Patients with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) experience impaired health-related quality of life (HRQL). The objective of this study was to evaluate HRQL in a nation-wide sample.
MethodsThis is a prospective, multicenter, non-interventional study of HRQL including 139 (89%) PAH and 17 (11%) CTEPH patients (women 70.5%; mean age, 52.2) recruited from 21 Spanish hospitals. 55% had idiopathic PAH, 34% other PAH and 11% CTEPH. HRQL was measured using the Short Form 36 Health Survey (SF-36) and EuroQoL-5D (baseline and after 6 months).
ResultsHRQL in the patients with PAH or CTEPH was impaired. The physical component of SF-36 and the EuroQol-5D correlated with the functional class (FC). Mean EuroQol-5D visual analogical scale (EQ-5D VAS) scores were 73.5±18.4, 62.9±20.7 and 51.3±16.0 (P<.0001) in patients with FC I, II and III, respectively. Every increase of one FC represented a loss of 4.0 on the PCS SF-36 and a loss of 9.5 on the EQ-5D VAS. Eight patients who died or received a transplant during the study period presented poorer initial HRQL compared with the rest of the population. No significant changes in HRQL were observed in survivors after 6 months of follow-up.
ConclusionsHRQL is impaired in this population, especially in PAH/CTEPH patients near death. HRQL measurements could help predict the prognosis in PAH and CTPH and provide additional information in these patients.
La hipertensión arterial pulmonar (HAP) y la hipertensión pulmonar tromboembólica crónica (HPTEC) provocan una reducción en la calidad de vida relacionada con la salud (CVRS). El objetivo del estudio fue evaluar la CVRS en una muestra nacional.
Pacientes y métodosEstudio prospectivo y no intervencionista en 139 (89%) pacientes con HAP y 17 (11%) pacientes con HPTEC (mujeres, 70,5%; edad media, 52,2 años) reclutados en 21 hospitales españoles. El 55% presentaban HAP idiopática, el 34% otras HAP y el 11%, HPTEC. La CVRS se midió utilizando los cuestionarios Short Form 36 (SF-36) y EuroQoL-5D (inicial y 6meses).
ResultadosLa CVRS en pacientes con HAP o HPTEC está deteriorada. El componente físico del SF-36 y el EuroQol-5D se correlacionó con la clase funcional. Las puntuaciones medias de la escala visual analógica del EuroQol-5D (EQ-5D EVA) fueron 73,5±18,4, 62,9±20,7 y 51,3±16,0 (p<0,0001) en pacientes con una clase funcional (CF) I, II y III, respectivamente. Cada aumento de una CF representa una pérdida de 4.0 en el PCS SF-36 y de 9,5 en el EQ-5D EVA. En 8pacientes que murieron o recibieron un trasplante durante el estudio, la CVRS fue peor al inicio, comparado con el resto. No se observaron cambios significativos en la CVRS en los supervivientes tras 6meses de seguimiento.
ConclusionesLa CVRS está deteriorada en esta población, especialmente en los pacientes con HAP/HPTEC al borde de la muerte. Las mediciones de CVRS ayudarían a predecir el pronóstico en la HAP y la HPTEC, así como proporcionar información adicional en estos pacientes.
The term pulmonary arterial hypertension (PAH) refers to a group of incurable disorders associated with a progressive increase in pulmonary vascular resistance (PVR), reduced exercise capacity and an end characterized by right ventricular failure and premature death.1 Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare and possibly underdiagnosed disease, with probably similar behavior when pulmonary endarterectomy is not feasible. Two decades ago, it was observed that the mean survival in untreated PAH was 2.8 years after diagnosis.2 However, in the last 15 years and after extensive research with over 25 multicenter clinical trials and two meta-analyses, survival benefits have been demonstrated with new treatments.3,4 The primary objective in most of these trials was exercise capacity, while HRQL was included as a secondary endpoint in only a few.5–7 Since new treatments have led to CTEPH and PAH becoming “manageable chronic diseases”, improvements in HRQL are increasingly important for physicians and patients when evaluating their impact.6,8 Measuring the HRQL is also useful for evaluating the cost-benefit.9 Aside from some clinical trials, there are few studies on HRQL in PAH10,11 or CTEPH.12–14 It is known that, compared to healthy individuals, patients with PAH have reduced physical mobility, lower energy levels, increased pain and greater social isolation.12
The aim of this multicenter, non-interventional, descriptive study was to prospectively evaluate HRQL in a cohort of Spanish patients diagnosed with PAH or CTEPH in an unselected population from hospitals of different sizes and expertise. As a further objective, it was also proposed to determine which factors influence HRQL in these patients.
Patients and MethodsStudy DesignThis was a prospective, non-interventionist, descriptive study conducted in 21 Spanish hospitals which recruited at least five consecutive outpatients diagnosed with PAH or CTEPH in February 2008. Participating centers were selected with the requirement to follow up at least five patients per month. Recruitment in the participating centers and data collection for analysis were carried out by Pfizer. Participating centers (see Appendix A) included all the most active hospitals according to the Spanish Register of Pulmonary Arterial Hypertension (REHAP). Data analysis and interpretation were performed independently by the authors. The study included two visits, one at the beginning (baseline) and another six months later. The investigators’ center was made aware of the recruitment period two weeks in advance, in an attempt to avoid any bias, and to be able to recruit consecutive and not selected PAH or CTEPH outpatients.
