To estimate the cumulative incidence of COVID-19 and its determinants among a nationally representative sample of adults from Spain who smoke.
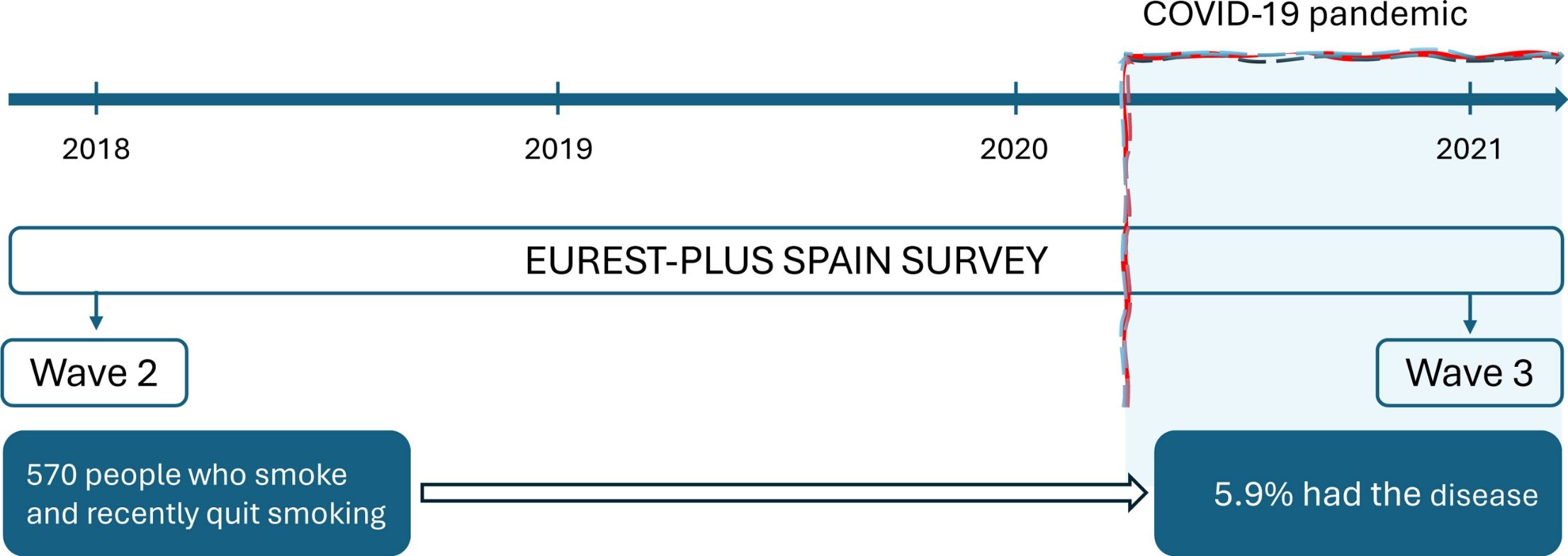
MethodsThis is a prospective cohort study that uses data from two waves (Wave 2 in 2018 and Wave 3 in 2021) of the ITC EUREST-PLUS Spain Survey. At baseline (Wave 1 in 2016), all respondents were adults (aged ≥18) who smoked. In total, 1008 respondents participated in Wave 2, and 570 out of 888 eligible participants were followed up in Wave 3 (64.2%). We estimated the cumulative incidence and the relative risk of COVID-19 (RR) and 95% confidence intervals (CI) during follow-up using self-reported information on sociodemographic, smoking-related and health-related characteristics and identified associated factors using multivariable Poisson models with robust variance adjusted for the independent variables.
ResultsThe overall cumulative incidence of self-reported COVID-19 was 5.9% (95% CI: 3.9–8.0%), with no significant differences between males (6.3%; 95% CI: 3.6–9.0%) and females (5.6%; 95% CI: 3.2–8.0%). After adjusting for age, sex, and educational level, COVID-19 incidence was positively associated with moderate nicotine dependence (RR: 2.37; 95% CI: 1.04–5.40) and negatively associated with having a partner who smoked (RR: 0.12; 95% CI: 0.03–0.42), and having friends but not a partner who smoked (RR: 0.28; 95% CI: 0.14–0.56).
ConclusionThe correlates of having had COVID-19 among people who smoke should be considered when tailoring information and targeted non-pharmacological preventive measures.
Since the onset of the COVID-19 pandemic, the effects of active smoking and exposure to second-hand tobacco smoke (SHS) on the risk of SARS-CoV-2 infection and the severity and mortality from COVID-19 have remained undetermined.1–7 An early review and meta-analysis of data1 from studies conducted all over the world was controversial and produced inconclusive results, reporting no association between smoking and risk of COVID-19, but pointing to a link between smoking and the risk of severity, hospitalization, and mortality. In Europe, the studies contributing to the above-mentioned results were from France,8 Italy,9 the United Kingdom,10–12 and Spain.13
A subsequent meta-analysis14 found a significant association between current smoking and COVID-19 mortality. Moreover, a multicenter study from Italy reported an association of COVID-19 progression among people exposed to SHS.15 Evidence indicates that COVID-19 mortality is associated with old age, being male, and having several comorbidities, particularly hypertension, kidney disease, diabetes mellitus, obesity, and cancer.13 In light of this evidence, it is particularly important to investigate the relationship between smoking and SHS with COVID-19, as stated since the onset of the pandemic.16,17
The International Tobacco Control Six European Countries (ITC 6E) Survey, part of the ITC EUREST-PLUS Project,18 aimed to prospectively evaluate the psychosocial and behavioral impact of the European Union (EU) Tobacco Products Directive (TPD) and the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) on several tobacco-related domains. To this end, a cohort of adults who smoke in Germany, Greece, Hungary, Poland, Romania, and Spain19 was established in 2016 (Wave 1), just before the transposition of the TPD, and respondents were recontacted in 2018 (Wave 2), following TPD transposition. In 2021, a third wave was conducted only in Spain (Wave 3), as part of the ITC EUREST-PLUS Spain Project, which coincided with the initiation of the fifth wave of the COVID-19 pandemic in Spain.
