Critically ill patients with COVID-19 are at increased risk of complications such as cardiovascular events, coagulation disorders, acute respiratory distress syndrome (ARDS), and nosocomial infections that could contribute to poorer clinical outcomes. It has been reported that the rate of nosocomial infections in critically ill COVID-19 patients ranges from 30 to 50%.1–3 A variety of factors have been suggested as contributors to this increased risk of developing nosocomial infections: an impaired immune system triggered by SARS-CoV-2 infection, encompassing phenomena such as immune hyper-response and inflammation; the increased risk of ARDS; the need for invasive ventilation; the use of empirical antibiotic therapy; and long ICU stays. Interestingly, recent studies found hyperglycemia to be a risk factor for adverse outcomes and mortality in severe COVID-19,4,5 but there is still a scarcity of data on the impact of hyperglycemia on the risk of nosocomial infections in critically ill COVID-19 patients. Thus, we aimed to investigate the association between hyperglycemia at ICU admission and the risk of nosocomial bacterial pneumonia in COVID-19 in the ICU setting, along with the risk of in-hospital mortality.
The CIBERESUCICOVID is a prospective, multicenter, and observational study that included consecutive adult patients admitted to 55 Spanish ICUs for severe COVID-19 between 5 February and 21 December 2021. Data was collected as previously described.6,7 Descriptive statistics were used for the basic features of the study data. Categorical variables were compared using the chi-squared test or Fisher's exact test, whereas continuous variables were compared using the non-parametric Mann–Whitney U test. We analyzed the association between hyperglycemia (serum glucose>126mg/dL)8 at ICU admission and nosocomial bacterial pneumonia9 by means of a mixed-effects multivariable model,10 defined by a binomial probability distribution and a logit link function, with centers as a random effect. To evaluate the effect of hyperglycemia on in-hospital mortality, a Fine-Gray competing risks model11 stratified on the centre variable was used, considering discharge from hospital as competing risk for mortality. The study received approval from the Institution's Internal Review Board (Comité Ètic d’Investigació Clínica, registry number HCB/2020/0370) and we obtained informed consent from either patients or their relatives.