Inclusion CriteriaInclusion criteria: consecutive outpatients aged ≥18 years old, with written informed consent and a diagnosis of pulmonary hypertension (PH), belonging to the PH classification group. The definition of PH used in this study was mean pulmonary arterial pressure (mPAP)≥25mmHg at rest, pulmonary capillary wedge pressure (PCWP) 15mmHg and PVR>3 Wood units. The availability of diagnostic hemodynamic data was one of the inclusion criteria.
Exclusion CriteriaPatients with PH who did not belong to groups I or IV, patients who were unable to read and understand the HRQL questionnaires, and those who did not agree to participate in the study.
EvaluationsHRQL was evaluated at baseline and after 6 months using the self-administered Short Form 36 and EuroQoL-5D questionnaires. The HRQL results were analyzed according to the FC, 6MWT, type of disease, sex, age (<50 years; ≥50 years), time since PAH diagnosis, clinical status, patients who worsened/did not worsen from baseline, patients who died/received a transplant (or not) during the 6 months of the study and those who received different PH treatments (oral treatment alone compared to non-oral treatment) at baseline and after 6 months. Clinical worsening was defined as any of the following situations: (1) death, lung transplant or hospitalization due to disease worsening; (2) worsening of the functional class (FC); (3) any treatment changes including dosage increases or change of drug due to clinical impression of worsening.
This study met all the ethical considerations of the Declaration of Helsinki (1964) and subsequent amendments. All information recorded was obtained following standard clinical guidelines and patients were not exposed to any therapeutic or diagnostic experiments. The study followed standard safety and confidentiality measures, fully complying with Spanish legislation on data protection (Organic Law 15/99). The Hospital 12 de Octubre (Madrid) clinical research ethics committee approved this study as reference committee.