In Spain, these prospective cohort data provide an opportunity to assess the COVID-19 risk among a nationally representative sample of adults who smoke. The purpose of this study was to estimate the cumulative incidence of self-reported COVID-19 and its determinants among Spanish adults who smoke.
MethodsStudy Design and ParticipantsThe ITC EUREST-PLUS Spain Survey is a prospective cohort study of adults who smoke. The current research is based on data from two waves of the cohort (Wave 2 in 2018 and Wave 3 in 2021). Details about the ITC EUREST-PLUS Spain Survey have been published elsewhere.20,21 In brief, a multistage sampling was used with geographical stratification, including all Spanish Autonomous Communities, except the Canary Islands, Ceuta, and Melilla. All respondents from the 2018 Wave 2 survey were invited to participate in Wave 3 in 2021.
An information letter was sent to Wave 2 respondents who had agreed to be recontacted (n=888), followed by a telephone call. Those who were not available were visited at their homes. Computer-assisted interviews were conducted in-person (CAPI) or by telephone (mCATI) if respondents had symptoms of COVID-19, had recently tested positive, or had health concerns and preferred not to have an in-person interview. All respondents were adults (aged ≥18), smoked at least monthly at first recruitment, and had smoked at least 100 cigarettes in their lifetime.
Ethical ConsiderationsThe ITC EUREST-PLUS Spain Survey received ethics approval from the Research Ethics Board of the Bellvitge University Hospital in Spain (PR100/16) and the University of Waterloo in Canada (REB#41105). All respondents received information on data confidentiality and security and the potential risks and benefits of their participation, and gave consent to participate.
MeasuresThe dependent variable, having had COVID-19, was gathered from the Wave 3 follow-up interview in 2021. The question used was: “Have you had COVID-19?” and the response options were: (1) “Yes, it was confirmed with a test (polymerase chain reaction (PCR), antigen or antibody test)”; (2) “Yes, it was confirmed by a physician”; (3) “I think so, but I haven’t seen a physician or used a test (PCR, antigen or antibody test)”; (4) “No, it was confirmed with a test (PCR, antigen or antibody test)”; (5) “No, but I haven’t been tested”. Self-reported infection was considered positive when the response to the question was any of the options 1, 2, or 3, and it was considered negative when the answer was 4 or 5. “Refused” and “Don’t know” responses were excluded from analysis (n = 4; 0.4%).
The independent variables were collected from Wave 2 in 2018. Smoking status and the type of tobacco product used were defined with the following three categories: (1) smoking only cigarettes; (2) smoking cigarettes and/or electronic cigarettes, and/or heated tobacco products; or (3) former smoking. We assessed cigarette dependence with the Heaviness of Smoking Index (categorized as low: 0–2; moderate: 3–4; and high: 5–6).22 We also asked if their partners or friends smoked and categorized this variable as: (1) having a partner who smoked; (2) not having a partner who smoked but having friends who smoked; and (3) having neither partners nor friends who smoked.
Exposure to SHS at home was assessed with the question: “Which of the following statements best describes smoking inside your home? NOT on the balcony, terrace, or other outdoor areas”. With the following options: (1) “Smoking is allowed anywhere inside your home”; (2) “Smoking is allowed in some rooms inside your home”; (3) “Smoking is never allowed anywhere inside your home”; (4) “Smoking is not allowed inside your home except under special circumstances”. The response options 1 and 2 indicated SHS exposure at home and the response options 3 and 4 indicated no exposure. One “Refused” response was excluded from analysis (0.1%).
Exposure to SHS in cars with children was assessed among those who own a car and carry children in it with the question: “What are the rules about smoking in your car or cars when there are children in the car? Smoking is…” With the following options: (1) “Never allowed when children are in my car”; (2) “Sometimes allowed when children are in my car”; (3) “Always allowed when children are in my car”; (4) “I do not have a car”; (5) “Children are never in my car”. Exposure was considered positive with response options 2 and 3. “Refused” and “Don’t know” responses were excluded from the analysis (n=2; 0.2%).
Exposure to SHS at work was assessed with the question: “In the last 30 days, have people smoked in indoor areas where you work?” With the following options: (1) “Yes”; (2) “No”. “Refused” and “Don’t know” responses were excluded from the analysis (n=1; 0.1%).
The question to assess SHS exposure in bars/pubs/discos and restaurants independently was: “The last time you visited [the setting], were people smoking inside?”. The options were: (1) “Yes”; (2) “No”. “Refused” and “Don’t know” responses were excluded from the analysis (n = 2; 0.2%).
We also gathered information about their self-perceived health (poor/fair, good, very good/excellent); self-reported chronic diseases, defined as (a) respiratory diseases: asthma, chronic obstructive pulmonary disease or emphysema, chronic bronchitis, tuberculosis; and (b) non-respiratory diseases: depression, anxiety, alcohol problems, chronic pain, diabetes, heart disease, lung cancer, other cancer and severe obesity.