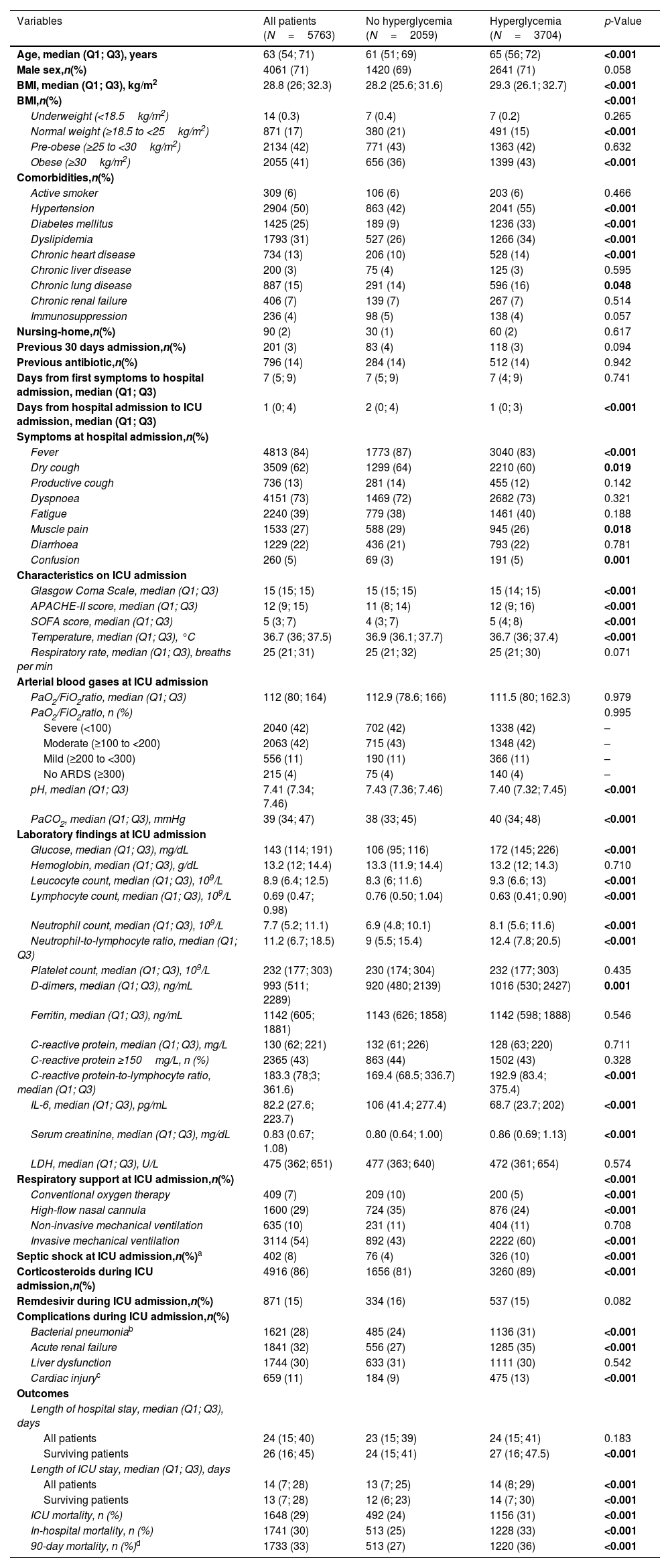
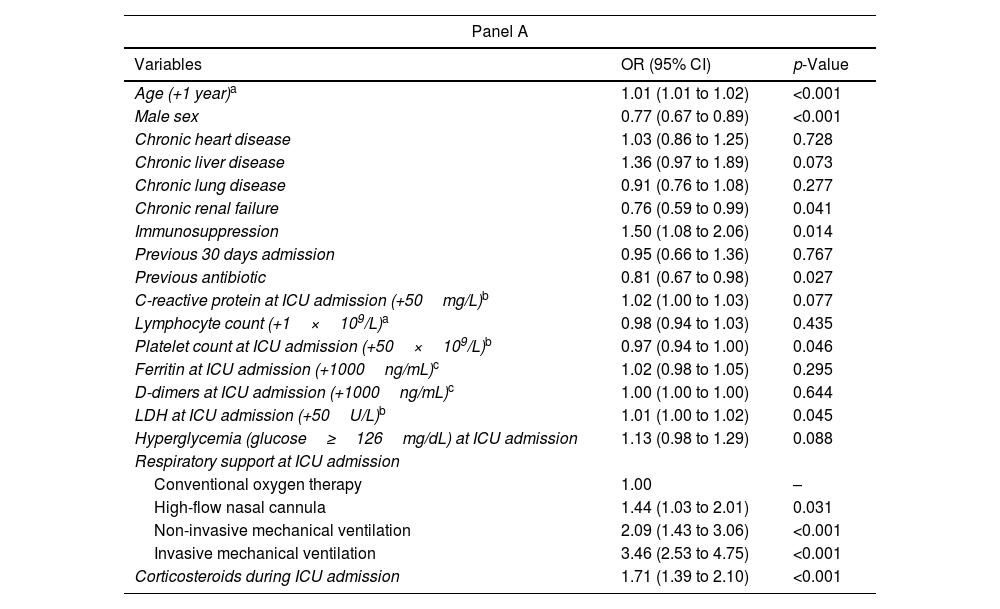
During the study period, 6225 patients were admitted to ICU in the 55 Spanish hospitals due to COVID-19. We included 5763 patients in this analysis; of these, 3704 (64%) presented hyperglycemia at ICU admission and 2059 (36%) did not. Baseline characteristics, clinical features, complications, and outcomes of the cohort according to the presence of hyperglycemia at ICU admission are shown in Table 1. Median age was 65 (56–72) years and 71% of patients were male. Hypertension (55%), obesity (43%), diabetes mellitus (33%), and chronic lung disease (16%) were the most frequent comorbidities. Patients were admitted to the ICU after a median of 74–9 days from the presentation of symptoms and of 1 (0–3) days from hospital admission. Patients with hyperglycemia were more frequently older and had more comorbidities (33% were diabetic, for example, compared to 9% of those not presenting hyperglycemia), and, overall, they presented more severe COVID-19 at ICU admission, as measured by the APACHE II and SOFA scores. Moreover, patients with hyperglycemia received corticosteroids more frequently and presented significantly more complications, e.g., a need for invasive mechanical ventilation, ARDS, acute renal failure, nosocomial pneumonia, and acute cardiac injury. The lengths of hospital and ICU stays were also significantly longer, and in-hospital, ICU, and 90-day mortality rates were significantly higher in patients with hyperglycemia. As regards the primary outcomes – namely rates of nosocomial bacterial pneumonia and in-hospital mortality, both were significantly higher in patients with hyperglycemia than in those without it (31% vs. 24% and 33% vs. 25%, respectively, both p<0.001). Several variables assessed at ICU admission were associated with an increased risk of developing nosocomial bacterial pneumonia in the multivariable analysis (Table 2, Panel A), notably male gender, older age, administration of antibiotics prior to ICU admission, mechanical ventilation, and corticosteroids, but hyperglycemia was not one of them. However, the factors associated with in-hospital mortality (Table 2, Panel B) did include hyperglycemia at ICU admission.
Demographic and clinical characteristics of the study population by hyperglycemia.
