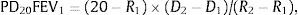The first guidelines on nonspecific bronchial tests sponsored by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) were published in 1987.1 Some years later, in 2004, these were updated under the format of a Procedure manual.2 A reasonable length of time having now passed, the SEPAR Scientific and Research Committee recently requested the Asthma Area to prepare a new update on the topic, attempting to gather together what daily practice and accumulated experience had proven valuable. Accordingly, this document is aimed at the description and methodology of tests employed in the study of bronchial hyperresponsiveness in asthma patients using direct and non-direct nonspecific stimuli, focusing on those most widely used in clinical practice: methacholine, adenosine, mannitol, exercise, eucapnic hyperventilation and hypertonic saline. However, before going into further detail, some general and key points related with the subject in question should be reviewed.
Bronchial Hyperresponsiveness: General ConsiderationsBronchial hyperresponsiveness (BH) is the term given to the excessive narrowing of the airway lumen when physical or chemical stimuli are applied that normally only cause little or no reduction in the airway calibre.3 Although this anomalous behaviour characterises one of the major singularities of asthma, it is not a unique feature of the disease.3,4 BH, transient or persistent, can also be detected accompanying other conditions (exposure to environmental pollutants and irritants, viral respiratory tract infections, chronic infections, rhinitis, sarcoidosis, mitral stenosis, bronchopulmonary dysplasia, etc.) or even in apparently healthy subjects.3,4 In fact, epidemiological studies conducted in the general population have shown that it occurs in 16%–30% of children and 10%–16% of adults, and that the degree of bronchial response has a continuous unimodal log-normal distribution; asthmatic subjects occupy the extreme end of the curve where higher levels of hyperresponsiveness are observed.5–7
Although this distribution pattern may lead us to think that BH is not a pathological condition per se, the fact is that the prevalence of respiratory symptoms is higher when it is present,8,9 and that asymptomatic and BH individuals have a high risk of developing asthma in the future, especially if there is concomitant atopy.9–13 However, BH and atopy are not two invariably associated events (there is atopy without BH and BH without atopy), suggesting that both have, at least in part, independent genetic determinants.9
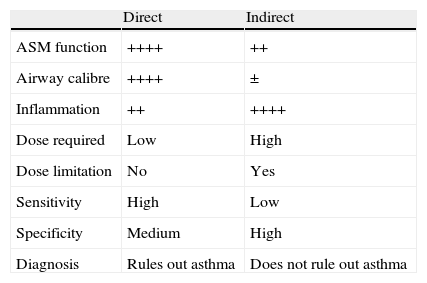
Types of StimuliIn any event, despite the lack of specificity, it is in the field of asthma where the analysis of BH has been best studied. Its assessment is a very helpful tool in the diagnosis of BH in daily practice, and allows the therapeutic response and degree of control achieved to be monitored.14,15 The study is carried out in the pulmonary function laboratory using substances which, like methacholine or histamine, cause bronchoconstriction by acting directly on the cells involved in causing airflow limitation (airway smooth muscle (ASM), vascular endothelium, mucous-producing cells, etc.).14,15 However, BH can also be demonstrated using agents (allergens, adenosine, mannitol, exercise, etc.) that indirectly cause a reduction in the airway calibre, by initially stimulating inflammatory or neuronal cells which, once activated, release the appropriate mediators to bring about narrowing of the bronchial lumen.16,17 This difference explains the far from perfect degree of correlation between direct and indirect stimuli, and most importantly, the information provided by each of them provides complementary views of abnormalities in the respiratory tract of the asthmatic patient.14,15 The direct methods are best for monitoring the function of the ASM, while the indirect methods reflect the magnitude of the inflammatory load more accurately. Similarly, and in contrast to the direct stimuli, the bronchospasm induced by indirect stimuli is prevented by taking chromones, inhaled furosemide or heparin, and the phenomenon of tachyphylaxis after a second exposure is much more pronounced and occurs earlier.16Table 1 summarises the characteristics that best differentiate the direct from the indirect stimuli.18
Comparison Between Direct and Indirect Stimuli in the Bronchial Provocation Test in Asthma (Adapted From Reference18).
| Direct | Indirect | |
| ASM function | ++++ | ++ |
| Airway calibre | ++++ | ± |
| Inflammation | ++ | ++++ |
| Dose required | Low | High |
| Dose limitation | No | Yes |
| Sensitivity | High | Low |
| Specificity | Medium | High |
| Diagnosis | Rules out asthma | Does not rule out asthma |
ASM, airway smooth muscle.