Sociodemographic variables included age (<40, 40–54, >54), sex (male, female), educational level (low: ≤lower secondary; moderate: upper secondary to short cycle; high: bachelor's degree or higher) and children living at home (yes, no).
Statistical AnalysisWe conducted a descriptive bivariate analysis and estimated the crude self-reported cumulative incidence rates of COVID-19 per 100 respondents (%) over the 1.5-year follow-up period between the start of the pandemic in Spain (the first COVID-19 case was recorded on 31st of January 2020) and the Wave 3 survey in June 2021. Given the small sample sizes in the stratified analysis, we computed the relative standard errors (RSE) of the estimated incidence rates to identify estimates having high sampling variability (RSE>30%).
We estimated the crude and adjusted relative risks (RR) and their 95% confidence intervals (CI) of having had COVID-19 and identified its associated factors using multivariable Poisson regression with robust variance. We computed a model adjusted for sex, age and education, and another model adjusted for all the independent variables. In both cases, age was used as a continuous variable.
Sensitivity AnalysesGiven that having had COVID-19 relied on self-reported information, we conducted sensitivity analyses using different definitions of the outcome variable: first, having had a test (PCR, antigen or antibody test)+physician diagnosis+belief in having had COVID-19; second, having had a test (PCR, antigen or antibody test)+physician diagnosis; third, having a test only (PCR, antigen or antibody test).
All the analyses incorporated longitudinal sampling weights derived from the complex sampling design to assure the sample representability of the adult Spanish smoking population. We used longitudinal bootstrap weights from Wave 2 to Wave 3 for all the analysis. We used Stata v. 14 (Texas, USA) to perform all analyses and statistical significance was set at p<0.05. We endorsed the STROBE guidance for observational research23 (STROBE checklist is available in the Supplementary Materials).
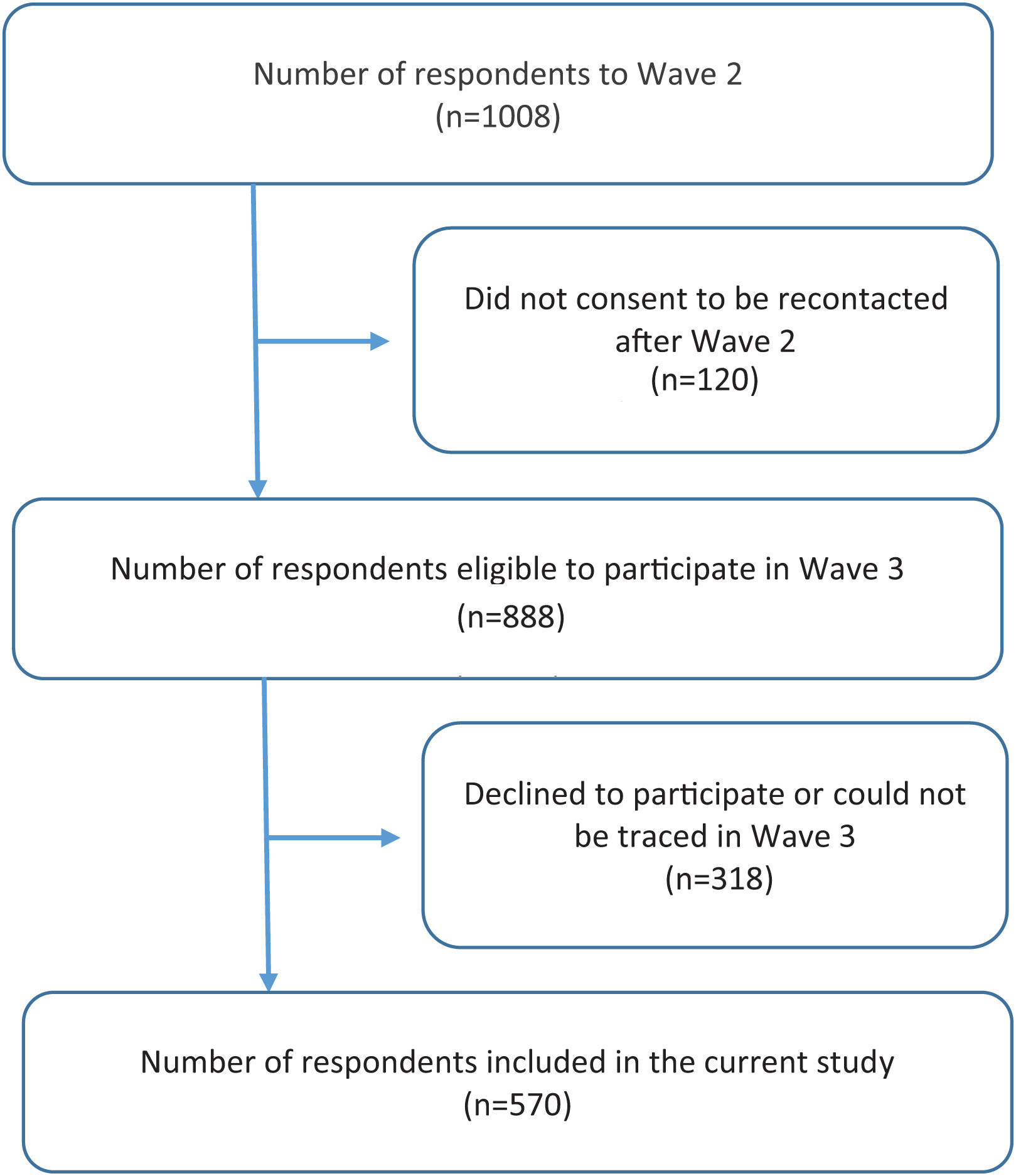
ResultsCharacteristics of ParticipantsOf 1008 respondents who participated in Wave 2 (interviewed between 12th of February and 14th of April 2018), 888 were eligible to participate in Wave 3 (i.e., they gave their consent to be recontacted in the future) and 570 (64.2%) effectively participated in Wave 3 (interviewed between 9th of June and 5th of August 2021; Fig. 1). The 570 respondents’ mean age was 43.1 years (standard deviation (SD): 13.9); 43.9 (SD: 14.3) in males and 43.0 (SD: 12.5) in females. There were no differences in sociodemographic, smoking or health-related characteristics between those who were followed up and those who were not (Supplementary Table S1).