| Variables | All patients (N=5763) | No hyperglycemia (N=2059) | Hyperglycemia (N=3704) | p-Value |
|---|---|---|---|---|
| Age, median (Q1; Q3), years | 63 (54; 71) | 61 (51; 69) | 65 (56; 72) | <0.001 |
| Male sex,n(%) | 4061 (71) | 1420 (69) | 2641 (71) | 0.058 |
| BMI, median (Q1; Q3), kg/m2 | 28.8 (26; 32.3) | 28.2 (25.6; 31.6) | 29.3 (26.1; 32.7) | <0.001 |
| BMI,n(%) | <0.001 | |||
| Underweight (<18.5kg/m2) | 14 (0.3) | 7 (0.4) | 7 (0.2) | 0.265 |
| Normal weight (≥18.5 to <25kg/m2) | 871 (17) | 380 (21) | 491 (15) | <0.001 |
| Pre-obese (≥25 to <30kg/m2) | 2134 (42) | 771 (43) | 1363 (42) | 0.632 |
| Obese (≥30kg/m2) | 2055 (41) | 656 (36) | 1399 (43) | <0.001 |
| Comorbidities,n(%) | ||||
| Active smoker | 309 (6) | 106 (6) | 203 (6) | 0.466 |
| Hypertension | 2904 (50) | 863 (42) | 2041 (55) | <0.001 |
| Diabetes mellitus | 1425 (25) | 189 (9) | 1236 (33) | <0.001 |
| Dyslipidemia | 1793 (31) | 527 (26) | 1266 (34) | <0.001 |
| Chronic heart disease | 734 (13) | 206 (10) | 528 (14) | <0.001 |
| Chronic liver disease | 200 (3) | 75 (4) | 125 (3) | 0.595 |
| Chronic lung disease | 887 (15) | 291 (14) | 596 (16) | 0.048 |
| Chronic renal failure | 406 (7) | 139 (7) | 267 (7) | 0.514 |
| Immunosuppression | 236 (4) | 98 (5) | 138 (4) | 0.057 |
| Nursing-home,n(%) | 90 (2) | 30 (1) | 60 (2) | 0.617 |
| Previous 30 days admission,n(%) | 201 (3) | 83 (4) | 118 (3) | 0.094 |
| Previous antibiotic,n(%) | 796 (14) | 284 (14) | 512 (14) | 0.942 |
| Days from first symptoms to hospital admission, median (Q1; Q3) | 7 (5; 9) | 7 (5; 9) | 7 (4; 9) | 0.741 |
| Days from hospital admission to ICU admission, median (Q1; Q3) | 1 (0; 4) | 2 (0; 4) | 1 (0; 3) | <0.001 |
| Symptoms at hospital admission,n(%) | ||||
| Fever | 4813 (84) | 1773 (87) | 3040 (83) | <0.001 |
| Dry cough | 3509 (62) | 1299 (64) | 2210 (60) | 0.019 |
| Productive cough | 736 (13) | 281 (14) | 455 (12) | 0.142 |
| Dyspnoea | 4151 (73) | 1469 (72) | 2682 (73) | 0.321 |
| Fatigue | 2240 (39) | 779 (38) | 1461 (40) | 0.188 |
| Muscle pain | 1533 (27) | 588 (29) | 945 (26) | 0.018 |
| Diarrhoea | 1229 (22) | 436 (21) | 793 (22) | 0.781 |
| Confusion | 260 (5) | 69 (3) | 191 (5) | 0.001 |
| Characteristics on ICU admission | ||||
| Glasgow Coma Scale, median (Q1; Q3) | 15 (15; 15) | 15 (15; 15) | 15 (14; 15) | <0.001 |
| APACHE-II score, median (Q1; Q3) | 12 (9; 15) | 11 (8; 14) | 12 (9; 16) | <0.001 |
| SOFA score, median (Q1; Q3) | 5 (3; 7) | 4 (3; 7) | 5 (4; 8) | <0.001 |
| Temperature, median (Q1; Q3), °C | 36.7 (36; 37.5) | 36.9 (36.1; 37.7) | 36.7 (36; 37.4) | <0.001 |
| Respiratory rate, median (Q1; Q3), breaths per min | 25 (21; 31) | 25 (21; 32) | 25 (21; 30) | 0.071 |
| Arterial blood gases at ICU admission | ||||
| PaO2/FiO2ratio, median (Q1; Q3) | 112 (80; 164) | 112.9 (78.6; 166) | 111.5 (80; 162.3) | 0.979 |
| PaO2/FiO2ratio, n (%) | 0.995 | |||
| Severe (<100) | 2040 (42) | 702 (42) | 1338 (42) | – |
| Moderate (≥100 to <200) | 2063 (42) | 715 (43) | 1348 (42) | – |
| Mild (≥200 to <300) | 556 (11) | 190 (11) | 366 (11) | – |
| No ARDS (≥300) | 215 (4) | 75 (4) | 140 (4) | – |
| pH, median (Q1; Q3) | 7.41 (7.34; 7.46) | 7.43 (7.36; 7.46) | 7.40 (7.32; 7.45) | <0.001 |
| PaCO2, median (Q1; Q3), mmHg | 39 (34; 47) | 38 (33; 45) | 40 (34; 48) | <0.001 |
| Laboratory findings at ICU admission | ||||
| Glucose, median (Q1; Q3), mg/dL | 143 (114; 191) | 106 (95; 116) | 172 (145; 226) | <0.001 |
| Hemoglobin, median (Q1; Q3), g/dL | 13.2 (12; 14.4) | 13.3 (11.9; 14.4) | 13.2 (12; 14.3) | 0.710 |