Statistical AnalysisDescriptive statistics were used for all variables, including measures of central tendency and dispersion for quantitative variables, and relative and absolute frequencies for qualitative variables, with 95% confidence intervals in both cases. The distribution type of the quantitative variables was studied and their adjustment to a Gaussian distribution was assessed using the Kolmogorov–Smirnov test. Student's t-test for quantitative variables was used to compare the independent data of the descriptive variables. The chi-squared or Mantel–Haenszel test (in the case of analysis groups with ordered categories) was used for qualitative variables. Student's t-test was used for the quantitative variables in the case of paired data analyses (final scores versus baseline scores on the scales). To compare changes in the scores of the scales between independent data from the different groups, analysis of covariance (ANCOVA) was carried out, adjusted for the baseline values. A multiple linear regression model was performed to determine which factors could potentially influence the HRQL measurements. Tests were two-sided with a 5% significance level. Data were analyzed using the statistical software package SAS® 8.2 (SAS Institute, Cary, NC).
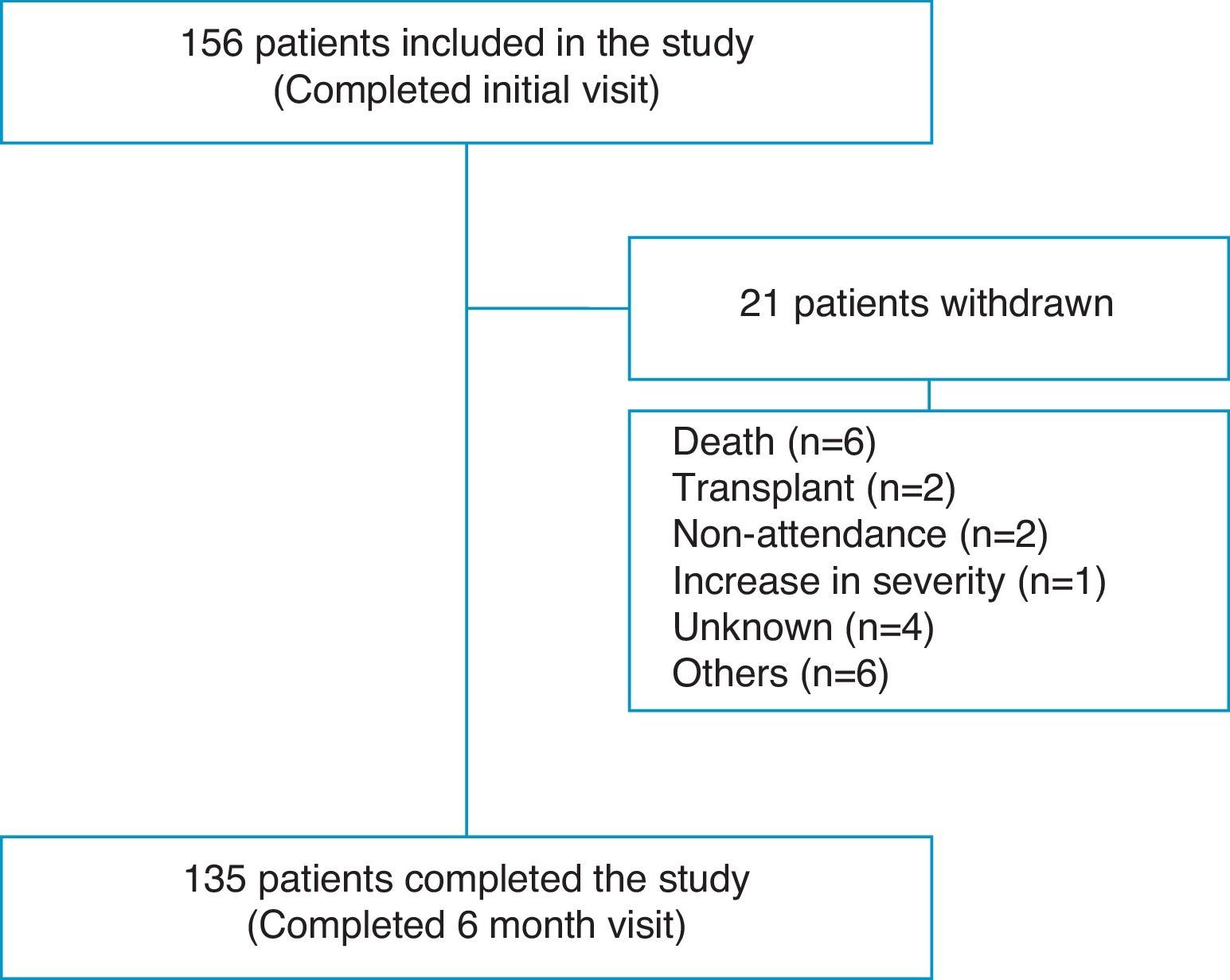
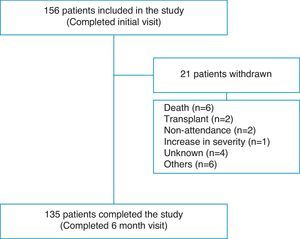
ResultsPatient Flow and Initial DemographyOne hundred fifty-six patients completed the evaluation at the baseline visit and 135 (86.5%) completed the end of study visit after 6 months (Fig. 1). Overall, 70.5% were women, with a mean age of 52.2 (17.2) years. The diagnostic categories were as follows: 139 (89.2%) PAH and 17 (10.8%) CTEPH. Seventy-six patients (48.7%) had idiopathic PAH (IPAH); 5 (3.2%) hereditary PAH; 5 (3.2%) drugs or toxins-associated PAH; 2 (1.2%) veno-occlusive disease; 23 (14.7%) connective tissue disease-associated PAH; 8 (5.1%) congenital heart disease-associated PAH; 9 (5.7%) portopulmonary hypertension; 6 (3.8%) human immunodeficiency virus-associated PAH, and 17 (10.8%) CTEPH. Twenty percent of patients were diagnosed in the 12 months prior to the start of the study and 80% were diagnosed earlier. During the 6-month follow-up, 8 (5.1%) patients died or received a lung transplant. A further 13 patients did not complete the study for various reasons, as shown in Table 1.
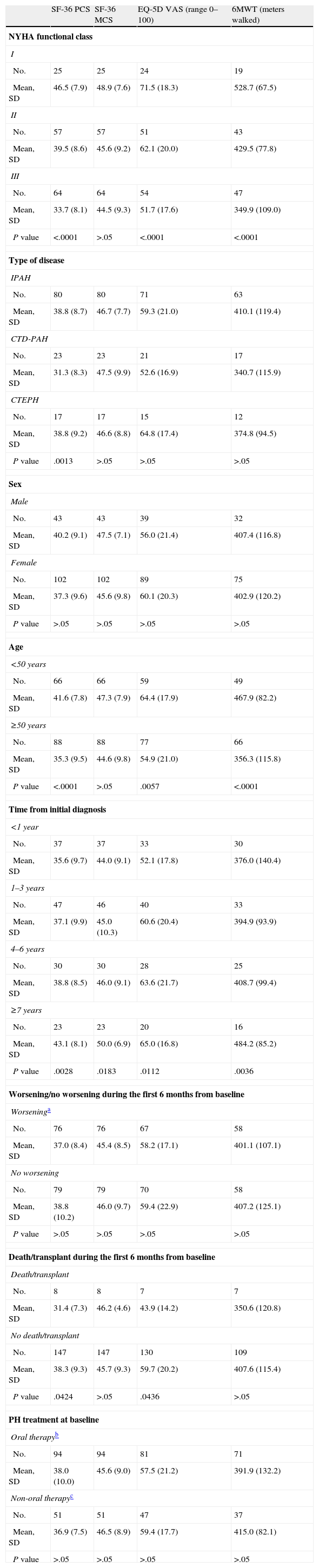
Scores for SF-36, EQ-5D Visual Analogue Scale (VAS) and 6-Minute Walk Test (6MWT), Stratified According to the Baseline Characteristics of the Population.