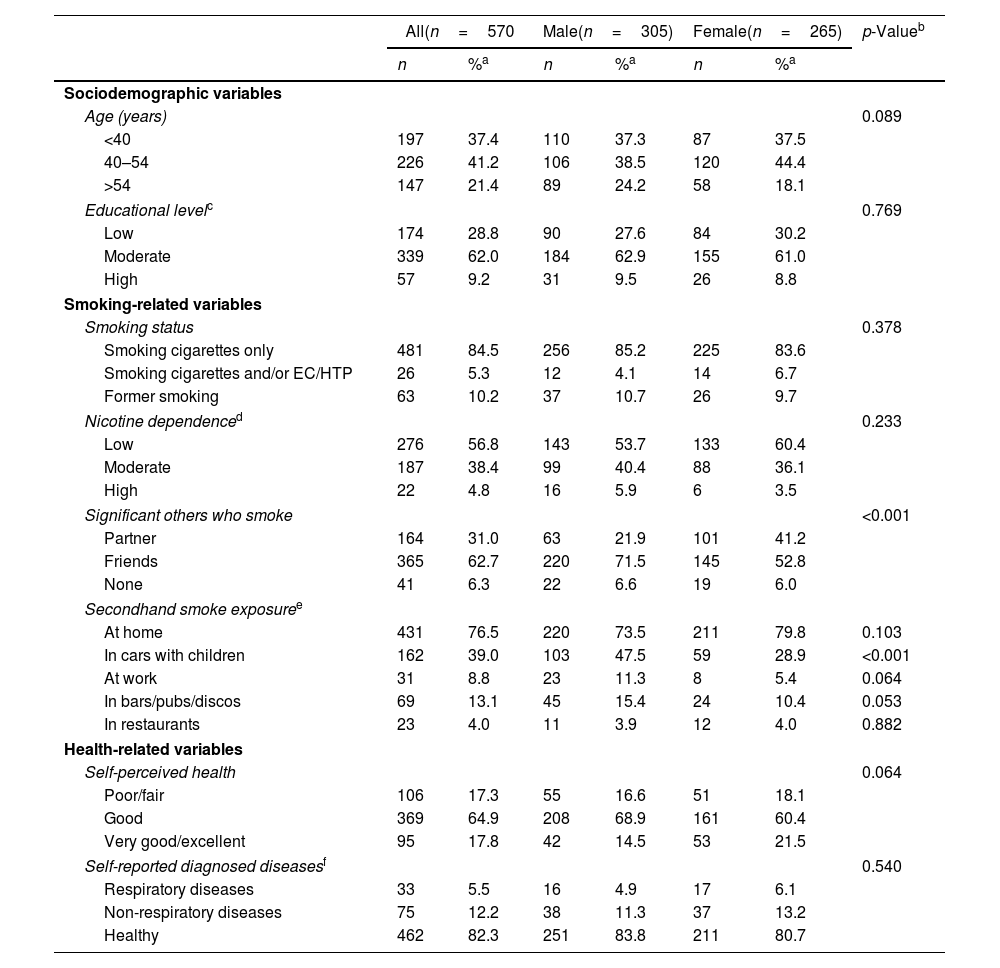
As shown in Table 1, there were no significant differences by sex according to most of the sociodemographic variables, smoking characteristics, and health-related variables. However, males reported having more friends who smoke (71.5%) than females (52.8%, p<0.001), and a greater percentage of females reported having partners who smoke (41.2%) than males (21.9%, p<0.001). Also, a greater percentage of males (47.5%) than females (28.9%, p<0.001) reported being exposed to SHS in cars with children (Table 1).
Characteristics of Respondents in 2018 by Sex. ITC EUREST-PLUS Spain Surveys, 2018–2021.