| Leucocyte count, median (Q1; Q3), 109/L | 8.9 (6.4; 12.5) | 8.3 (6; 11.6) | 9.3 (6.6; 13) | <0.001 |
| Lymphocyte count, median (Q1; Q3), 109/L | 0.69 (0.47; 0.98) | 0.76 (0.50; 1.04) | 0.63 (0.41; 0.90) | <0.001 |
| Neutrophil count, median (Q1; Q3), 109/L | 7.7 (5.2; 11.1) | 6.9 (4.8; 10.1) | 8.1 (5.6; 11.6) | <0.001 |
| Neutrophil-to-lymphocyte ratio, median (Q1; Q3) | 11.2 (6.7; 18.5) | 9 (5.5; 15.4) | 12.4 (7.8; 20.5) | <0.001 |
| Platelet count, median (Q1; Q3), 109/L | 232 (177; 303) | 230 (174; 304) | 232 (177; 303) | 0.435 |
| D-dimers, median (Q1; Q3), ng/mL | 993 (511; 2289) | 920 (480; 2139) | 1016 (530; 2427) | 0.001 |
| Ferritin, median (Q1; Q3), ng/mL | 1142 (605; 1881) | 1143 (626; 1858) | 1142 (598; 1888) | 0.546 |
| C-reactive protein, median (Q1; Q3), mg/L | 130 (62; 221) | 132 (61; 226) | 128 (63; 220) | 0.711 |
| C-reactive protein ≥150mg/L, n (%) | 2365 (43) | 863 (44) | 1502 (43) | 0.328 |
| C-reactive protein-to-lymphocyte ratio, median (Q1; Q3) | 183.3 (78;3; 361.6) | 169.4 (68.5; 336.7) | 192.9 (83.4; 375.4) | <0.001 |
| IL-6, median (Q1; Q3), pg/mL | 82.2 (27.6; 223.7) | 106 (41.4; 277.4) | 68.7 (23.7; 202) | <0.001 |
| Serum creatinine, median (Q1; Q3), mg/dL | 0.83 (0.67; 1.08) | 0.80 (0.64; 1.00) | 0.86 (0.69; 1.13) | <0.001 |
| LDH, median (Q1; Q3), U/L | 475 (362; 651) | 477 (363; 640) | 472 (361; 654) | 0.574 |
| Respiratory support at ICU admission,n(%) | <0.001 | |||
| Conventional oxygen therapy | 409 (7) | 209 (10) | 200 (5) | <0.001 |
| High-flow nasal cannula | 1600 (29) | 724 (35) | 876 (24) | <0.001 |
| Non-invasive mechanical ventilation | 635 (10) | 231 (11) | 404 (11) | 0.708 |
| Invasive mechanical ventilation | 3114 (54) | 892 (43) | 2222 (60) | <0.001 |
| Septic shock at ICU admission,n(%)a | 402 (8) | 76 (4) | 326 (10) | <0.001 |
| Corticosteroids during ICU admission,n(%) | 4916 (86) | 1656 (81) | 3260 (89) | <0.001 |
| Remdesivir during ICU admission,n(%) | 871 (15) | 334 (16) | 537 (15) | 0.082 |
| Complications during ICU admission,n(%) | ||||
| Bacterial pneumoniab | 1621 (28) | 485 (24) | 1136 (31) | <0.001 |
| Acute renal failure | 1841 (32) | 556 (27) | 1285 (35) | <0.001 |
| Liver dysfunction | 1744 (30) | 633 (31) | 1111 (30) | 0.542 |
| Cardiac injuryc | 659 (11) | 184 (9) | 475 (13) | <0.001 |
| Outcomes | ||||
| Length of hospital stay, median (Q1; Q3), days | ||||
| All patients | 24 (15; 40) | 23 (15; 39) | 24 (15; 41) | 0.183 |
| Surviving patients | 26 (16; 45) | 24 (15; 41) | 27 (16; 47.5) | <0.001 |
| Length of ICU stay, median (Q1; Q3), days | ||||
| All patients | 14 (7; 28) | 13 (7; 25) | 14 (8; 29) | <0.001 |
| Surviving patients | 13 (7; 28) | 12 (6; 23) | 14 (7; 30) | <0.001 |
| ICU mortality, n (%) | 1648 (29) | 492 (24) | 1156 (31) | <0.001 |
| In-hospital mortality, n (%) | 1741 (30) | 513 (25) | 1228 (33) | <0.001 |
| 90-day mortality, n (%)d | 1733 (33) | 513 (27) | 1220 (36) | <0.001 |
Abbreviations: ICU indicates intensive care unit; Q1, first quartile; Q3, third quartile; BMI, body mass index; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; LDH, lactate dehydrogenase. Percentages calculated on non-missing data. p-Values marked in bold indicate numbers that are statistically significant on the 95% confidence limit.
Criteria for the Sepsis-3 definition of septic shock include vasopressor treatment and a lactate concentration >2mmol/L.
Clinically or radiologically diagnosed bacterial pneumonia managed with antimicrobials. Bacteriological confirmation was not required.