| SF-36 PCS | SF-36 MCS | EQ-5D VAS (range 0–100) | 6MWT (meters walked) | |
| NYHA functional class | ||||
| I | ||||
| No. | 25 | 25 | 24 | 19 |
| Mean, SD | 46.5 (7.9) | 48.9 (7.6) | 71.5 (18.3) | 528.7 (67.5) |
| II | ||||
| No. | 57 | 57 | 51 | 43 |
| Mean, SD | 39.5 (8.6) | 45.6 (9.2) | 62.1 (20.0) | 429.5 (77.8) |
| III | ||||
| No. | 64 | 64 | 54 | 47 |
| Mean, SD | 33.7 (8.1) | 44.5 (9.3) | 51.7 (17.6) | 349.9 (109.0) |
| P value | <.0001 | >.05 | <.0001 | <.0001 |
| Type of disease | ||||
| IPAH | ||||
| No. | 80 | 80 | 71 | 63 |
| Mean, SD | 38.8 (8.7) | 46.7 (7.7) | 59.3 (21.0) | 410.1 (119.4) |
| CTD-PAH | ||||
| No. | 23 | 23 | 21 | 17 |
| Mean, SD | 31.3 (8.3) | 47.5 (9.9) | 52.6 (16.9) | 340.7 (115.9) |
| CTEPH | ||||
| No. | 17 | 17 | 15 | 12 |
| Mean, SD | 38.8 (9.2) | 46.6 (8.8) | 64.8 (17.4) | 374.8 (94.5) |
| P value | .0013 | >.05 | >.05 | >.05 |
| Sex | ||||
| Male | ||||
| No. | 43 | 43 | 39 | 32 |
| Mean, SD | 40.2 (9.1) | 47.5 (7.1) | 56.0 (21.4) | 407.4 (116.8) |
| Female | ||||
| No. | 102 | 102 | 89 | 75 |
| Mean, SD | 37.3 (9.6) | 45.6 (9.8) | 60.1 (20.3) | 402.9 (120.2) |
| P value | >.05 | >.05 | >.05 | >.05 |
| Age | ||||
| <50 years | ||||
| No. | 66 | 66 | 59 | 49 |
| Mean, SD | 41.6 (7.8) | 47.3 (7.9) | 64.4 (17.9) | 467.9 (82.2) |
| ≥50 years | ||||
| No. | 88 | 88 | 77 | 66 |
| Mean, SD | 35.3 (9.5) | 44.6 (9.8) | 54.9 (21.0) | 356.3 (115.8) |
| P value | <.0001 | >.05 | .0057 | <.0001 |
| Time from initial diagnosis | ||||
| <1 year | ||||
| No. | 37 | 37 | 33 | 30 |
| Mean, SD | 35.6 (9.7) | 44.0 (9.1) | 52.1 (17.8) | 376.0 (140.4) |
| 1–3 years | ||||
| No. | 47 | 46 | 40 | 33 |
| Mean, SD | 37.1 (9.9) | 45.0 (10.3) | 60.6 (20.4) | 394.9 (93.9) |
| 4–6 years | ||||
| No. | 30 | 30 | 28 | 25 |
| Mean, SD | 38.8 (8.5) | 46.0 (9.1) | 63.6 (21.7) | 408.7 (99.4) |
| ≥7 years | ||||
| No. | 23 | 23 | 20 | 16 |
| Mean, SD | 43.1 (8.1) | 50.0 (6.9) | 65.0 (16.8) | 484.2 (85.2) |
| P value | .0028 | .0183 | .0112 | .0036 |
| Worsening/no worsening during the first 6 months from baseline | ||||
| Worseninga | ||||
| No. | 76 | 76 | 67 | 58 |
| Mean, SD | 37.0 (8.4) | 45.4 (8.5) | 58.2 (17.1) | 401.1 (107.1) |
| No worsening | ||||
| No. | 79 | 79 | 70 | 58 |
| Mean, SD | 38.8 (10.2) | 46.0 (9.7) | 59.4 (22.9) | 407.2 (125.1) |
| P value | >.05 | >.05 | >.05 | >.05 |
| Death/transplant during the first 6 months from baseline | ||||
| Death/transplant | ||||
| No. | 8 | 8 | 7 | 7 |
| Mean, SD | 31.4 (7.3) | 46.2 (4.6) | 43.9 (14.2) | 350.6 (120.8) |
| No death/transplant | ||||
| No. | 147 | 147 | 130 | 109 |
| Mean, SD | 38.3 (9.3) | 45.7 (9.3) | 59.7 (20.2) | 407.6 (115.4) |
| P value | .0424 | >.05 | .0436 | >.05 |
| PH treatment at baseline | ||||
| Oral therapyb | ||||
| No. | 94 | 94 | 81 | 71 |
| Mean, SD | 38.0 (10.0) | 45.6 (9.0) | 57.5 (21.2) | 391.9 (132.2) |
| Non-oral therapyc | ||||
| No. | 51 | 51 | 47 | 37 |
| Mean, SD | 36.9 (7.5) | 46.5 (8.9) | 59.4 (17.7) | 415.0 (82.1) |
| P value | >.05 | >.05 | >.05 | >.05 |
CTEPH, chronic thromboembolic pulmonary hypertension; CTD-PAH, connective tissue disease-associated PAH; IPAH, idiopathic pulmonary arterial hypertension.
The FC of the 152 patients who were seen initially was distributed as follows: FC I, 25 (16.4%); FC II, 58 (38.2%); FC III, 64 (42.1%) and FC IV, 5 (3.3%). The functional class at baseline for the 21 patients who did not complete the six-month visit was as follows: FC I, 2; FC II, 7; FC III 9 and FC IV 2 patients. Eight of these 21 patients died or received a transplant during the study period and presented the following baseline FC: FC II 2; FC III 4 and FC IV 2 patients. The 6MWT was 404.1 (115.9) m and 426 (112.5) m at baseline and after 6 months follow-up, respectively.