Leaving aside their underlying mechanism (direct or indirect), stimuli that reveal a state of BH are traditionally classified into specific and nonspecific. Specific stimuli only give positive results in individuals who are sensitive or intolerant to them. Direct (methacholine, histamine) and indirect nonspecific stimuli (adenosine, mannitol, exercise, eucapnic hyperventilation, iso-hypertonic aerosols, etc.) indicate hyperresponsiveness in any subject with BH, regardless of the underlying cause.18,19 The term “nonspecific” does not mean that the effects of a stimulus always correlate with those induced by another. For example, some asthmatics, especially those who are intolerant to non-steroidal anti-inflammatory drugs, are relatively more sensitive to methacholine than to prostaglandin F2a, and many patients with chronic obstructive pulmonary disease (COPD) respond to methacholine but not to hyperventilation.19
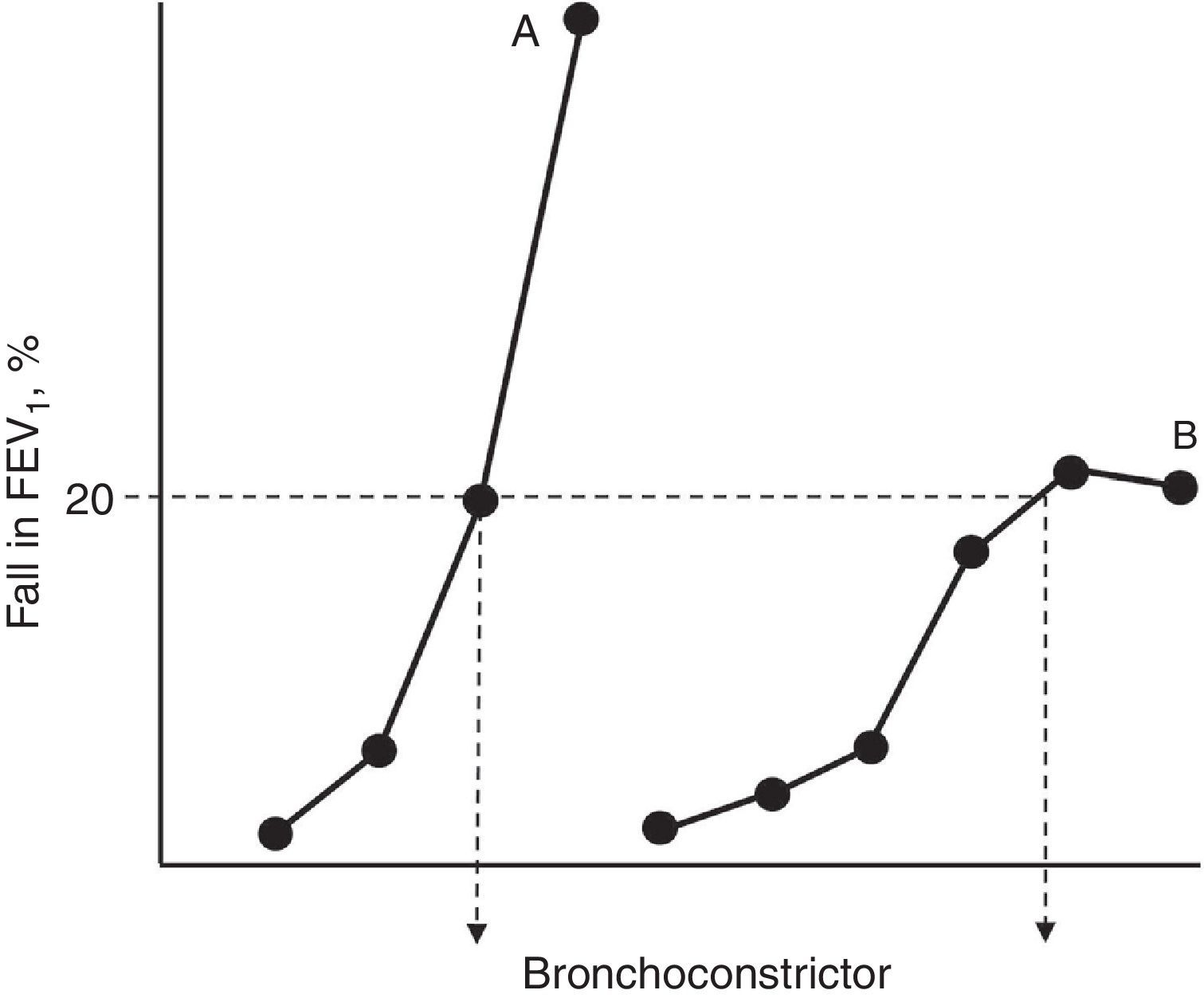
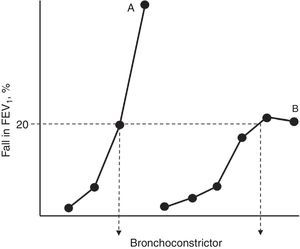
Pathogenesis of Bronchial Hyperresponsiveness in AsthmaAll authors agree that, as occurs in in vitro models of drug-receptor interaction, BH is the corollary of two well defined events: hypersensitivity and hyperreactivity.19–21 The increase in sensitivity (reduction in the pharmacological reagent when there are lower stimulus concentrations than under normal conditions) involves: (a) modifications in the number and/or affinity of the receptors to contractants; (b) changes in their absorption or metabolism; or (c) local circumstances that facilitate the accessibility of the ligand to its action sites. For its part, hyperreactivity (the development of a stronger contractile response) is caused by modifications in the properties and behaviour of the excitable tissue itself (more muscle, presence of factors that facilitate its shortening and dysfunctions in the contractile machinery).19–21 Hypersensitivity and hyperreactivity are identifiable in the functional examination laboratory if we construct “complete” dose–response curves for a bronchoconstrictor agonist in a normal subject and an asthmatic subject, and compare their position along the y-axis and their morphologies (Fig. 1). The appearance of a plateau in the maximum observed effect may not appear if the asthma is moderate–severe, and its absence reflects a particular condition of excessive airway narrowing, with higher morbidity.21
Simplified representation of dose–response curves to a bronchoconstrictor agonist in an asthmatic patient (A) and a normal subject (B). The shift towards the left of curve A and its steeper slope with respect to curve B reflect greater hypersensitivity and hyperreactivity, respectively. The dose that causes a 20% fall in the forced expiratory volume in the first second (FEV1) for both curves (PD20) is shown at their crosspoint with the horizontal dashed line.