Multivariable models assessing predictors of bacterial pneumonia using mixed-effects regression analysis (Panel A) and predictors of in-hospital mortality using competing risks survival analysis (Panel B).
| Panel A | ||
|---|---|---|
| Variables | OR (95% CI) | p-Value |
| Age (+1 year)a | 1.01 (1.01 to 1.02) | <0.001 |
| Male sex | 0.77 (0.67 to 0.89) | <0.001 |
| Chronic heart disease | 1.03 (0.86 to 1.25) | 0.728 |
| Chronic liver disease | 1.36 (0.97 to 1.89) | 0.073 |
| Chronic lung disease | 0.91 (0.76 to 1.08) | 0.277 |
| Chronic renal failure | 0.76 (0.59 to 0.99) | 0.041 |
| Immunosuppression | 1.50 (1.08 to 2.06) | 0.014 |
| Previous 30 days admission | 0.95 (0.66 to 1.36) | 0.767 |
| Previous antibiotic | 0.81 (0.67 to 0.98) | 0.027 |
| C-reactive protein at ICU admission (+50mg/L)b | 1.02 (1.00 to 1.03) | 0.077 |
| Lymphocyte count (+1×109/L)a | 0.98 (0.94 to 1.03) | 0.435 |
| Platelet count at ICU admission (+50×109/L)b | 0.97 (0.94 to 1.00) | 0.046 |
| Ferritin at ICU admission (+1000ng/mL)c | 1.02 (0.98 to 1.05) | 0.295 |
| D-dimers at ICU admission (+1000ng/mL)c | 1.00 (1.00 to 1.00) | 0.644 |
| LDH at ICU admission (+50U/L)b | 1.01 (1.00 to 1.02) | 0.045 |
| Hyperglycemia (glucose≥126mg/dL) at ICU admission | 1.13 (0.98 to 1.29) | 0.088 |
| Respiratory support at ICU admission | ||
| Conventional oxygen therapy | 1.00 | – |
| High-flow nasal cannula | 1.44 (1.03 to 2.01) | 0.031 |
| Non-invasive mechanical ventilation | 2.09 (1.43 to 3.06) | <0.001 |
| Invasive mechanical ventilation | 3.46 (2.53 to 4.75) | <0.001 |
| Corticosteroids during ICU admission | 1.71 (1.39 to 2.10) | <0.001 |
| Panel B | ||
|---|---|---|
| Variables | sHR (95% CI) | p-Value |
| Age (+1 year)a | 1.06 (1.05 to 1.06) | <0.001 |
| Male sex | 0.85 (0.76 to 0.95) | 0.005 |
| Chronic heart disease | 1.17 (1.03 to 1.34) | 0.019 |
| Chronic liver disease | 1.07 (0.83 to 1.38) | 0.62 |
| Chronic lung disease | 1.23 (1.08 to 1.40) | 0.002 |
| Chronic renal failure | 1.56 (1.32 to 1.85) | <0.001 |
| Immunosuppression | 1.60 (1.28 to 2.00) | <0.001 |
| Previous 30 days admission | 1.42 (1.13 to 1.78) | 0.002 |
| C-reactive protein at ICU admission (+50mg/L)b | 1.01 (1.00 to 1.02) | 0.062 |
| Lymphocyte count (+1×109/L)a | 1.01 (0.98 to 1.03) | 0.64 |
| Platelet count at ICU admission (+50×109/L)b | 0.93 (0.91 to 0.96) | <0.001 |
| Ferritin at ICU admission (+1000ng/mL)c | 1.00 (0.97 to 1.03) | 0.96 |
| D-dimers at ICU admission (+1000ng/mL)c | 1.00 (1.00 to 1.01) | 0.005 |
| LDH at ICU admission (+50U/L)b | 1.02 (1.01 to 1.02) | <0.001 |
| Hyperglycemia (glucose≥126mg/dL) at ICU admission | 1.14 (1.03 to 1.27) | 0.016 |
| Bacterial pneumonia during ICU admission | 1.07 (0.96 to 1.20) | 0.20 |
| Respiratory support at ICU admission | ||
| Conventional oxygen therapy | 1.00 | – |
| High-flow nasal cannula | 0.85 (0.66 to 1.10) | 0.22 |
| Non-invasive mechanical ventilation | 1.38 (1.04 to 1.82) | 0.023 |
| Invasive mechanical ventilation | 1.59 (1.25 to 2.03) | <0.001 |
| Corticosteroids during ICU admission | 0.79 (0.67 to 0.93) | 0.004 |
| Remdesivir during ICU admission | 0.82 (0.71 to 0.94) | 0.006 |
Abbreviations: OR indicates odds ratio; CI, confidence interval; ICU, intensive care unit; LDH, lactate dehydrogenase; sHR indicates subdistribution hazard ratio. In Panel A data are shown as estimated ORs (95% CIs) of the explanatory variables in the bacterial pneumonia group and the p-value is based on the null hypothesis that all ORs relating to an explanatory variable equal unity (no effect). Area under the ROC curve, AUC=0.75 (95% CI 0.73–0.76). In Panel B data are shown as estimated sHRs (95% CIs) of the explanatory variables in the in-hospital mortality group and the p-value is based on the null hypothesis that all sHRs relating to an explanatory variable equal unity (no effect).
