. Its prevalence increases with age and it is traditionally more frequent in men.3 Tobacco is its main risk factor.11 However, a relevant percentage (25%–45%) of all COPD patients are never smokers.12 Other risk factors have been associated to the development of COPD, and can be divided in genetic conditions and environmental factors, which appear in early life or due to adult exposures during adult life.13 Environmental exposures that have shown a strong association with COPD are: biomass smoke as a result of domestic solid fuel use, occupational exposure to dust and fumes, secondhand smoke, outdoor air pollution mainly related to traffic emissions, chronic asthma and tuberculosis.11,13
Radon is the most important source of ionizing radiation from natural origin to humans.14 It is a radioactive gas that appears as a decay product of the uranium disintegration chain contained in rocks that are present in the earth crust.15 This gas emerges from rocks and may accumulate gradually on dwellings and workplaces.16 It was recognized in 1988 as a human carcinogen by IARC,17 and it is considered the most important risk factor associated to lung cancer following tobacco consumption. When radon gas is inhaled its solid decay products are retained in lungs and irradiate alpha particles to the cell lining of the lungs. This causes molecular changes and may damage DNA.
A few number of studies have investigated indoor radon exposure as a potential risk factor associated with COPD. A systematic review published in 2020 found a possible trend toward the existence of such association,18 although no definite conclusion could be made. Two studies conducted in general population showed an association between residential radon and COPD.19,20 Galicia is a radon-prone area due to the granitic nature of the bedrock. It is estimated that 21.3% of Galician dwellings have radon concentrations above the US Environmental Protection Agengy (USEPA) action level (148Bq/m3).21
The main objective of this study is to analyze if indoor radon exposure may be associated with the onset of COPD and to asses if the association between COPD and tobacco consumption may be modified by radon exposure in a radon-prone area.
Material and MethodsDesign, Subjects and SettingsWe performed a multicenter, hospital-based, case-control study in the northwest of Spain, classified previously as a radon-prone area.22 Cases and controls were recruited from 5 different hospitals. Recruitment started in May 2018 and ended in December 2019.
Cases were recruited from the reference hospitals located in each healthcare area, and all cases had to have a previous spirometric diagnosis of COPD. COPD diagnosis had to be confirmed in the last 10 years, with the spirometric criteria proposed by the Spanish guide GesEPOC.23 This criterion consisted in a FEV1/FVC ratio below 0.70 after administration of bronchodilators. In addition, all COPD cases should have had at least one spirometry performed in the last 3 years previous to the inclusion in the study. Additionally, cases with a COPD diagnosis longer than 10 years than the inclusion date were not allowed, in order to include mainly incident cases. All cases and controls had to be older than 30 years. In addition, all participants should have lived at least 15 years in the same dwelling. Participants with neoplastic history were discarded from the study.
Controls were recruited from individuals attending minor surgery or ambulatory major surgery non related to tobacco smoking in the same hospitals from which cases were collected. Some causes for the recruitment of controls were lipoma removal, orthopedic surgery, dermatological interventions, etc.
The study protocol and consent forms were approved by the Santiago de Compostela-Lugo Ethics Committee (REF 2017/526). All participants gave written consent for inclusion.
Information Retrieval and Radon MeasurementCases were recruited and interviewed by participating pulmonologists while controls were interviewed by nursing staff specially trained for the study. Both cases and controls were interviewed at hospital by using a questionnaire which included sociodemographic information, with special emphases on tobacco consumption (age of starting and stopping tobacco consumption, number of cigarettes per day and environmental tobacco smoke exposure for never-smokers).
Radon measurements were performed using alpha-track detectors (CR-39 type). Radon detectors were placed individually by the participants in their homes. They were placed preferentially in the main bedroom, between 60 and 180cm off the floor, and at least 15cm separated from walls, and away from doors, windows and electronic devices. The detectors were placed for at least 3 months, and then were sent to the Galician Radon Laboratory (University of Santiago de Compostela, School of Medicine) where they were read. All participants received two phone calls to assure the placement and sending of the radon device once the measurement period ended. The Galician Radon Laboratory (www.radon.gal) is one of the three Spanish laboratories certified by the National Entity of Accreditation (ENAC) to measure indoor radon in air. All participants were informed by letter regarding the results of the radon measurement.