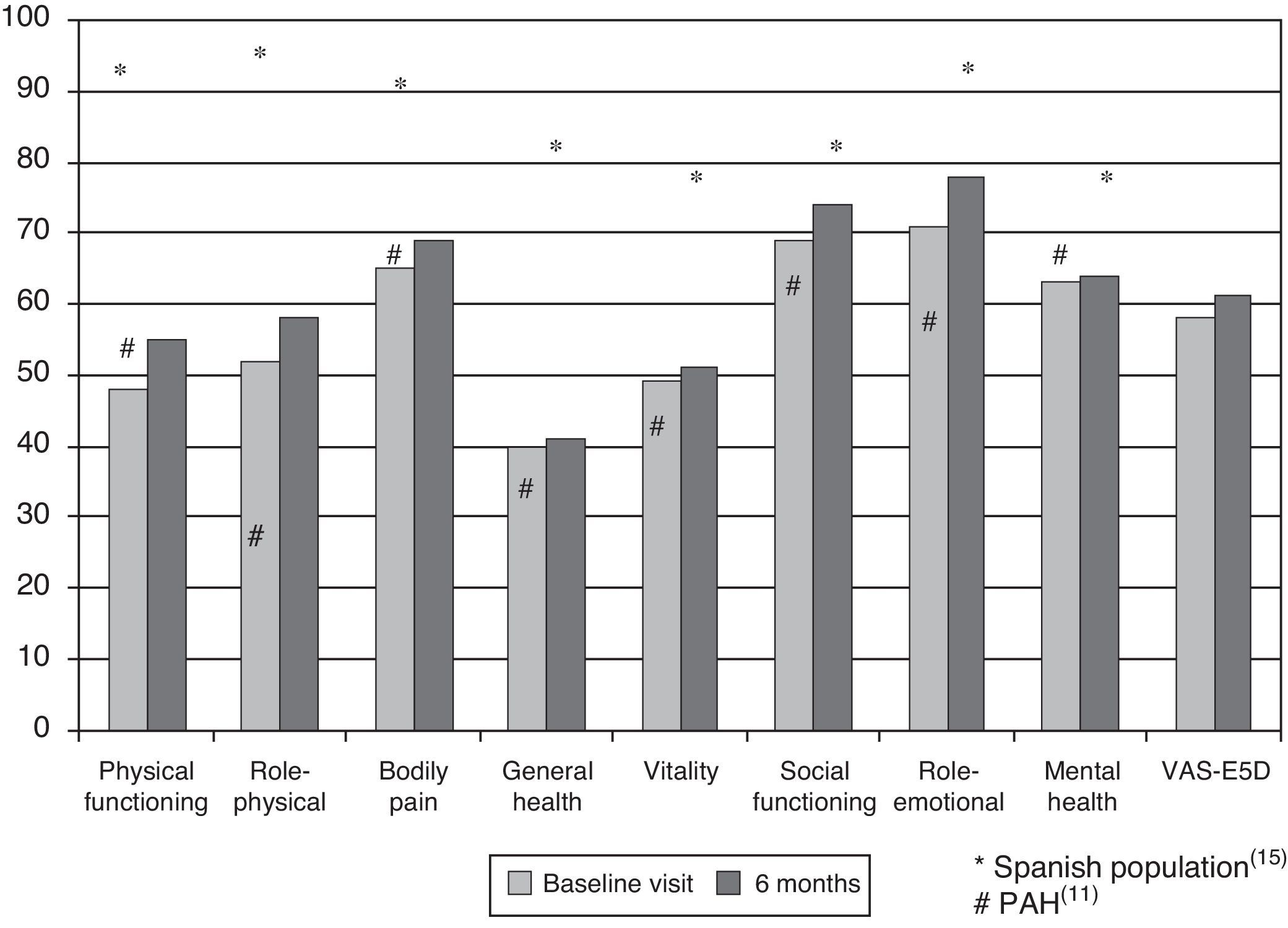
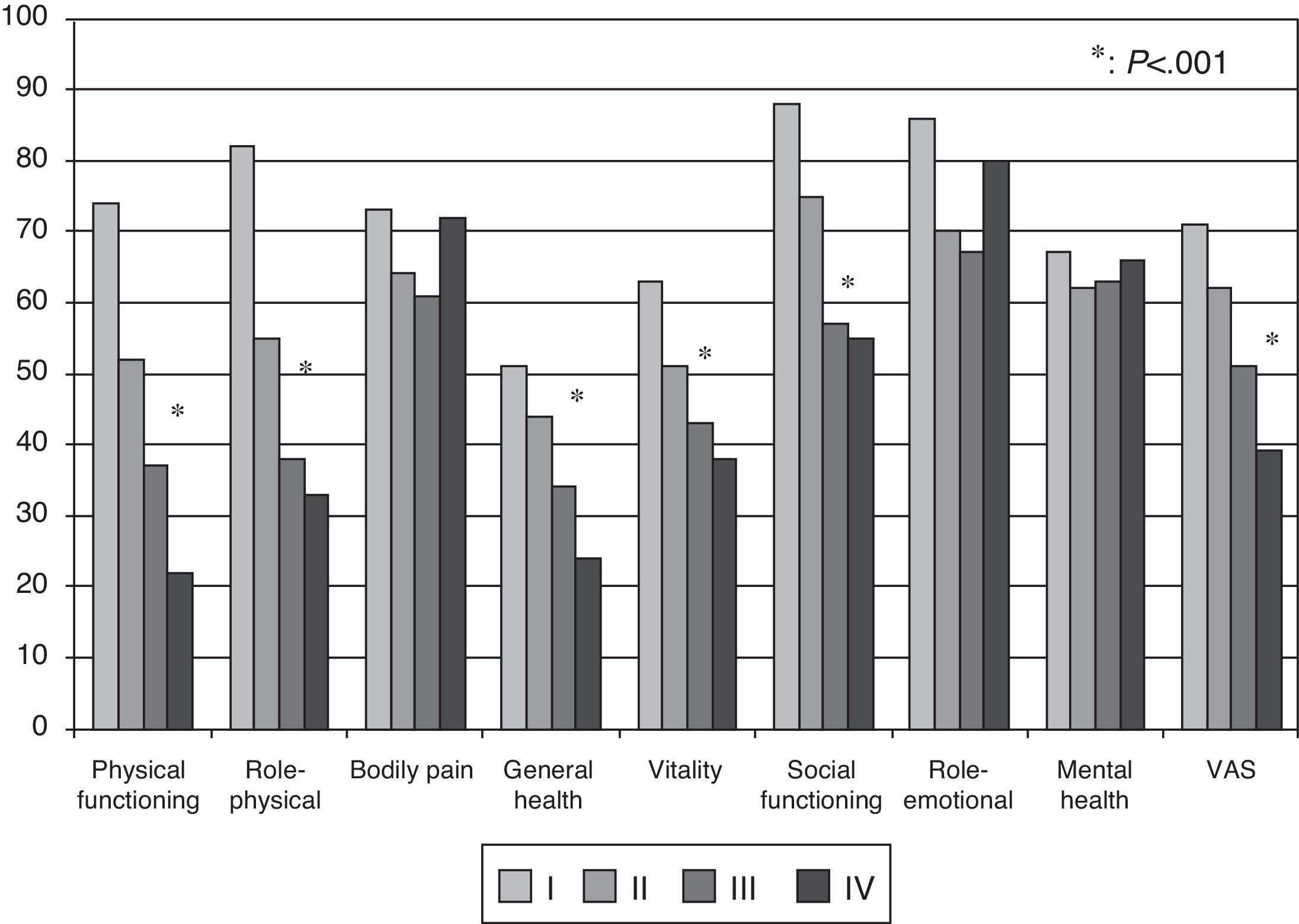
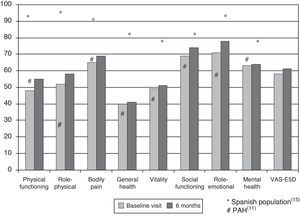
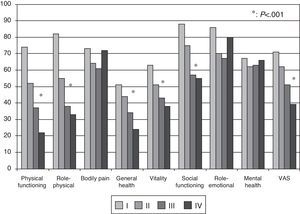
Health-related Quality of Life ResultsAt baseline, the mean (SD) PCS of the SF-36, MCS of the SF-36, VAS of the EQ-5D and the 6MWT were 37.9 (9.3), 45.7 (9.1) and 58.9 (20.2), respectively. These HRQL measures were considerably worse than those published for the normal population. No significant changes were observed when the baseline values were compared to those of the survivors after six months follow-up (Fig. 2). The SF-36 physical component PCS also differed significantly between functional classes at the baseline visit (Fig. 3); progressively worse PCS values were observed as the FC increased (Table 1). Fig. 2 shows that the differences observed with respect to the FC in the SF-36 were seen only in the physical domains, as the SF-36 mental values were not significantly different between groups at baseline (P=.0531; Table 1). A significant association (P<.0001) was observed at the baseline visit between the patients’ perception of their own health at that time (EQ-5D VAS) and their functional class (Table 1). In the regression analysis, every increase of one FC represented a loss of 4.0 on the physical component of the SF-36 and a loss of 9.5 on the EQ-5D VAS. In addition, with this stepwise regression model, every increase of one meter in the 6MWT resulted in an increase of 0.032 in the physical component of the SF-36.
Although there were no significant differences at baseline with respect to the 6MWT between IPAH, CTD-PAH and CTEPH, statistically significant differences (P=.0013) were observed in the physical component of the SF-36 between disease types at baseline. Patients with CTD-PAH obtained worse scores than patients with IPAH or CTEPH (Table 1). Patients with IPAH and CTEPH showed a modest improvement in the physical component of the SF-36 between baseline and the survivors at the 6-month visit (+1.9, P=.0149; +3.6, P=.0421, respectively), but no significant changes occurred in patients with CTD-PAH (P=.7892).