Likewise, most authors agree when stating that, in the case of asthma, the origin of BH is linked to the inflammation/repair that the bronchial wall undergoes during the course of the disease, with two forms of hyperresponsiveness existing here: one baseline and relatively persistent and the other transient.21,22 The baseline form is present in the majority of patients with chronic asthma and the transient or variable form occurs superimposed on this as a product of exposure to environmental events (allergens, respiratory tract infections, occupational agents). The variable component reflects the airway inflammation at a given moment, while the baseline BH is more closely related with the structural changes that accompany the remodelling.22
Taking these general hypotheses as a starting point, a number of pathogenic alternatives have been proposed over the years, divided into three non-mutually exclusive research lines: (a) the involvement of mechanical determinants; (b) ASM dysfunction; and (c) the loss of some limiting component of normal ASM contractility. Readers interested in more detailed understanding of these hypotheses can consult references.3,4,19,20,22–26
Bronchial Provocation Tests With Direct Nonspecific Stimuli: MethacholineBasic FoundationsMethacholine and histamine have been the two substances traditionally administered as direct nonspecific stimuli and, in similar measurement conditions, they obtain comparable effects.27,28 However, methacholine is more widely used today, as it offers two major advantages27–29: (a) it can be used at concentrations of up to 200mg/ml without side effects, while histamine concentrations greater than 32mg/ml can cause facial erythema, headache, nausea, dysphonia and even oedema of the glottis; and (b) the reproducibility of the bronchial provocation test (BPT) with methacholine is better than with histamine. Therefore, the choice of methacholine is recommended as a bronchoconstrictor agent instead of histamine.
Methacholine is a synthetic derivative of acetylcholine (acetyl-ß-methylcholine) with no nicotinic action, which acts on the cholinergic receptors of the bronchial tree and is biotransformed, like acetylcholine, by acetyl cholinesterase but at a slower rate.28 This allows its effects to be prolonged and facilitates measurement of the responses. Methacholine hydrochloride is classed as category C for pregnancy. It is not known whether it can cause foetal damage or if it affects fertility. It should only be administered to pregnant women if clearly needed and should not be used in breastfeeding mothers, as it is not known whether it passes into breast milk when inhaled.
Methacholine comes as a crystalline powder (acetyl-methyl-choline chloride or bromide salts) and has been approved for use in humans (Provocholine™, 20ml/100mg vials).28–30 Methacholine powder is hygroscopic and should be stored in a tightly closed container in a dry place, preparing the required dilutions as they are to be used.30
Addresses of interest: Methacholine distributor: DIATER, S.A. Avda. Gregorio Peces Barba 2, Parque Tecnológico de Leganés, Leganés, 28918-Madrid. Contact telephone number: +349149660 13. Fax: +34914966012. Website: http://www.diater.com. Email: info@diater.com.
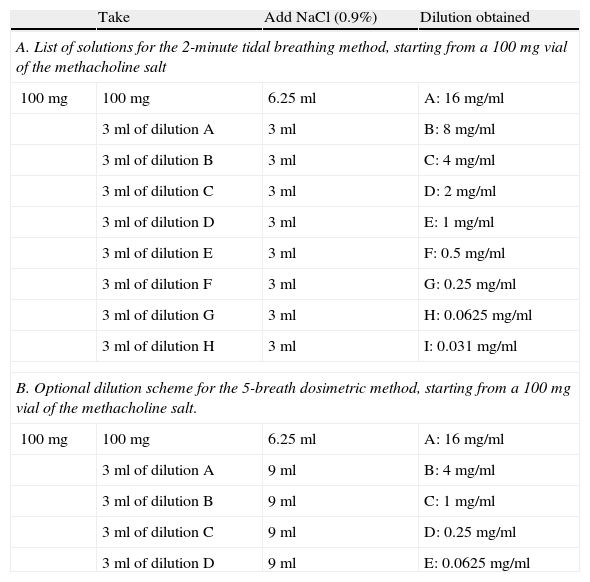
Technical FoundationsPreparation of the DilutionsDilutions should be made with 0.9% sodium chloride for injection, using empty, sterile, type I borosilicate glass vials, shaking after adding the diluent until a clear solution is obtained. This task should preferably be performed by the hospital Pharmacy Department. Methacholine dilutions in isotonic saline solution greater than 0.125mg/ml are stable for 3 months when stored at 4°C.28,31 If the solvent contains a preservative (e.g. 0.4% phenol to inhibit bacterial growth), the stability is reduced and the solution should be used within two weeks. The pH of the methacholine in the saline solution is moderately or weakly acidic, depending on the concentration of the methacholine itself.28,31 Reconstituted vials should be stored in cool conditions (4°C), avoiding exposure to sunlight, and used at room temperature. No special precautions are required for disposal of excess product.30Table 2 shows the schemes for preparing dilutions.