Statistical AnalysisA bivariate analysis was first performed to compare the characteristics of cases and controls. Then, we conducted a non-conditional multivariate logistic regression in which the case or control status was considered the dependent variable and radon concentration as the independent variable broken down in 4 categories (≤50Bq/m3; 51–147Bq/m3; 148–199Bq/m3 and >199Bq/m3). This regression was adjusted by age, sex, and education. A second regression model was performed adjusted for tobacco consumption. Results were presented as Odds Ratio (OR) with their 95% confidence interval. We repeated this analysis only for men, because it was not possible for women due to the low number of COPD cases. We also performed a further model creating a new variable combining tobacco consumption and indoor radon exposure. This variable was the independent variable with 12 categories, combining 4 categories of radon exposure (≤50Bq/m3; 51–147Bq/m3; 148–199Bq/m3 and >199Bq/m3) and 3 of tobacco consumption (never and light smokers: <33 pack-year; moderate smokers: 34–66 pack-years; heavy smokers: >66 pack-years). Never and light smokers had to be collapsed due to the low number of never smoking cases. We calculated the Synergism index (S – the most reliable measure of additive interaction when adjusting for confounding variables) between radon exposure and tobacco smoking. Therefore, S and its 95% CI were calculated to assess the additive interaction between tobacco consumption and indoor radon. S was obtained from the ratio of the combined effects to the sum of the individual effects of tobacco consumption and indoor radon (two categories each, radon with a cutpoint in 148Bq/m3 and never/light smokers and moderate/heavy smokers). All analyses were performed with IBM SPSS v22 (IBM, Armonk, NY, USA) and R statistical package.
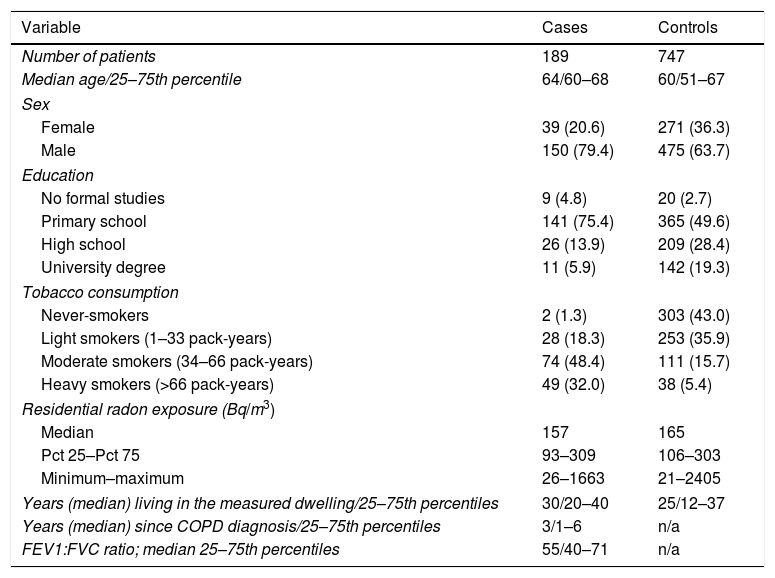
ResultsThe study included 189 cases and 747 controls, with a mean age of 64 and 60 years, respectively. There was a higher percentage of men than women among cases, with cases having a worse education compared to controls. Tobacco consumption was much more frequent and with a higher intensity in cases than in controls. Median indoor radon concentration was slightly smaller for cases (157 vs 165Bq/m3). Cases received COPD diagnosis a median time of 3 years before inclusion, with a median FEV1/FVC ratio of 55. A brief description of the sample characteristics is shown in Table 1.
Sample Description Broken Down by Case–control Status (n=936).
| Variable | Cases | Controls |
|---|---|---|
| Number of patients | 189 | 747 |
| Median age/25–75th percentile | 64/60–68 | 60/51–67 |
| Sex | ||
| Female | 39 (20.6) | 271 (36.3) |
| Male | 150 (79.4) | 475 (63.7) |
| Education | ||
| No formal studies | 9 (4.8) | 20 (2.7) |
| Primary school | 141 (75.4) | 365 (49.6) |
| High school | 26 (13.9) | 209 (28.4) |
| University degree | 11 (5.9) | 142 (19.3) |
| Tobacco consumption | ||
| Never-smokers | 2 (1.3) | 303 (43.0) |
| Light smokers (1–33 pack-years) | 28 (18.3) | 253 (35.9) |
| Moderate smokers (34–66 pack-years) | 74 (48.4) | 111 (15.7) |
| Heavy smokers (>66 pack-years) | 49 (32.0) | 38 (5.4) |
| Residential radon exposure (Bq/m3) | ||
| Median | 157 | 165 |
| Pct 25–Pct 75 | 93–309 | 106–303 |
| Minimum–maximum | 26–1663 | 21–2405 |
| Years (median) living in the measured dwelling/25–75th percentiles | 30/20–40 | 25/12–37 |
| Years (median) since COPD diagnosis/25–75th percentiles | 3/1–6 | n/a |
| FEV1:FVC ratio; median 25–75th percentiles | 55/40–71 | n/a |
Data are presented as n (%) unless otherwise stated.