| All(n=570 | Male(n=305) | Female(n=265) | p-Valueb | ||||
|---|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | ||
| Sociodemographic variables | |||||||
| Age (years) | 0.089 | ||||||
| <40 | 197 | 37.4 | 110 | 37.3 | 87 | 37.5 | |
| 40–54 | 226 | 41.2 | 106 | 38.5 | 120 | 44.4 | |
| >54 | 147 | 21.4 | 89 | 24.2 | 58 | 18.1 | |
| Educational levelc | 0.769 | ||||||
| Low | 174 | 28.8 | 90 | 27.6 | 84 | 30.2 | |
| Moderate | 339 | 62.0 | 184 | 62.9 | 155 | 61.0 | |
| High | 57 | 9.2 | 31 | 9.5 | 26 | 8.8 | |
| Smoking-related variables | |||||||
| Smoking status | 0.378 | ||||||
| Smoking cigarettes only | 481 | 84.5 | 256 | 85.2 | 225 | 83.6 | |
| Smoking cigarettes and/or EC/HTP | 26 | 5.3 | 12 | 4.1 | 14 | 6.7 | |
| Former smoking | 63 | 10.2 | 37 | 10.7 | 26 | 9.7 | |
| Nicotine dependenced | 0.233 | ||||||
| Low | 276 | 56.8 | 143 | 53.7 | 133 | 60.4 | |
| Moderate | 187 | 38.4 | 99 | 40.4 | 88 | 36.1 | |
| High | 22 | 4.8 | 16 | 5.9 | 6 | 3.5 | |
| Significant others who smoke | <0.001 | ||||||
| Partner | 164 | 31.0 | 63 | 21.9 | 101 | 41.2 | |
| Friends | 365 | 62.7 | 220 | 71.5 | 145 | 52.8 | |
| None | 41 | 6.3 | 22 | 6.6 | 19 | 6.0 | |
| Secondhand smoke exposuree | |||||||
| At home | 431 | 76.5 | 220 | 73.5 | 211 | 79.8 | 0.103 |
| In cars with children | 162 | 39.0 | 103 | 47.5 | 59 | 28.9 | <0.001 |
| At work | 31 | 8.8 | 23 | 11.3 | 8 | 5.4 | 0.064 |
| In bars/pubs/discos | 69 | 13.1 | 45 | 15.4 | 24 | 10.4 | 0.053 |
| In restaurants | 23 | 4.0 | 11 | 3.9 | 12 | 4.0 | 0.882 |
| Health-related variables | |||||||
| Self-perceived health | 0.064 | ||||||
| Poor/fair | 106 | 17.3 | 55 | 16.6 | 51 | 18.1 | |
| Good | 369 | 64.9 | 208 | 68.9 | 161 | 60.4 | |
| Very good/excellent | 95 | 17.8 | 42 | 14.5 | 53 | 21.5 | |
| Self-reported diagnosed diseasesf | 0.540 | ||||||
| Respiratory diseases | 33 | 5.5 | 16 | 4.9 | 17 | 6.1 | |
| Non-respiratory diseases | 75 | 12.2 | 38 | 11.3 | 37 | 13.2 | |
| Healthy | 462 | 82.3 | 251 | 83.8 | 211 | 80.7 | |
EC: electronic cigarette; HTP: heated tobacco product.
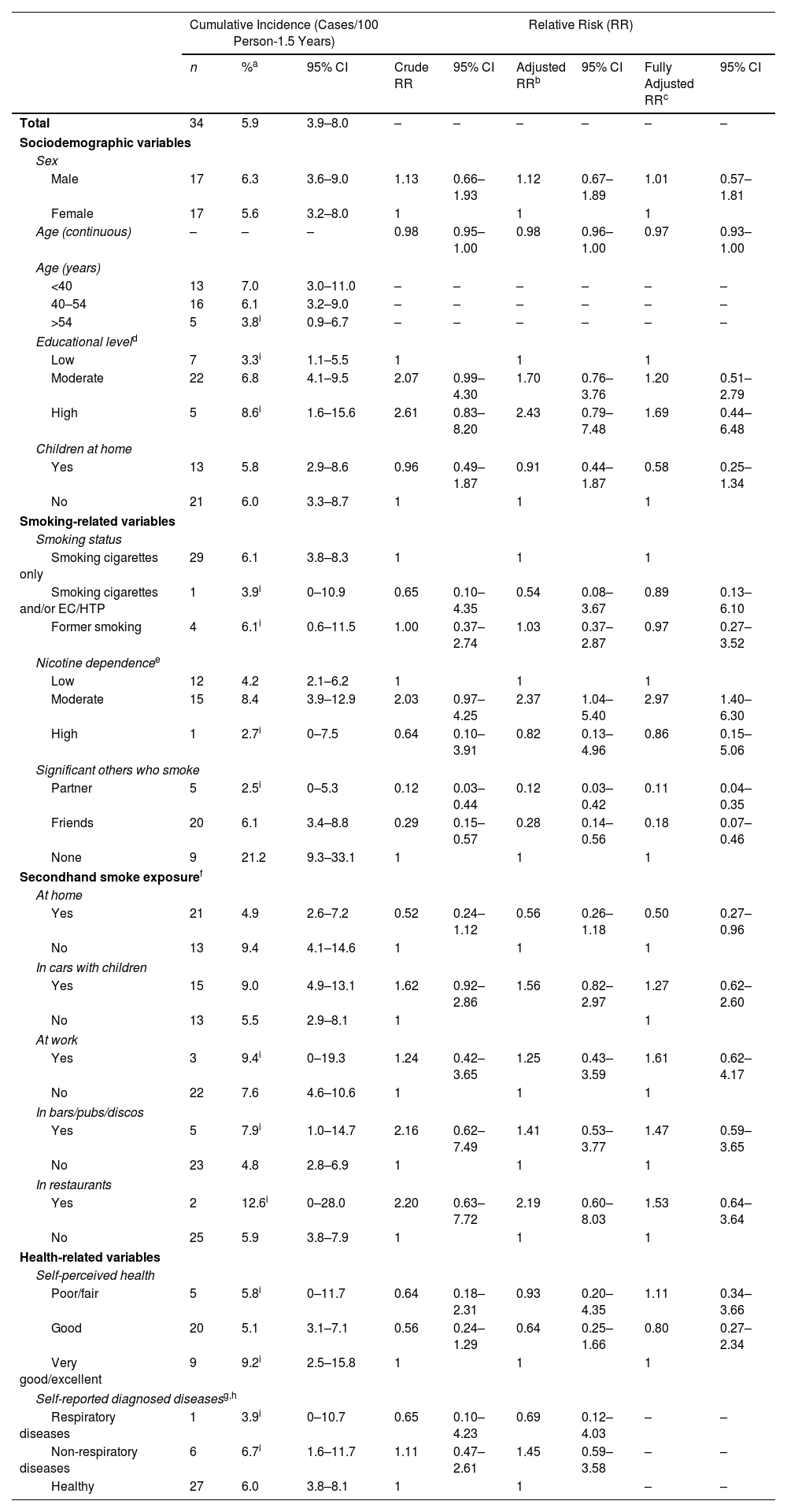
Among the 570 respondents who were followed up, 34 (5.9%; 95% CI: 3.9-8.0%) reported having had COVID-19, with no significant differences between males (6.3%; 95% CI: 3.6–9.0%) and females (5.6%; 95% CI: 3.2–8.0%; Table 2). COVID-19 cumulative incidence was significantly higher among those without a partner or friend who smoked (21.2%; 95% CI: 9.3–33.1%) compared with those with a partner who smoked (2.5%; 95% CI: 0–5.3%) and those with only friends who smoked (6.1%; 95% CI: 3.4–8.8%; Table 2).