When we separately analyzed diabetic and non-diabetic patients according to the presence of hyperglycemia at ICU admission, we found that the prevalence of hyperglycemia amongst the former was 86.7%, as against 56.9% in the latter. Furthermore, hyperglycemia did not impact outcomes in patients with diabetes, who, overall, presented poorer outcomes than non-diabetic patients (Supplementary Tables 1 and 2). Moreover, when multivariable analyses of risk factors for bacterial pneumonia and in-hospital mortality were run for both groups separately, hyperglycemia was not found to predict either outcome in diabetic or non-diabetic patients (Supplementary Tables 3 and 4). A receiving operating curve showed a cut-off of 150mg/dL for hyperglycemia, allowing for a better discrimination of outcomes than 126mg/dL in the overall cohort (Supplementary Figure 1), although it did not predict bacterial pneumonia in the multivariable analysis (Supplementary Table 5).
The prevalence of hyperglycemia at ICU admission in our cohort was found to be strikingly high, even though only a quarter of the patients were diabetic. Moreover, although a higher percentage of patients in the hyperglycemia group received systemic corticosteroids, the vast majority of patients only started receiving corticosteroids once they had been admitted to the ICU – i.e., after hyperglycemia was detected. The most likely explanation is the systemic inflammation induced by COVID-19 itself, which has been widely demonstrated in several previous studies.12 However, we did not assess the percentage of non-diabetic patients who developed, during admission for COVID-19, de novo diabetes that persisted after discharge or until death. This omission prevented us from comparing our results with those of Cromer and colleagues,13 who recently found that diabetes diagnosed at the presentation of COVID-19 is associated with lower glucose but higher inflammatory markers and a greater likelihood of ICU admission, suggesting that stress hyperglycemia is a significant physiological mechanism. Interestingly, these authors found that 31.2% of their patients were diabetic – in line with our results –, whereas only 13.2% of their patients fulfilled the criteria for newly diagnosed diabetes at some point. Approximately half of these individuals experienced a regression of DM.13 Remarkably, however, although hyperglycemia was found to be a risk factor for in-hospital mortality in the overall cohort, only non-diabetic patients developed significantly poorer outcomes when presenting hyperglycemia at admission. This finding is crucial to the interpretation of our findings and the subsequent extraction of clinical conclusions, and it also presents the ability to predict mortality more accurately, with a higher cut-off than that of the traditional definition of hyperglycemia (i.e., 150mg/dL than 126mg/dL).
Interestingly, while the prevalence of bacterial pneumonia in our cohort was high overall, presentation of hyperglycemia did not predict bacterial pneumonia in the whole cohort or in patients with diabetes, whereas non-diabetic patients with hyperglycemia did have a significantly higher prevalence of bacterial pneumonia than those not presenting hyperglycemia at admission. The higher incidence of bacterial nosocomial pneumonia found in COVID-19 patients compared to other critically ill has mostly been linked to the long duration of invasive mechanical ventilation, the high incidence of ARDS, and immune-suppressive treatment.14 The non-diabetic patients in our cohort with hyperglycemia had higher rates of invasive mechanical ventilation and immune-suppressive treatment, but not ARDS. While the quality of evidence on the risk factors for ventilator-associated hospital-acquired pneumonia unrelated to COVID-19 is moderate-good, this is not the case for pneumonia not associated with the use of ventilators.15 Furthermore, systematic reviews and meta-analyses have consistently found corticosteroids to be a risk factor, along with invasive mechanical ventilation and diabetes, whereas this is not true of hyperglycemia.14 Further studies are needed to investigate the risk factors for developing nosocomial pneumonia, particularly in non-ventilated critically ill COVID-19 patients.
Our study is limited by the lack of data on antidiabetic therapy, disease baseline control in diabetics (e.g., glycosylated hemoglobin), and the rate of new diagnoses of diabetes during the index episode.
In summary, we found that, overall, patients presenting with hyperglycemia at ICU admission had more aggressive and severe COVID-19 (i.e., less time between hospital and ICU admissions), as well as poorer outcomes, including in-hospital mortality. Hyperglycemia appears to be a better predictor of poor outcomes in non-diabetic than in diabetic patients. Early detection and management of hyperglycemia is required in hospitalized COVID-19 patients.
Authors’ contributionsStudy concept and design: CC, AM, AT; data collection: CC, AM, AP, TC, AC; statistical analysis: AG; analysis and interpretation of data: CC, AM, JP, TC, AT; drafting of the manuscript: CC, AM, JP, AT; critical revision of the manuscript for important intellectual content: CC, AM, JP, AT; and study supervision: AT. AT had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. CIBERESUCICOVID consortium participated in data collection.
FundingThis study was supported by the Instituto de Salud Carlos III de Madrid (COV20/00110, ISCIII); Fondo Europeo de Desarrollo Regional (FEDER); “Una manera de hacer Europa”; and Centro de Investigación Biomédica En Red – Enfermedades Respiratorias (CIBERES). DdGC has received financial support from the Instituto de Salud Carlos III (Miguel Servet 2020: CP20/00041), co-funded by European Social Fund (ESF)/“Investing in your future”. CC received a grant from the Fondo de Investigación Sanitaria (PI19/00207), Instituto de Salud Carlos III, co-funded by the European Union.