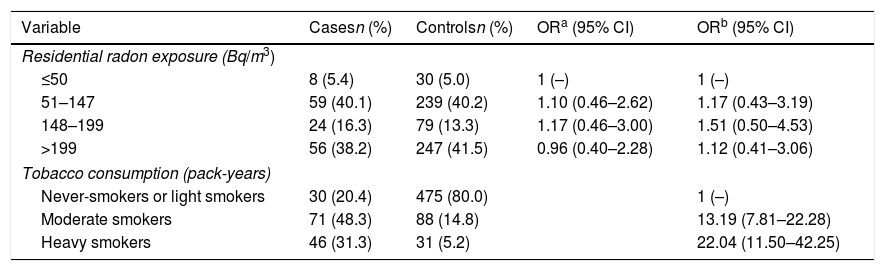
Table 2 shows the Odds Ratio for COPD appearance in relation to radon concentrations adjusted for sex, age, level of education and tobacco consumption. Residential radon concentration does not seem to influence the risk of COPD, even at the highest concentrations. Nevertheless, heavy smokers multiply their risk of COPD by 22 compared to never and light smokers. When analyzing the data exclusively for men, the results are very similar, as it can be seen in Table 3. The risk seems to increase slightly, although far from statistical significance.
Residential Radon Exposure and Risk of COPD.
| Variable | Casesn (%) | Controlsn (%) | ORa (95% CI) | ORb (95% CI) |
|---|---|---|---|---|
| Residential radon exposure (Bq/m3) | ||||
| ≤50 | 8 (5.4) | 30 (5.0) | 1 (–) | 1 (–) |
| 51–147 | 59 (40.1) | 239 (40.2) | 1.10 (0.46–2.62) | 1.17 (0.43–3.19) |
| 148–199 | 24 (16.3) | 79 (13.3) | 1.17 (0.46–3.00) | 1.51 (0.50–4.53) |
| >199 | 56 (38.2) | 247 (41.5) | 0.96 (0.40–2.28) | 1.12 (0.41–3.06) |
| Tobacco consumption (pack-years) | ||||
| Never-smokers or light smokers | 30 (20.4) | 475 (80.0) | 1 (–) | |
| Moderate smokers | 71 (48.3) | 88 (14.8) | 13.19 (7.81–22.28) | |
| Heavy smokers | 46 (31.3) | 31 (5.2) | 22.04 (11.50–42.25) | |
Residential Radon Exposure and Risk of COPD. Only Males (n=476).
| Variable | Casesn (%) | Controlsn (%) | ORa (95% CI) | ORb (95% CI) |
|---|---|---|---|---|
| Residential radon exposure (Bq/m3) | ||||
| ≤50 | 6 (5.4) | 17 (4.5) | 1 (–) | 1 (–) |
| 51–147 | 39 (34.8) | 147 (39.2) | 1.09 (0.39–3.06) | 1.26 (0.40–3.98) |
| 148–199 | 22 (19.6) | 58 (15.5) | 1.43 (0.48–4.27) | 2.06 (0.60–7.13) |
| >199 | 45 (40.2) | 153 (40.8) | 1.14 (0.41–3.18) | 1.58 (0.50–4.98) |
| Tobacco consumption (pack-years) | ||||
| Never-smokers or light smokers | 18 (16.1) | 269 (71.9) | 1 (–) | |
| Moderate smokers | 52 (46.4) | 76 (20.3) | 9.15 (4.96–16.86) | |
| Heavy smokers | 42 (37.5) | 29 (7.8) | 17.52 (8.64–35.56) | |
Table 4 presents the results for the different combinations of radon exposure and tobacco use on the onset of COPD. It can be observed that for the same category of tobacco consumption, the risk of COPD increases when radon exposure increases. Thus, in heavy smokers exposed to less than 50Bq/m3, the risk of COPD is 12.8 (95%CI 1.56–94.68) compared to 23.53 (95%CI 4.40–125.64) for those heavy smokers exposed to more than 200Bq/m3, and compared to light smokers and never smokers exposed to less than 50Bq/m3. Therefore, in heavy smokers the effect is twofold when moving from the lowest radon concentration to the highest exposure category. A similar effect seems to exist in moderate smokers, although not so evident for the highest category of radon exposure. The synergy index showed a significant additive interaction between radon exposure and tobacco consumption, with an S-index of 11.60 (95%CI 3.71–36.26).