Cumulative Incidence and Relative Risk of COVID-19 and Related Factors. ITC EUREST-PLUS Spain Surveys, 2018–2021.
| Cumulative Incidence (Cases/100 Person-1.5 Years) | Relative Risk (RR) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | %a | 95% CI | Crude RR | 95% CI | Adjusted RRb | 95% CI | Fully Adjusted RRc | 95% CI | |
| Total | 34 | 5.9 | 3.9–8.0 | – | – | – | – | – | – |
| Sociodemographic variables | |||||||||
| Sex | |||||||||
| Male | 17 | 6.3 | 3.6–9.0 | 1.13 | 0.66–1.93 | 1.12 | 0.67–1.89 | 1.01 | 0.57–1.81 |
| Female | 17 | 5.6 | 3.2–8.0 | 1 | 1 | 1 | |||
| Age (continuous) | – | – | – | 0.98 | 0.95–1.00 | 0.98 | 0.96–1.00 | 0.97 | 0.93–1.00 |
| Age (years) | |||||||||
| <40 | 13 | 7.0 | 3.0–11.0 | – | – | – | – | – | – |
| 40–54 | 16 | 6.1 | 3.2–9.0 | – | – | – | – | – | – |
| >54 | 5 | 3.8i | 0.9–6.7 | – | – | – | – | – | – |
| Educational leveld | |||||||||
| Low | 7 | 3.3i | 1.1–5.5 | 1 | 1 | 1 | |||
| Moderate | 22 | 6.8 | 4.1–9.5 | 2.07 | 0.99–4.30 | 1.70 | 0.76–3.76 | 1.20 | 0.51–2.79 |
| High | 5 | 8.6i | 1.6–15.6 | 2.61 | 0.83–8.20 | 2.43 | 0.79–7.48 | 1.69 | 0.44–6.48 |
| Children at home | |||||||||
| Yes | 13 | 5.8 | 2.9–8.6 | 0.96 | 0.49–1.87 | 0.91 | 0.44–1.87 | 0.58 | 0.25–1.34 |
| No | 21 | 6.0 | 3.3–8.7 | 1 | 1 | 1 | |||
| Smoking-related variables | |||||||||
| Smoking status | |||||||||
| Smoking cigarettes only | 29 | 6.1 | 3.8–8.3 | 1 | 1 | 1 | |||
| Smoking cigarettes and/or EC/HTP | 1 | 3.9i | 0–10.9 | 0.65 | 0.10–4.35 | 0.54 | 0.08–3.67 | 0.89 | 0.13–6.10 |
| Former smoking | 4 | 6.1i | 0.6–11.5 | 1.00 | 0.37–2.74 | 1.03 | 0.37–2.87 | 0.97 | 0.27–3.52 |
| Nicotine dependencee | |||||||||
| Low | 12 | 4.2 | 2.1–6.2 | 1 | 1 | 1 | |||
| Moderate | 15 | 8.4 | 3.9–12.9 | 2.03 | 0.97–4.25 | 2.37 | 1.04–5.40 | 2.97 | 1.40–6.30 |
| High | 1 | 2.7i | 0–7.5 | 0.64 | 0.10–3.91 | 0.82 | 0.13–4.96 | 0.86 | 0.15–5.06 |
| Significant others who smoke | |||||||||
| Partner | 5 | 2.5i | 0–5.3 | 0.12 | 0.03–0.44 | 0.12 | 0.03–0.42 | 0.11 | 0.04–0.35 |
| Friends | 20 | 6.1 | 3.4–8.8 | 0.29 | 0.15–0.57 | 0.28 | 0.14–0.56 | 0.18 | 0.07–0.46 |
| None | 9 | 21.2 | 9.3–33.1 | 1 | 1 | 1 | |||
| Secondhand smoke exposuref | |||||||||
| At home | |||||||||
| Yes | 21 | 4.9 | 2.6–7.2 | 0.52 | 0.24–1.12 | 0.56 | 0.26–1.18 | 0.50 | 0.27–0.96 |
| No | 13 | 9.4 | 4.1–14.6 | 1 | 1 | 1 | |||
| In cars with children | |||||||||
| Yes | 15 | 9.0 | 4.9–13.1 | 1.62 | 0.92–2.86 | 1.56 | 0.82–2.97 | 1.27 | 0.62–2.60 |
| No | 13 | 5.5 | 2.9–8.1 | 1 | 1 | ||||
| At work | |||||||||
| Yes | 3 | 9.4i | 0–19.3 | 1.24 | 0.42–3.65 | 1.25 | 0.43–3.59 | 1.61 | 0.62–4.17 |
| No | 22 | 7.6 | 4.6–10.6 | 1 | 1 | 1 | |||
| In bars/pubs/discos | |||||||||
| Yes | 5 | 7.9i | 1.0–14.7 | 2.16 | 0.62–7.49 | 1.41 | 0.53–3.77 | 1.47 | 0.59–3.65 |
| No | 23 | 4.8 | 2.8–6.9 | 1 | 1 | 1 | |||
| In restaurants | |||||||||
| Yes | 2 | 12.6i | 0–28.0 | 2.20 | 0.63–7.72 | 2.19 | 0.60–8.03 | 1.53 | 0.64–3.64 |
| No | 25 | 5.9 | 3.8–7.9 | 1 | 1 | 1 | |||
| Health-related variables | |||||||||
| Self-perceived health | |||||||||
| Poor/fair | 5 | 5.8i | 0–11.7 | 0.64 | 0.18–2.31 | 0.93 | 0.20–4.35 | 1.11 | 0.34–3.66 |
| Good | 20 | 5.1 | 3.1–7.1 | 0.56 | 0.24–1.29 | 0.64 | 0.25–1.66 | 0.80 | 0.27–2.34 |
| Very good/excellent | 9 | 9.2i | 2.5–15.8 | 1 | 1 | 1 | |||
| Self-reported diagnosed diseasesg,h | |||||||||
| Respiratory diseases | 1 | 3.9i | 0–10.7 | 0.65 | 0.10–4.23 | 0.69 | 0.12–4.03 | – | – |
| Non-respiratory diseases | 6 | 6.7i | 1.6–11.7 | 1.11 | 0.47–2.61 | 1.45 | 0.59–3.58 | – | – |