Competing interestsThe authors declare that they have no competing interests.
We are indebted to all participating medical and nursing colleagues for their assistance and cooperation in this study.
Víctor D. Gumucio- Sanguino, Rafael Mañez: Hospital Universitario de Bellvitge, Barcelona. Jordi Solé-Violan, Felipe Rodríguez de Castro: Hospital Dr. Negrín, Las Palmas. Fernando Suarez-Sipmann: Hospital Universitario La Princesa, Madrid. Ruth Noemí Jorge García, María Mora Aznar: Hospital Nuestra Señora de Gracia, Zaragoza. Mateu Torres, María Martinez, Cynthia Alegre, Jordi Riera, Sofía Contreras: Hospital Universitari Vall d’Hebron, Barcelona. Jesús Caballero, Javier Trujillano, Montse Vallverdú, Miguel León, Mariona Badía, Begoña Balsera, Lluís Servià, Judit Vilanova, Silvia Rodríguez, Neus Montserrat, Silvia Iglesias, Javier Prados, Sula Carvalho, Mar Miralbés, Josman Monclou, Gabriel Jiménez, Jordi Codina, Estela Val, Pablo Pagliarani, Jorge Rubio, Dulce Morales, Andrés Pujol, Àngels Furro, Beatriz García, Gerard Torres, Javier Vengoechea, David de Gozalo Calvo, Jessica González, Silvia Gomez: Hospital Universitari Arnau de Vilanova, Lleida. José M. Gómez: Hospital General Universitario Gregorio Marañón, Madrid. Nieves Franco: Hospital Universitario de Móstoles, Madrid. José Barberán: Hospital Universitario HM Montepríncipe. Guillermo M. Albaiceta, Lorena Forcelledo Espina, Emilio García Prieto, Paula Martín Vicente, Cecilia del Busto Martínez: Hospital Universitario Central de Asturias, Oviedo. Pablo Vidal: Complexo Hospitalario Universitario de Ourense, Ourense. José Luis García Garmendia, María Aguilar Cabello, Carmen Eulalia Martínez Fernández: Hospital San Juan de Dios del Aljarafe, Sevilla. Nieves Carbonell, María Luisa Blasco Cortés, Ainhoa Serrano Lázaro, Mar Juan Díaz: Hospital Clínic Universitari de València, Valencia. Aaron Blandino Ortiz: Hospital Universitario Ramón y Cajal, Madrid. Rosario Menendez: Hospital La Fe de Valencia. Luis Jorge Valdivia: Hospital Universitario de León, León. María Victoria Boado: Hospital Universitario de Cruces, Barakaldo. Susana Sancho Chinesta: Hospital Universitario y Politécnico La Fe, Valencia. Maria del Carmen de la Torre: Hospital de Mataro. Ignacio Martínez Varela, María Teresa Bouza Vieiro, Inés Esmorís Arijón: Hospital Universitario Lucus Augusti, Lugo. David Campi Hermoso., Rafaela Nogueras Salinas, Teresa Farre Monjo, Ramon Nogue Bou, Gregorio Marco Naya, Carme Barberà, Núria Ramon Coll: Hospital Universitari de Santa Maria, Lleida. Mercedes Catalán-González, Juan Carlos Montejo-González: Hospital Universitario 12 de Octubre, Madrid. Gloria Renedo Sanchez-Giron, Juan Bustamante-Munguira, Elena Bustamante-Munguira, Ramon Cicuendez Avila, Nuria Mamolar Herrera: Hospital Clínico Universitario, Valladolid. Raquel Almansa: Instituto de Investigación Biomédica de Salamanca (IBSAL). Víctor Sagredo: Hospital Universitario de Salamanca, Salamanca. Jose Añon, Alexander Agrifoglio, Lucia Cachafeiro, Emilio Maseda: Hospital Universitario La Paz-Carlos III, Madrid. Lorenzo Socias, Mariana Andrea Novo, Albert Figueras, Maria Teresa Janer, Laura Soliva, Marta Ocón, Luisa Clar, J. Ignacio Ayestarán: Hospital Universitario Son Espases, Palma de Mallorca. Yhivian Peñasco, Sandra Campos Fernández: Hospital Universitario Marqués de Valdecilla, Santander. Mireia Serra-Fortuny, Eva Forcadell-Ferreres, Immaculada Salvador-Adell, Neus Bofill, Berta Adell-Serrano, Josep Pedregosa Díaz, Núria Casacuberta-Barberà, Luis Urrelo-Cerrón, Àngels Piñol-Tena, Ferran Roche-Campo: Hospital Verge de la Cinta de Tortosa, Tortosa. Amalia Martínez de la Gándara, Pablo Ryan Murúa, Covadonga Rodríguez Ruíz, Laura Carrión García, Juan I. Lazo Álvarez: Hospital Universitario Infanta Leonor,Madrid. José Ángel Lorente: Hospital Universitario de Getafe. Ana Loza-Vázquez, Desire Macias