Interaction Among Tobacco Consumption and Residential Radon Exposure and Risk of COPD.
| Tobacco Consumption (Cases, Controls); ORa (95% CI) | |||
|---|---|---|---|
| Residential Radon Exposure (Bq/m3) | Never and Light Smokers | Moderate Smokers | Heavy Smokers |
| ≤50 | 2, 231 (–) | 2, 37.04 (0.67–74.2) | 4, 412.8 (1.56–94.68) |
| 51–147 | 14, 1880.95 (0.20–4.52) | 30, 3710.24 (2.16–48.49) | 15, 1412.37 (2.35–65.01) |
| 148–199 | 4, 640.80 (0.13–4.75) | 13, 1214.94 (2.74–81.51) | 7, 325.76 (3.43–193.33) |
| >199 | 10, 2000.62 (0.13–3.06) | 26, 369.61 (2.01–45.91) | 20, 1023.53 (4.40–125.64) |
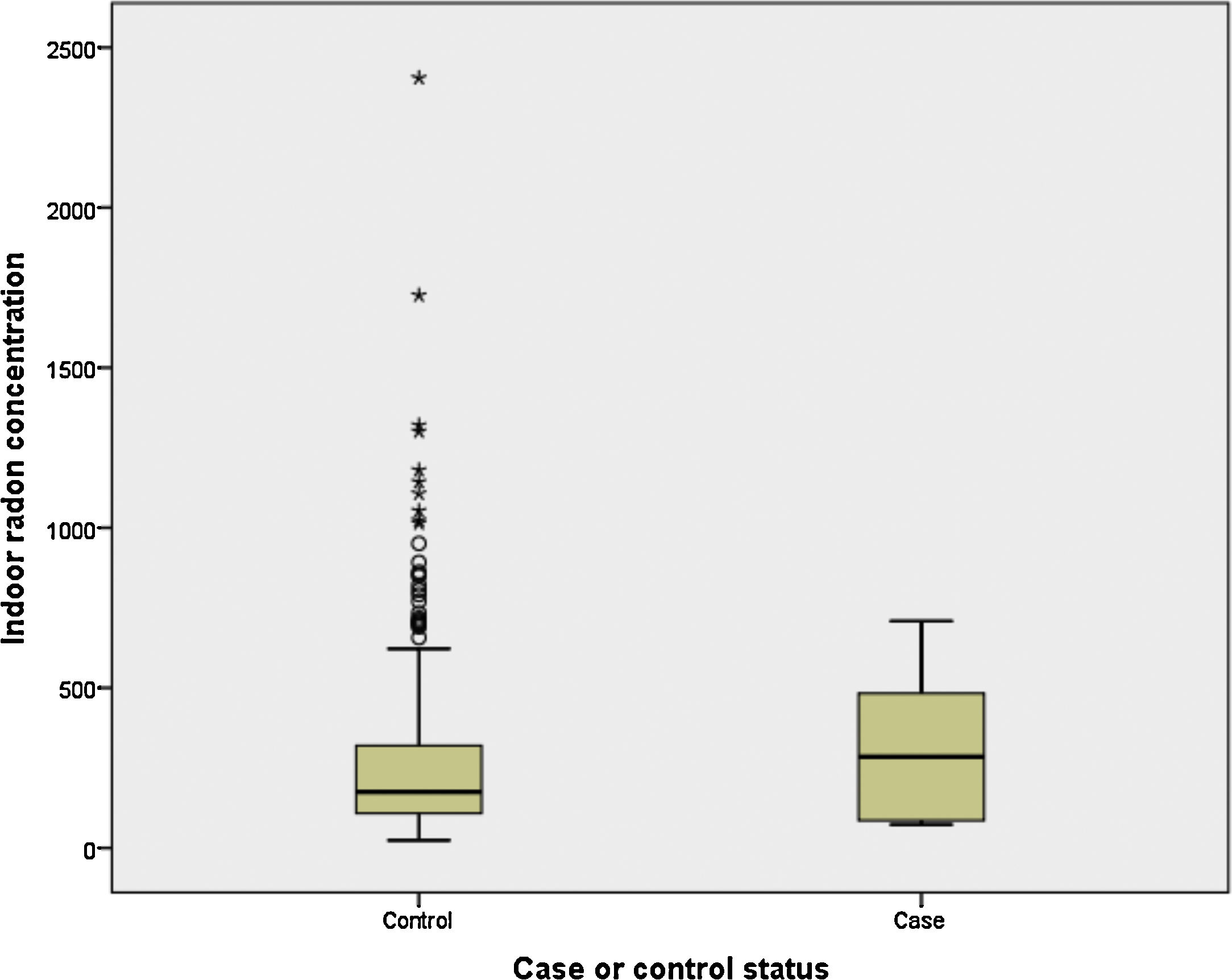
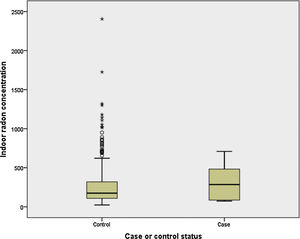
Finally, we have compared indoor radon concentration by case-control status for light and never smokers (<10 pack-years). It can be observed in Fig. 1 that cases (n=6) have a much higher indoor radon concentration, with medians being 285 and 176Bq/m3 for cases and controls, respectively (P>.05).