| Healthy | 27 | 6.0 | 3.8–8.1 | 1 | 1 | – | – | ||
CI: confidence interval; EC: electronic cigarettes; HTP: heated tobacco products; RR: relative risk.
Educational level: low: ≤lower secondary; moderate: upper secondary to short cycle; high: bachelor's degree or higher.
Assessed with the Heaviness of Smoking Index only among respondents who smoked daily: low (0–2), moderate (3–4), and high (5–6).
After adjusting for age, sex, and educational level, COVID-19 incidence was positively associated with moderate nicotine dependence compared with low dependence, with an adjusted RR (aRR) of 2.37 (95% CI 1.04–5.40), and was negatively associated with having a partner who smoked (aRR=0.12; 95% CI 0.03–0.42) and having not a partner, but friends who smoked (aRR=0.28; 95% CI 0.14–0.56) compared with not having a partner or friends who smoked. Further adjustment including all the independent variables confirmed the associations found.
We conducted a sensitivity analysis to assess the impact of different definitions of the COVID-19 occurrence on the estimated incidence rates as it was self-reported. When we considered the definition of COVID-19 occurrence as “having a positive test or having a physician diagnosis” (n=29), the cumulative incidence of COVID-19 was 4.9% (95% CI 3.1–6.8), and with the definition “only having a test” (n=16), the cumulative incidence was 2.7% (95% CI 1.2–4.2). No meaningful impact of the COVID-19 occurrence definition was observed in the adjusted RR estimates (Supplementary Table S2).
DiscussionIn our study, 5.9% of adults who smoke or had recently quit smoking reported having had COVID-19 within the 1.5-year period since the onset of the COVID-19 pandemic in Spain. Since then, Spain suffered five consecutive waves (with peaks in March 2020, October 2020, February 2021, April 2021, and July 2021). A cumulative incidence of 5.9% over the 1.5-year study period is equivalent to a cumulative incidence of 136 cases/100,000 person-14 days, i.e., close to the peak of 200 cases/100,000 person-14 days reached during the first wave in Spain and well below the peaks of the subsequent waves (between 600 and 1000 cases/100,000 person-14 days).24 Although these figures should be compared with caution, given that the cumulative incidence assumes a constant rate of disease occurrence over time, they could suggest that the 1.5-year cumulative incidence among people who smoke may be lower than the 14-day cumulative incidence in the general population at the time of the survey. Other studies have found a lower percentage of people who smoke among COVID-19 diagnosed cases than among the general population,25 a phenomenon that has been named the “smoking paradox”.26
The only factor positively associated with having had COVID-19 was moderate nicotine dependence. In contrast, the factor inversely associated with the cumulative incidence of COVID-19 was having a partner or friends who smoked. This finding is difficult to explain, but one possible hypothesis is that respondents who had partners who smoked increased their efforts to protect themselves from COVID-19. Similarly, respondents who reported only having friends who smoked may have had an increased awareness of their own risk and protected themselves from infection, for instance, by means of non-pharmacological practices, such as maintaining a safe social distance, wearing face masks, increasing indoor ventilation, or receiving COVID-19 vaccinations. Furthermore, no association was found between SHS exposure at home and COVID-19 incidence.