Scheme for the Methacholine Dilutions to be Prepared for the Two Recommended Methods.28
| Take | Add NaCl (0.9%) | Dilution obtained | |
| A. List of solutions for the 2-minute tidal breathing method, starting from a 100mg vial of the methacholine salt | |||
| 100mg | 100mg | 6.25ml | A: 16mg/ml |
| 3ml of dilution A | 3ml | B: 8mg/ml | |
| 3ml of dilution B | 3ml | C: 4mg/ml | |
| 3ml of dilution C | 3ml | D: 2mg/ml | |
| 3ml of dilution D | 3ml | E: 1mg/ml | |
| 3ml of dilution E | 3ml | F: 0.5mg/ml | |
| 3ml of dilution F | 3ml | G: 0.25mg/ml | |
| 3ml of dilution G | 3ml | H: 0.0625mg/ml | |
| 3ml of dilution H | 3ml | I: 0.031mg/ml | |
| B. Optional dilution scheme for the 5-breath dosimetric method, starting from a 100mg vial of the methacholine salt. | |||
| 100mg | 100mg | 6.25ml | A: 16mg/ml |
| 3ml of dilution A | 9ml | B: 4mg/ml | |
| 3ml of dilution B | 9ml | C: 1mg/ml | |
| 3ml of dilution C | 9ml | D: 0.25mg/ml | |
| 3ml of dilution D | 9ml | E: 0.0625mg/ml | |
Two inhalation methods can be used to perform the BPT: continuous aerosol generation systems and the dosimetric technique.28,31
Continuous generation systems use a jet impactor nebuliser equipped with a one-way valve (with inlet to the nebuliser and outlet with filter to the outside) attached to a compressed air source with the flow adjusted to obtain a constant output of between 0.13 and 0.16ml/min.28 The nebuliser must dispense particles with a median aerodynamic mass of 1–3µm, so that approximately 80% reach the most peripheral bronchi.28,31 Although there are different procedures for calibrating the nebuliser, the “double weighing” method is recommended due to its availability and simplicity. It consists of weighing the nebuliser before and after dispensing 3ml of solution, over 2min, at room temperature and with a given airflow.28 Calibration is necessary before and after using each device, and at regular intervals, as the output changes when it is used and cleaned. The continuous generation system thus requires relatively simple equipment but has some disadvantages: (a) difficulty in ensuring that anxious patients inhale the solution at tidal volume; and (b) it is impossible to know the amount of agonist inhaled for certain (see below).
In the case of the dosimetric technique, the aerosol is generated only during a period of the inspiration by means of a solenoid valve activated electrically when the patient inhales through the nebuliser.28 The use of dosimeters allows better adjustment of the dose administrated, which is determined based on the output of the nebuliser, the agonist concentration, nebulisation time and number of breaths. In addition to pulse nebulisation, some dosimeters allow continuous nebulisation during the entire inspiration, interrupting the administration of the agonist automatically once the programmed dose has been reached. The dosimetric technique also has certain disadvantages: (a) more sophisticated equipment; (b) significant deposition of the agonist in the upper airways if the inspiratory flows are not controlled; and (c) possible bronchoprotective effect of the maximal inspiratory manoeuvre.28,31,32 Dosimetric systems require periodic calibration following the manufacturer's instructions.
Finally, the following are worth bearing in mind: (a) that the results and cut-off points obtained by both methods are not completely comparable (the dosimetric method may underestimate the degree of BH); and (b) that to be able to interpret the BPT, it is essential to detail the characteristics of the nebuliser and the use or not of a dosimeter.32 Despite the validity of the aforementioned methods, in order to homogenise the procedure, these guidelines recommend the use of the dosimeter.
Recording the ResponseThe response induced by a bronchoconstrictor stimulus can be evaluated by recording the changes detected in various lung function parameters, among which are the forced expiratory volume in the first second (FEV1), peak flow or measurement of airway resistance and specific conductance.33 Of these, the most widely used in daily practice is the FEV1. Even with certain limitations (it requires forced expiration manoeuvres capable of inducing variations in the airway calibre per se and does not provide information on the behaviour of the small airways), it is a very reproducible measure, with a low coefficient of variation (<8%), easy to carry out and does not require complex equipment.33 The spirometer and method used to obtain the spirometry must meet the conditions specified in the corresponding guidelines.34,35
In small children or subjects whose cooperation cannot be obtained, the use of transcutaneous oxygen tension or the forced oscillation technique may be considered as alternatives for recording the response.28 These should only be used when valid spirometries cannot be obtained, and in laboratories with experience in their use and interpretation.