DiscussionThis study analyzes, as far as we know for the first time in general population and using individual radon exposure, the effect of residential radon on the COPD onset. Though it can be concluded that indoor radon does not have an association with COPD onset, there might be some effect modification when indoor radon is combined with tobacco consumption in heavy smokers. The risk of COPD for heavy smokers seems to be higher when high indoor radon concentrations are present. In fact, the formal testing for synergy has revealed this synergism between both variables. It is necessary to mention the interesting finding that indoor radon concentration is much higher in never or light smokers (<10 pack-years) compared to their control counterparts.
Our results differ from the other two previous epidemiological studies that evaluated the relationship between indoor radon concentrations and mortality or prevalence of COPD in general population.19,20 The first one was a prospective cohort study carried out in 811,961 participants from the American Cancer Prevention Study II, and the second one was an ecological study which analyzed 237 municipalities from Galicia, Spain. These two studies observed an association of a COPD-related outcome with residential radon concentration. The CPSII study found a significant trend for COPD mortality rate (HR 1.13, 95% CI 1.05–1.21), while the ecological study found a significant association between COPD-hospital admissions and radon concentrations at a municipal level (RR 1.04, 95% CI 1.00–1.10). The results of the ecological study have to be interpreted with caution due to the ecological design.
These two studies19,20 did not use measurements of residential radon concentrations taken ad hoc. Although the American study was a cohort study, in which the unit of observation were individual participants, residential radon concentrations were assigned in an ecological-basis. Each participant was matched with a radon measurement that was the mean concentration of the county where they lived. This fact means that both the cohort and the ecological study may be affected by the ecological fallacy since indoor radon exposure missclassification can be present. Our study obtained for the first time direct radon concentrations from the participants’ dwellings using alpha-track detectors. This means having a much more reliable information regarding radon exposure compared to both previous studies.
Other epidemiological studies were previously conducted in miners in order to evaluate the association between occupational exposure to radon and COPD. Three of them24–26 are in agreement with our study and found no significant association with COPD-related outcomes. All these studies were designed as cohort studies and used COPD mortality rate as the final outcome. Two of them were conducted in the German Wismut Mining company, firstly assessing the risk of death in all workers associated to silica or radon exposure, and then analyzing a smaller cohort of uranium miners selected from the initial cohort.24,25 Cumulative radon exposure could not be associated to death from COPD in the first study, while in the second one the excess of relative risk (ERR) due to cumulative exposure was not significant (ERR 3.28, 95%CI −2.33 to 8.89). The other cohort study was conducted in uranium processing workers from an American company,26 and did not find a significant increase in risk of death due to COPD. Other study that assessed the causal relationship between occupational exposure to radon decay products and COPD was conducted in the Colorado Plateau cohort in the United States.27 This investigation carried out in 3238 uranium miners found a positive association between radon and COPD mortality. Nevertheless, epidemiological studies conducted on miners can be hardly extrapolated to general population. These studies suffer from the called “healthy-worker” bias, which limits the external validity. Also, uranium mine workers are usually influenced by another harmful exposures associated to their jobs, such as silica, other dusts or diesel exhaust.27 In addition, all these studies are subject to confounding bias, since most of them have not been properly adjusted for posible confounding risk factors, mainly tobacco consumption.