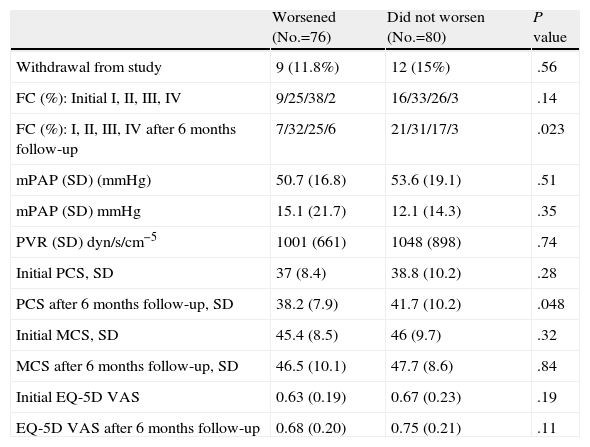
Health-related Quality of Life Stratified by Results at the 6-Month Follow-upEight patients who died (6) or underwent a lung transplant (2) during the study period presented a lower score in the physical component of the SF-36 and EQ-5D, but there were no differences in the 6MWT at the initial study visit, compared to the other patients (Table 1). Table 2 shows the comparison between 76 (48.7%) patients who worsened during the study period and 80 patients who did not worsen. Only a small difference was observed in the initial physical component between groups.
Comparison Between Patients who Worsened and Those who did not Worsen During Follow-up.
| Worsened (No.=76) | Did not worsen (No.=80) | P value | |
| Withdrawal from study | 9 (11.8%) | 12 (15%) | .56 |
| FC (%): Initial I, II, III, IV | 9/25/38/2 | 16/33/26/3 | .14 |
| FC (%): I, II, III, IV after 6 months follow-up | 7/32/25/6 | 21/31/17/3 | .023 |
| mPAP (SD) (mmHg) | 50.7 (16.8) | 53.6 (19.1) | .51 |
| mPAP (SD) mmHg | 15.1 (21.7) | 12.1 (14.3) | .35 |
| PVR (SD) dyn/s/cm−5 | 1001 (661) | 1048 (898) | .74 |
| Initial PCS, SD | 37 (8.4) | 38.8 (10.2) | .28 |
| PCS after 6 months follow-up, SD | 38.2 (7.9) | 41.7 (10.2) | .048 |
| Initial MCS, SD | 45.4 (8.5) | 46 (9.7) | .32 |
| MCS after 6 months follow-up, SD | 46.5 (10.1) | 47.7 (8.6) | .84 |
| Initial EQ-5D VAS | 0.63 (0.19) | 0.67 (0.23) | .19 |
| EQ-5D VAS after 6 months follow-up | 0.68 (0.20) | 0.75 (0.21) | .11 |
Of the initial 156 evaluable patients, 119 (76.3%) were on anticoagulant treatment, 64 (41%) took diuretics, 39 (25%) used oxygen at home and 27 (17.3%) were on digoxin. No percentage differences were observed in the use of these treatments at 6 months. Comparison between the 117 patients who were on oral or inhaled treatment at study inclusion versus 28 patients who received parenteral treatment showed that, among the latter, there was a higher percentage of women, patients were younger and had poorer hemodynamic parameters. There were more patients in the parenteral treatment group who died or received a lung transplant during the months of the study. However, comparison of HRQL between these groups at baseline and after 6 months follow-up did not show any differences.
DiscussionIn this prospective study, we have tried to describe the evolution of HRQL in a national Spanish cohort of patients with PAH or CTEPH. Two generic questionnaires were chosen as measuring instruments, the SF-36 and EQ-5D, basically because these have been the most widely used in PAH clinical trials, and also due to lack of availability of specific questionnaires on these diseases in Spanish. Among the advantages of this choice is that it allows us to make comparisons with the normal population or other serious pathologies. The first major result was that HRQL in PAH and CTEPH was worse than in the normal Spanish population,15 and did not change in the survivors at 6 months of follow-up. An Australian study of a national cohort of patients with PAH also showed a deterioration in HRQL, although in that case the patient population included was one that had started a specific treatment.16 All PAH patients included were on specific treatment and, therefore, our study suggests that the impact of new PAH treatments on HRQL is not progressive but could possibly be sustained over time. This idea has already been previously explored. Thus, in a study of 177 PAH patients,17 HRQL improved modestly after bosentan treatment; this improvement remained stable for three months. This result contrasts with the HRQL results in clinical trials with PAH-specific drugs. Of a total of 26 published clinical trials, 12 included HRQL as a secondary endpoint.6,18,19 Of the latter, some measured HRQL using a specific questionnaire for cardiovascular disease, while only a few did so using a generic questionnaire. HRQL measurements did not vary in one third of these studies, although they improved moderately in the remainder.6,10,20 Therefore, the absence of significant variation in HRQL over the six months of the study is consistent with previous information. The best comparison of our results can be made with the SUPER study,10 where the same questionnaires were used in 278 patients; the SF-36 showed results very similar to ours, and distinct from the normal Spanish population.15
With respect to HRQL in CTEPH, this has been evaluated in very few studies,13,14 none of which were classed as clinical trials. A Japanese study compared 43 patients treated medically with 40 patients treated surgically.14 The SF-36 scores in the group treated medically were very similar to the results obtained in this study. Moreover, our results support the idea that patients with CTEPH attended by doctors specializing in this condition in Spain have a HRQL similar to patients with idiopathic PAH. When we analyzed potential differences at baseline between patients who worsened in the follow-up compared to those who did not, we did not find any differences in the baseline data, including HRQL. This result highlights the difficulties in establishing a prognosis in PAH or CTEPH patients.
In this respect, information from the HRQL is particularly relevant for helping to predict which patients will die or require a lung transplant in the following weeks or months.