ProceduresTwo classical provocation methods remain: the tidal breathing method and the 5-breath dosimeter method, although various simplified dosimetric procedures have also been added.
Tidal Breathing MethodThis requires the use of a Wright jet impactor nebuliser or similar.36 Once the baseline spirometry has been performed, the patient should be asked to breathe quietly through a mouthpiece connected to the nebuliser while seated comfortably, with a nose clip in place. Alternatively, the use of a mask may be considered, in this case also using a nose clip. The test begins by inhaling the diluent and continues with the administration of increasing methacholine concentrations (from 0.03mg/ml to 16mg/ml) (Table 3). At each stage of the test, 3ml of the relevant concentration are placed in the nebuliser, and inhaled continuously by the patient while tidal breathing for 2min. After each inhalation, the patient should be removed from the equipment and after 30–90s, a spirometry should be performed (not more than three manoeuvres during the following 3min). If the FEV1 falls by 20% or more, the BPT is concluded. Otherwise, administer the following concentration until this decrease is reached or the remaining preparations have been used.27,28 To assure the cumulative effect of the doses, there should be no more than 5min between the administration of one concentration and the next. For safety reasons, once the test is finished, a short-acting ß2 adrenergic agonist must always be administered (salbutamol or terbutaline), preferably with a pressurised cartridge, in order to return the patient to his or her baseline situation.
Main Methacholine Bronchial Provocation Protocols.
| Step | Concentration, mg/ml | Nebulisation time, min |
| Tidal breathing method36 | ||
| Nebuliser flow: 0.13ml/min±10%; nebuliser output in each step: 90µl | ||
| 1 | 0.03 | 2 |
| 2 | 0.06 | 2 |
| 3 | 0.125 | 2 |
| 4 | 0.25 | 2 |
| 5 | 0.50 | 2 |
| 6 | 1 | 2 |
| 7 | 2 | 2 |
| 8 | 4 | 2 |
| 9 | 8 | 2 |
| 10 | 16 | 2 |
| Step | Concentration, mg/ml | No. inspirations | Nebulisation time, s |
| Five-breath dosimetric method38 | |||
| Nebuliser flow: 0.009 for 0.6 s±10%; nebuliser output in each step: 45µl | |||
| 1 | 0.0625 | 5 | 0.6 |
| 2 | 0.25 | 5 | 0.6 |
| 3 | 1 | 5 | 0.6 |
| 4 | 4 | 5 | 0.6 |
| 5 | 16 | 5 | 0.6 |
| Step | Concentration, mg/ml | No. inhalations | Dose, mg | Cumulative dose, mg |
| Chinn dosimetric method42 | ||||
| Mefar dosimeter: nebuliser output per inhalation: 0.010ml | ||||
| Simplified protocol | ||||
| 1 | 0.39 | 4 | 0.0156 | 0.0156 |
| 2 | 1.56 | 3 | 0.0468 | 0.0625 |
| 3 | 6.25 | 3 | 0.1875 | 0.25 |
| 4 | 12.5 | 6 | 0.750 | 1.0 |
| 5 | 12.5 | 8 | 1.0 | 2.0 |
| Long protocol | ||||
| 1 | 0.39 | 2 | 0.0078 | 0.0078 |
| 2 | 0.39 | 2 | 0.0078 | 0.0156 |
| 3 | 1.56 | 1 | 0.0156 | 0.0312 |
| 4 | 1.56 | 2 | 0.0312 | 0.0625 |
| 5 | 6.25 | 1 | 0.0625 | 0.125 |
| 6 | 6.25 | 2 | 0.25 | 0.25 |
| 7 | 12.5 | 2 | 0.25 | 0.5 |
| 8 | 12.5 | 4 | 0.5 | 1.0 |
| 9 | 12.5 | 8 | 1.0 | 2.0 |
| Step | Concentration, mg/ml | Nebulisation | Dose, mg | Cumulative dose, mg |
| Schulze dosimetric method44 | ||||
| APS pro dosimeter; nebuliser output: 160–240mg/min | ||||
| 1 | 16 | Pulse or continuous | 0.01 | 0.01 |
| 2 | 16 | Pulse or continuous | 0.10 | 0.11 |
| 3 | 16 | Pulse or continuous | 0.40 | 0.51 |
| 4 | 16 | Pulse or continuous | 0.80 | 1.31 |
| 5 | 16 | Pulse or continuous | 1.60 | 2.91 |
The diluent may be used to familiarise the subject with the technique and to identify the existence of bronchial lability. A greater than 10% fall in the FEV1 after inhaling the diluent indicates a positive response.28 The post-diluent FEV1 will be the reference value for the comparison following the administration of the different methacholine concentrations.