Considering the differences in SHS exposure in cars with children (male respondents reported higher exposure than females), we believe that developing intervention programs that bear these differences in mind is important to minimize the negative effects of the pandemic.27
Several potential limitations should be mentioned. First, since the COVID-19 incidence estimates are based on self-reported information, we cannot disregard a reporting bias from respondents who said they were not infected when they may have had mild symptoms or respondents who were more inclined to get tested because of their serious symptoms. For this reason, we performed a sensitivity analysis using three different definitions of a case (first, having had a test+physician diagnosis+belief in having had COVID-19; second, having had a test+physician's diagnosis; third, having had a test only). The analysis showed that the estimated RR of having had COVID-19 did not change, regardless of the lower number of cases when more restrictive criteria were applied. Furthermore, we believe that the risk of information bias is low, given the general awareness of the population of COVID-19 derived from the heavy impact of the pandemic in Spain and worldwide. Second, given that we do not know exactly when the respondents had COVID-19, we had to assume that the risk of infection was constant during the study period, although it clearly was not, given its seasonality, the existence of different variants of the virus, and vaccination uptake. Third, it is difficult to determine how self-reported SHS exposures during the period before the COVID-19 pandemic could be related to self-reported COVID-19 during the 1.5-year period after the onset of the COVID-19 pandemic. There is a possibility that smoking behavior changed between the surveys, and we cannot ignore the fact that some respondents who smoked in 2018 may have quit since then, either before or after having COVID-19. Given that the follow-up period includes five COVID-19 pandemic waves, such a bias would have a non-differential misclassification effect, thus biasing the RR estimates toward the null hypothesis. Fourth, attrition of the cohort could have biased the results if losses to follow-up were related with COVID-19. We do not know why some respondents were lost to follow-up, but assuming higher COVID-19 rates among Spanish residents, only 1 or 2 could have been infected. Given that the characteristics of those who were lost to follow-up did not differ from those who completed follow-up (Supplementary Table S1), selection bias is unlikely. Fifth, it is impossible to determine the extent of a respondent's exposure to SHS in different venues (bars, pubs, discos and restaurants) based on self-reported SHS exposure, since the respondent must have visited the particular venue and have seen someone smoking inside. Sixth, vaccine rollout had already begun by the time data were collected. Early on, the vaccine may have provided some protection from the variant circulating at the time, which could have influenced the results. Finally, the limited numbers of COVID-19 cases made it impossible to analyze other correlates of infection in depth.
Our focus on the incidence of COVID-19 among people who smoke is the main strength of this investigation. While other studies have analyzed information from subsets of cases detected in healthcare centers, we were able to study the cumulative incidence of COVID-19 among a nationally representative sample of people who smoke in Spain. Assessing the role of SHS exposure on COVID-19 was also considered very pertinent due to growing evidence of virus transmission through the exhalation of respiratory droplets.28 The potential of SARS-CoV-2 transmission in aerosols was the basis for Spanish COVID-19 “ad hoc” regulations29 and a ban on smoking in outdoor public places was instated that included outdoor terraces of bars and restaurants when a safe distance (set at 1.5m) could not be maintained. It should be noted, however, that this ban was only implemented in certain regions of Spain, with scant enforcement, and at a late stage of the pandemic.
In conclusion, the correlates of having had COVID-19 among people who smoke, i.e., who have moderate nicotine dependence (positive association) and among people with a partner who smoked or only had friends who smoked (negative association) should be considered when tailoring information and non-pharmacological preventive measures for people who smoke. In line with previous evidence,30 we endorse the potential of the COVID-19 pandemic as an opportunity to defeat nicotine addiction and promote national tobacco control programs to achieve a tobacco-free future. SHS exposure surveillance should be supported by proper coordination of tobacco control leaders and policy makers, in order to understand the impact of tobacco smoking in future outbreaks and epidemics.31
FundingThe EUREST-PLUS Spain Survey were partially funded by the European Union's Horizon 2020 research and innovation programme (Wave 2, grant 681109), the Instituto de Salud Carlos III (Wave 3, grant PI17/01338, co-funded by European Regional Development Fund (ERDF), a way to build Europe) and the Canadian Institutes of Health Research (Waves 2 and 3, grant FDN-148477). D. Carnicer-Pont, Y. Castellano, E. Fernández, M. Fu, and O. Tigova are partly supported by the Ministry of Universities and Research, Government of Catalonia (2021SGR00906). Additional support is provided by the Canadian Institutes of Health Research (FDN-148477) to A.C.K. Quah, P. Driezen, S.C. Kaai, and G.T. Fong for the work on this manuscript. Additional support to G.T. Fong is also provided by a Senior Investigator Grant from the Ontario Institute for Cancer Research (AI-004).
Authors’ ContributionsDCP: Conceptualization; methodology, writing – original draft, writing – review & editing, visualization.
MF: Conceptualization; methodology, writing – original draft, writing – review & editing, visualization, supervision, project administration, funding acquisition.
YC: Conceptualization; methodology, validation, formal analysis, writing – review & editing, visualization.
OT: Conceptualization; writing – review & editing, project administration.
PD: Methodology, validation, formal analysis, data curation, writing – review & editing.
ACKQ: Methodology, software, validation, investigation, resources, data curation, writing – review & editing, supervision, project administration.
SCK: Validation, investigation, writing – review & editing, supervision, project administration.
JBS: Conceptualization, writing – review & editing.
CIV: Conceptualization; investigation, writing – review & editing.
GTF: Conceptualization; methodology, validation, resources, data curation, writing – review & editing, supervision, project administration, funding acquisition.
EF: Conceptualization; methodology, formal analysis, writing – original draft, writing – review & editing, visualization, supervision, project administration, funding acquisition.
Conflicts of InterestG.T. Fong has been an expert witness or consultant for governments defending their country's policies or regulations in litigation. All other authors have no conflicts of interest to declare.
Data AvailabilityIn each country participating in the international Tobacco Control Policy Evaluation (ITC) Project, the data are jointly owned by the lead researcher(s) in that country and the ITC Project at the University of Waterloo. Requests for data used in this manuscript can be sent to: itc@uwaterloo.ca.
Artificial Intelligence InvolvementNo artificial intelligence was used in the production of any part of the manuscript.