In this study, this patient group presented an initial HRQL that was worse than the other patients, but with no differences in the 6MWT or FC. This information is relevant, and invites us to explore the design of new studies, as the value of HRQL as a potential prognostic predictor in PAH and CTEPH is not well established in the literature. The differences in HRQL observed between patients who died or required a lung transplant and the other subjects are not too far from the minimal important difference (MID), defined as the smallest change in the HRQL score that is considered significant for the patient. The largest study to attempt to define this MID was performed in the cohort of the SUPER study.21 The authors found that the MID for SF-36 physical functioning, role-physical, social functioning, and vitality scales and for 6MWD were 13, 25, 21, and 15 points, and 41 m, respectively. Although the study has numerous limitations recognized by the authors, including the fact that this was a post hoc analysis in a study with no statistical power for this objective, it is a first point of reference for establishing MID in HRQL in this pathology.
Various clinical determinants have been associated with the degree of impairment of HRQL in these patients, including the diagnosis and exercise capacity. We found that patients with PAH associated with a CTD, especially scleroderma, experienced poorer HRQL than patients with other disease forms (Table 2). Despite all the therapeutic measures available, these patients did not obtain the same level of benefits as the others, which is compatible with other studies.22–24 In our cohort, the physical component of the SF-36 and the score on the EQ-5D VAS correlated with the 6MWT; the scores worsened as the exercise capacity was reduced. This result is consistent with previously published data,22,25 and supports the importance of these clinical parameters in practice. Recently, an acceptable correlation has been observed between changes in the 6MWT and the physical component of the SF-36, which has served as a basis for setting the minimum clinically important difference in the 6MWT at 33 m.26 Patients diagnosed in the year prior to study inclusion had worse HRQL and 6MWT scores than patients who were diagnosed earlier. This has not been studied previously in the literature, but enables us to speculate about differences in survival between incident and prevalent patients. We also hypothesized that patients on complex parenteral treatment would have a poorer HRQL than those who were on simpler treatment. However, we did not find any differences in HRQL between patients who were receiving oral-inhaled medical treatment or another more invasive one. This result, although surprising, is consistent with published studies.12,23 Finally, it is a matter of speculation whether this reflects differences in the factors that determined the choice of therapy, or if these different treatments have the same impact on HRQL.
This study has the limitation of short follow-up, losses to follow-up and the use of only generic instruments to measure HRQL. Furthermore, we must bear in mind that the recruitment designed may represent a limitation in the external validity of the study and, in theory, may not be a good representation of the Spanish patient population. Finally, in future designs, it will be important to include specific questionnaires and longer follow-up to be able to see small changes in HRQL and their value for taking decisions in these patients.
In conclusion, our study systematically evaluated HRQL and its value in short-term follow-up in a national cohort of patients with PAH and CTEPH. We can confirm that there is serious impairment of HRQL in these patients, which varied little within six months. The most relevant result was the fact that HRQL measured using generic instruments was worse in patients who were near death. Future efforts and prospective studies will be required that include, as well as generic instruments, specific questionnaires to definitively establish the value of HRQL as a potential predictor of outcome in PAH and CTEPH.
Authors’ ContributionsAR has made substantial contributions to the conception, design, data collection, data analysis and interpretation, and drafting of the manuscript; JAB has made contributions to data collection and interpretation, and has provided a critical review of the manuscript; JSR has made contributions to data collection and interpretation, and has provided a critical review of the manuscript; RM has made contributions to the design, data collection, technical and logistic support, and data interpretation and provided a critical review of the manuscript; PE has made substantial contributions to the conception, design, data collection, data analysis and interpretation, and has provided a critical review of the manuscript.
Conflict of InterestsThis study is sponsored by Pfizer. Ms. Rocío Muñoz currently works in Laboratorios Pfizer, the company which sponsored this study. The other authors have no conflicts of interest and have ensured the impartiality of the article.
The authors would like to thank Xavier Masramon and Luz Samaniego for their contribution as statisticians, Josep Román for his contribution in the critical correction of the manuscript and Silvia Martínez for her contribution in reviewing and sending the article to the journal.
Hospital 12 de Octubre (Madrid), Hospital Arnau de Vilanova (Valencia), Hospital Clínic (Barcelona), Hospital de Cruces (Barakaldo-Bizkaia), Hospital de Galdakao (Galdakao-Bizkaia), Hospital de León (León), Hospital del Bierzo (Ponferrada-León), Hospital Dr. Negrín (Las Palmas de Gran Canaria), Hospital General Universitario de Alicante (Alicante), Hospital General Yagüe (Burgos), Hospital La Fe (Valencia), Hospital Marqués de Valdecilla (Santander), Hospital Miguel Servet (Zaragoza), Hospital Puerta de Hierro (Majadahonda-Madrid), Hospital Ramón y Cajal (Madrid), Hospital Reina Sofía (Córdoba), Hospital San Jorge (Huesca), Hospital Torrecárdenas (Almería), Hospital Universitario Dr. Peset (Valencia), Hospital Universitario Insular (Las Palmas de Gran Canaria), Hospital Universitario Virgen del Rocío (Seville), Hospital Vall d’Hebrón (Barcelona).
Please cite this article as: Roman A, et al. Calidad de vida relacionada con la salud en una cohorte nacional de pacientes con hipertensión arterial pulmonar o hipertensión pulmonar tromboembólica crónica. Arch Bronconeumol. 2013;49:181–8.