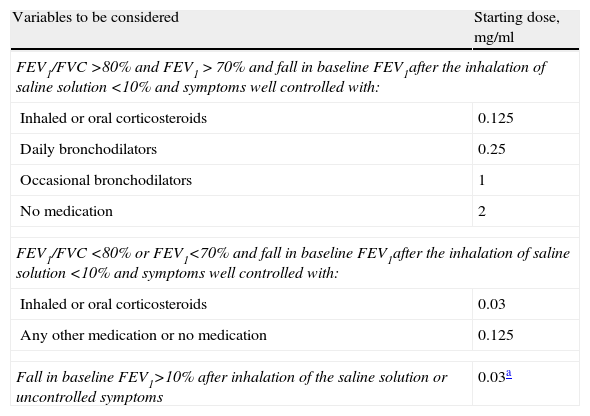
Since the whole procedure is quite time-consuming, modifications have been proposed that enable the BPT to be commenced with doses of bronchoconstriction agent that are higher than those recommended in the original protocol. Thus, the first concentration to be administered is decided considering the presence of respiratory symptoms, usual medication, baseline FEV1 and its fall after administering the diluent (Table 4).36 If there is no significant fall in the FEV1 (decrease less than 5% with respect to the reference value) after the first methacholine concentration and there are no respiratory symptoms, a dose may be skipped, omitting the concentration immediately afterwards.37 This simplified protocol should only be carried out by experienced technicians.
Rapid Protocol for Performing the Methacholine Bronchial Provocation Test. Adapted From Juniper et al.36
| Variables to be considered | Starting dose, mg/ml |
| FEV1/FVC >80% and FEV1>70% and fall in baseline FEV1after the inhalation of saline solution <10% and symptoms well controlled with: | |
| Inhaled or oral corticosteroids | 0.125 |
| Daily bronchodilators | 0.25 |
| Occasional bronchodilators | 1 |
| No medication | 2 |
| FEV1/FVC <80% or FEV1<70% and fall in baseline FEV1after the inhalation of saline solution <10% and symptoms well controlled with: | |
| Inhaled or oral corticosteroids | 0.03 |
| Any other medication or no medication | 0.125 |
| Fall in baseline FEV1>10% after inhalation of the saline solution or uncontrolled symptoms | 0.03a |
Subsequent concentrations may only be omitted if, after the previous concentration, the fall in FEV1 is less than 5% and no symptoms have appeared.
The main limitation of this method lies in its dependence on the respiratory pattern. In fact, variations in both the respiratory rate and tidal volume amplitude can modify the dose administered.
Five-breath Dosimeter MethodThis method uses a jet nebuliser connected to a dosimeter, programmed to nebulise for the first 0.6s of each inhalation, delivering an output of 9µl±10% in that period.27,28 In this method, the diluent and 5 increasing methacholine doses (from 0.0625 to 16mg/ml) are administered (Table 3).38
At end exhalation during tidal breathing, at functional residual capacity (FRC), the subject should be connected to the mouthpiece and must inhale slowly (for 5s) until total lung capacity (TLC). Once maximum inspiration has been reached, the apnoea should be maintained for 5s before exhaling slowly. This procedure should be repeated with intermediate periods of quiet breathing until 5 inhalations have been completed for each concentration, within a period of not more than 2min. There should be no longer than 5min between the administration of each concentration level so that a cumulative effect can be produced.27,28 The spirometry should be performed in the 30–90s following the inhalation of each concentration. The sequence of steps to be followed until the test is completed is identical to that previously described.
It has been suggested that the deep inhalations and apnoea phases that characterise this procedure may have a bronchoprotective effect in normal subjects and patients with mild hyperresponsiveness.39 Therefore, although there are no major differences between these two methods in most asthma patients, in subjects with mild hyperresponsiveness, deep inhalations may produce false negatives when using the dosimeter method with respect to the tidal breathing method.40,41
Simplified Dosimetric MethodsThese minimise the number of concentrations to use, thereby reducing the technician's work and possible error sources.
The Chinn dosimetric method was designed for the European Community Respiratory Health Survey II, as an abbreviated protocol (Table 3).42 It was developed for a Mefar dosimeter, which programmed for a nebulisation time of 1s, has an output of 0.01ml of aerosol each time it is activated.43 Each inhalation sequence requires that, once connected to the mouthpiece in FRC, the patient inhales slowly until TLC, maintaining apnoea for 3s, before disconnecting the mouthpiece immediately afterwards. The slow inspiration and subsequent apnoea should total at least 6s if possible. Once the baseline spirometry has been performed, at least three inhalations of the diluent should be taken, repeating the spirometry at 60s. If the FEV1 falls by >10% after the diluent, then the long protocol should be followed (Table 3). In the simplified protocol, 4 concentrations should be used to administer 5 doses, performing the spirometry 60s after each dose level, and interrupting the test if a =20% fall in the FEV1 with respect to the post-diluent value is recorded.42,43 A bronchodilator should be administered after the last dose in this case as well.