The effect modification observed for heavy smokers with the increase in radon exposure might be biologically plausible since alpha radiation could increase the inflammatory permanent status caused by tobacco consumption in the lungs. In agreement with our results, the American cohort study found that the association between radon exposure and COPD mortality was stronger in current smokers than in never or ex-smokers.19 However, they did not find any significant effect modification for the county radon level on the association between tobacco and COPD mortality. We have to highlight that COPD mortality studies lack of a reliable codification of the cause of death (something sometimes difficult for this disease),28 and that radon association might be blurred by the long time that patients suffer COPD. In this sense, it is easier to assess the relationship of radon exposure with COPD incidence compared to COPD mortality, but as far as we know this is the only study having analyzed incidence.
From a biological point of view, it may be logical that radon decay products could be involved in the development of chronic obstructive pulmonary disease in humans. COPD is a chronic condition that occurs after a long period of exposure of lung parenchyma to some toxic particles such as smoke irritants derived from tobacco consumption.3 This chronic exposure causes a chronic inflammation in lung microenvironment and leads to airways narrowing and lung parenchima destruction. Appart from tobacco smoke irritants, other substances have also been found to trigger excessive inflammation in lung epithelium, such as particulate matter, other ambient pollutants, biomass smoke, or dust and fumes derived from occupational exposures.11,29 When a person is chronically exposed to radon gas, as in the case of occupational or residential exposure, lung parenchyma is the organ receiving the highest doses of alpha radiation.30 Association between emphysema and COPD with the development of lung cancer has been widely studied,31 and COPD is considered to be an independent risk factor for lung cancer appearance. In COPD presentation, the excessive inflammatory response leads to over-production of some specific cytokines (IL-6, IL-8) which are able to regulate pro-oncogens and amplify mutagenic damage, as well as growth factors which favors tumor growth.32 This common inflammatory pathway on the development of COPD and lung cancer could be potentionally activated in both diseases by exposure to alpha particles derived from radon. Alpha particles have been shown to induce the production of reactive oxygen species (ROS) and inflammatory mediators such as IL-8 in lung microenvironment.33 This type of radiation triggers the alteration of more than 500 genes, some of them involved in respiratory diseases. In this way, BEIR IV report describes pathological findings in respiratory airways of animals exposed to high radon concentrations.34 Studies conducted on patients exposed to medical radiotherapy for cancer treatment concluded that radiation causes acute and late lung damage (Radiation-Induced-Lung-Injury) and a decrease in pulmonary function.35–37 Other studies conducted in nuclear workers also found a decline in respiratory function after a chronic exposure to radiation.38 However, the UNSCEAR 2016 report concluded that there is no strong evidence on the causal relationship between exposure to radionuclides in mine uranium workers and non malignant respiratory disease appearance.39
The present study has some advantages. Firstly, it has been performed in a radon-prone area,22 and therefore it could be easier to find a potential association between indoor radon and COPD. Galician population has shown also lower mobility than other populations, with a mean time of residence in the same dwelling of 30 years.40 This means that indoor radon concentrations are a good indicator of long-term exposures in our participants. Additionally, due to the universal coverage of the Spanish health system, COPD cases can be considered as highly representative of cases found in the community, reducing the possibility of a selection bias.
The main limitation of the present study is the retrospective design employed (a case-control study), but given the long induction period for COPD, a prospective study such a cohort study would have been difficult to perform. Other limitation could be the use of prevalent cases instead of strictly incident cases. Nevertheless, 50% of our cases had a COPD diagnoses in the previous 3 years. We have included a low number of women, but this represents the usual distribution of COPD in Spain,6 because women started smoking later than men and therefore their COPD incidence is still increasing.41 We did not use a consecutive sampling for cases to avoid a relevant workload for recruiting clinicians though this sampling type would have been preferable. Finally, we had a low number of never smoking cases, so our results cannot be extrapolable to this subpopulation of COPD cases.
To conclude, these results point out that no association seems to exist between residential radon exposure and COPD. Nevertheless, radon exposure might modify the effect of tobacco consumption for heavy smokers since there might be an additive synergism between both variables. The possible association between indoor radon exposure and COPD onset in never and light smokers needs to be further studied.
FundingSpanish Society of Neumology and Thoracic Surgery (SEPAR). Competitive project number 439. Call 2018.
This work is part of the research conducting to the PhD degree of Ana Pando Sandoval.
Conflict of InterestThe authors declare that they have no conflict of interest.