Guerrero: Hospital Universitario Virgen de Valme, Sevilla. Arturo Huerta, Daniel Tognetti: Clinica Sagrada Familia, Barcelona. Carlos García Redruello, David Mosquera Rodríguez, Eva María Menor Fernández, Sabela Vara Adrio, Vanesa Gómez Casal, Marta Segura Pensado, María Digna Rivas Vilas, Amaia García Sagastume: Hospital de Vigo, Vigo. Raul de Pablo Sánchez, David Pestaña Laguna, Tommaso Bardi: Hospital Universitario Ramón y Cajal, Madrid. Rosario Amaya Villar, Carmen Gómez Gonzalez, Maria Luisa Gascón Castillo: Hospital Universitario Virgen del Rocio, Sevilla. José Garnacho-Montero, María Luisa Cantón-Bulnes: Hospital Universitario Virgen Macarena, Sevilla. Judith Marin-Corral, Cristina Carbajales Pérez: Hospital Álvaro Cunqueiro, Vigo. Joan Ramon Masclans, Ana Salazar Degracia, Judit Bigas, Rosana Muñoz-Bermúdez, Clara Vilà-Vilardel, Francisco Parrilla, Irene Dot, Ana Zapatero, Yolanda Díaz, María Pilar Gracia, Purificación Pérez, Andrea Castellví, Cristina Climent: Hospital del Mar, Barcelona. Lidia Serra, Laura Barbena, Iosune Cano: Consorci Sanitari del Maresme, Barcelona. Pilar Ricart, Alba Herraiz, Pilar Marcos, Laura Rodríguez, Maria Teresa Sariñena, Ana Sánchez: Hospital Universitari Germans Trias i Pujol, Badalona. Alejandro Úbeda: Hospital Punta de Europa, Algeciras. María Cruz Martin Delgado: Hospital Universitario Torrejón-Universidad Francisco de Vitoria, Madrid. Elena Gallego, Juan Fernando Masa Jimenez: Hospital Universitario San Pedro de Alcántara, Cáceres. Gemma Gomà, Emi Díaz: Hospital Parc Taulí, Sabadell. Mercedes Ibarz, Diego De Mendoza: Hospital Universitari Sagrat Cor, Bacelona. Enric Barbeta, Victoria Alcaraz-Serrano, Joan Ramon Badia, Manuel Castella, Leticia Bueno, Adrian Ceccato, Andrea Palomeque, Laia Fernandez Barat, Catia Cillóniz, Pamela Conde, Javier Fernández, Albert Gabarrus, Karsa Kiarostami, Alexandre López- Gavín, Cecilia L. Mantellini, Carla Speziale, Nil Vázquez, Hua Yang, Minlan Yang, Carlos Ferrando, Pedro Castro, Marta Arrieta, Jose Maria Nicolas, Rut Andrea: Hospital Clinic, Barcelona. Marta Barroso, Raquel Pérez, Sergio Álvarez, Dario Garcia-Gasulla, Adrián Tormos: Barcelona supercomputing Center, Barcelona. Luis Tamayo Lomas, Cesar Aldecoa, Rubén Herrán-Monge, José Ángel Berezo García, Pedro Enríquez Giraudo: Hospital Rio Hortega, Valladolid. Pablo Cardinal Fernández, Alberto Rubio López, Orville Báez Pravia: Hospitales HM, Madrid. Juan López Messa, Leire Pérez Bastida, Antonjo Alvarez Ruiz: Complejo Asistencial Universitario de Palencia, Palencia. José Trenado, Anna Parera Pous: Hospital Universitari MutuaTerrassa, Terrassa. Cristóbal Galbán, Ana López Lago, Eva Saborido Paz, Patricia Barral Segade: Hospital de Santiago de Compostela, Santiago. Ana Balan Mariño, Manuel Valledor Mendez: Hospital San Agustin, Aviles. Raúl de Frutos, Luciano Aguilera: Hospital Basurto, Basurto. Felipe Pérez-García, Esther López-Ramos, Ángela Leonor Ruiz-García, Belén Beteré: Hospital Universitario Principe Asturias, Alcala de Henares. Rafael Blancas: Hospital Universitario del Tajo, Aranjuez. Cristina Dólera, Gloria Perez Planelles, Enrique Marmol Peis, Maria Dolores Martinez Juan, Miriam Ruiz Miralles, Eva Perez Rubio, Maria Van der Hofstadt Martin-Montalvo, Ángel Sánchez-Miralles, Tatiana Villada Warrington: Hospital Universitario Sant Joan d’Alacant, Alicante. Juan Carlos Pozo-Laderas: Hospital Universitario Reina Sofia. Angel Estrella, Sara Guadalupe Moreno Cano: Hospital de Jerez, Jerez. Federico Gordo: Hospital Universitario del Henares, Coslada. Basilisa Martinez Palacios: Hospital Universitario Infanta Cristina, Parla. Maite Nieto, Maria Teresa Nieto: Hospital de Segovia, Segovia. Sergio Ossa: Hospital de Burgos, Burgos. Ana Ortega: Hospital Montecelo, Pontevedra. Miguel Sanchez: Hospital Clinico, Madrid. Bitor Santacoloma: Hospital Galdakao, Galdakao.