The Schulze dosimetric method is an even greater simplification, as it uses a single concentration (16mg/min) to generate 5 increasing doses (Table 3) using APS dosimeters, which allow both pulse and continuous nebulisation.44–46 While there are no differences between both procedures in adults, continuous nebulisation is recommended in children due to the greater irregularity of the respiratory cycle.46 In this method, the nebulisation is performed while the patient is tidal breathing, with a direct view of his or her respiratory tracing. To guarantee better aerosol deposition in the airways, the inspiratory flow should not exceed 0.5l/s.46 Two minutes after the administration of each dose, the spirometry is carried out, maintaining the same performance and end of procedure characteristics as in the other protocols.
Measurement and Interpretation of ResultsThe result of the methacholine BPT is represented by a dose–response curve in which the x-axis reflects the cumulative dose or concentration of the inhaled agonist and the y-axis the change observed in lung function.1,2,28,31,33
The most classic parameter to be estimated is the PD20, defined as the cumulative methacholine dose that reduces the FEV1 by 20% (PD20FEV1) with respect to the value obtained after administration of the solvent.1,2,28,31,33 It is calculated using the formula:
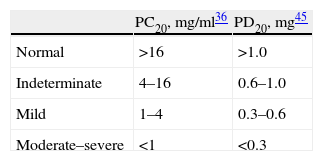
where R1 is the percentage FEV1 immediately before the =20% fall, R2 is the percentage fall in FEV1 =20%, D1 the cumulative methacholine dose inhaled in R1 and D2 is the cumulative methacholine dose inhaled in R2.1,2,28,31,33,36The PD20 values can only be calculated when a dosimeter is used. In continuous generation systems, since the dose administered is not known for sure, the previous formula can be used but substituting D1 and D2 for the previous agonist cumulative concentration (C1) and the concentration that induces the fall in FEV1 =20 (C2), respectively. The resulting index is known as the PC20. PD20FEV1 and PC20FEV1 can also be identified by direct interpolation from the results graph.28,36 Both the PD20FEV1 and the PC20FEV1 values allow the severity of the BH to methacholine to be classified (Table 5).28
Other parameters of interest are the slope of the dose–response curve (which is obtained by applying a linear regression model and provides information about the degree of bronchial hyperreactivity) and the shape of the curve itself, specified by the maximum response (MR) values or percentage of maximum fall in the FEV1 that does not increase despite inhalation of increasing doses of agonist.31 The MR is expressed as the mean of the falls in FEV1 that maintain a variation less than 5% in three increasing agonist concentrations.
In recent years, it has been proposed to work with other additional indices: deltaFVC, the bronchial reactivity index and the continuous response index. DeltaFVC represents the % decrease in the forced vital capacity when the FEV1 falls by 20% with respect to baseline. DeltaFVC may differentiate the levels of asthma severity better than PD20FEV1 or PC20FEV1, and reflects not only the hypersensitivity, but also involvement of the small airways and air trapping.47 For their part, the bronchial reactivity index and continuous response index could be more useful for discriminating between patients with BH associated with asthma or COPD,48 as well as maintaining a better relationship with health-related quality of life in asthmatics.49
Indications, Contraindications and General PrecautionsThe fundamental indication for nonspecific BPTs in clinical practice is to establish or exclude a diagnosis of asthma, especially when neither the symptoms nor spirometry with a bronchodilator can confirm or rule out this possibility. It has already been noted above that a BPT with direct stimuli is more useful for excluding asthma (except in cases of seasonal or occupational asthma without exposure to the causal agent), as it has a greater negative than positive predictive capacity.18,28 The maximum negative predictive value for ruling out asthma in a negative methacholine BPT occurs when the previous probabilities of asthma vary between 30% and 70%.28,50 Nonspecific BPTs using inhaled agents can be used equally for monitoring the therapeutic response and degree of control of the disease, and are often used in epidemiological studies, controlled clinical trials and in the assessment of occupational respiratory diseases (diagnosis and monitoring of occupational asthma).28 The absence of a bronchial response to methacholine in a patient who has been exposed to his work environment in the previous two weeks rules out a diagnosis of occupational asthma.51 On the contrary, increases in the response to methacholine, after natural exposure or provoked by an agent, suggest a causal role.
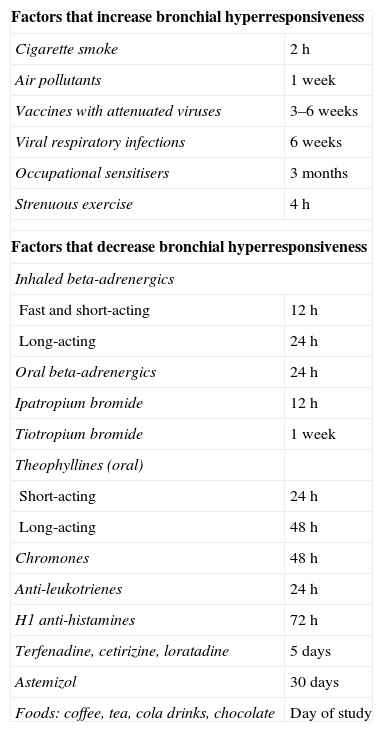
Once the indication has been established and the patient properly informed, he or she must sign the corresponding consent form after reading it and having any relevant questions answered. They will also be instructed on foods to avoid, stimulating beverages, medicinal products and other factors that could modify the bronchomotor response (Table 6). At the time of the test, the patient should be stable and free from airway infections for the previous six weeks.1,2,28
Factors That Alter the Bronchial Dynamics and Indication of the Minimal Time That Should Pass Between Exposure to These Factors and Performing a BPT.1,2,28
| Factors that increase bronchial hyperresponsiveness | |
| Cigarette smoke | 2h |
| Air pollutants | 1 week |
| Vaccines with attenuated viruses | 3–6 weeks |
| Viral respiratory infections | 6 weeks |
| Occupational sensitisers | 3 months |
| Strenuous exercise | 4h |
| Factors that decrease bronchial hyperresponsiveness | |
| Inhaled beta-adrenergics | |
| Fast and short-acting | 12h |
| Long-acting | 24h |
| Oral beta-adrenergics | 24h |
| Ipatropium bromide | 12h |
| Tiotropium bromide | 1 week |
| Theophyllines (oral) | |
| Short-acting | 24h |
| Long-acting | 48h |
| Chromones | 48h |
| Anti-leukotrienes | 24h |
| H1 anti-histamines | 72h |
| Terfenadine, cetirizine, loratadine | 5 days |
| Astemizol | 30 days |
| Foods: coffee, tea, cola drinks, chocolate | Day of study |
Note: Routinely withholding inhaled or oral corticosteroids (2–3 weeks) is not recommended, although their anti-inflammatory effect may decrease bronchial hyperresponsiveness.
The BPT should be carried out in a well-ventilated, spacious room, with cardiorespiratory resuscitation equipment nearby. Nursing staff in charge of performing the test should have at least 6 months training in an accredited lung function laboratory, have the required knowledge to detect and help resolve a respiratory emergency and have previously performed at least 25 supervised tests.28 The doctor in charge of the laboratory must remain close to the site where the test is to be carried out, to attend any unexpected events that could arise. Staff should not be asthmatic.
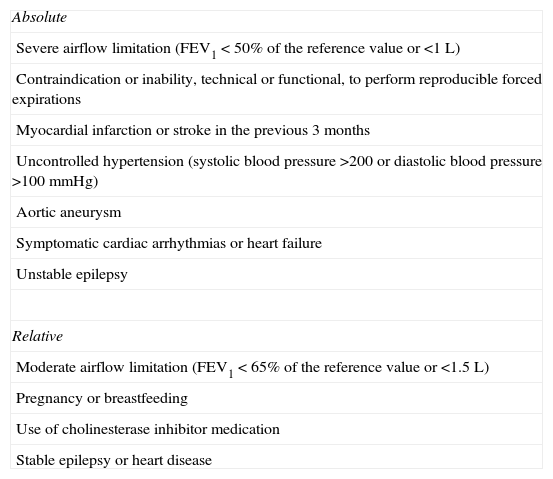
With respect to contraindications, these are subdivided into absolute and relative (see Table 7).1,2,28 Some patients may present pharyngeal discomfort and dry cough. The effects are temporary.28,31 The onset of an excessive bronchoconstrictive response is only rarely detected,52 especially with the short protocols, but can be prevented if the nitric oxide levels in exhaled air (FENO) can be determined prior to performing the BPT. It has been noted that FENO values less than 19.5ppb rule out the development of this event with reasonable certainty (sensitivity 80%, specificity 77%, negative predictive value 88%).53
Contraindications for Performing the Methacholine Bronchial Provocation Test.1,2,28
| Absolute |
| Severe airflow limitation (FEV1<50% of the reference value or <1L) |
| Contraindication or inability, technical or functional, to perform reproducible forced expirations |
| Myocardial infarction or stroke in the previous 3 months |
| Uncontrolled hypertension (systolic blood pressure >200 or diastolic blood pressure >100mmHg) |
| Aortic aneurysm |
| Symptomatic cardiac arrhythmias or heart failure |
| Unstable epilepsy |
| Relative |
| Moderate airflow limitation (FEV1<65% of the reference value or <1.5L) |
| Pregnancy or breastfeeding |
| Use of cholinesterase inhibitor medication |
| Stable epilepsy or heart disease |
Adenosine is an endogenous nucleoside involved in numerous physiological processes (control of vascular and intestinal tone, neurotransmission, neurosecretion, etc.) and inflammatory reactions.54 Synthesis takes place inside the cells from the hydrolysis of adenosine 5'-monophosphate (AMP) by 5'-nucleotidase, or by the catabolism of S-adenosyl homocysteine. When the intracellular AMP concentration increases (hypoxia, cell activation), it crosses to the outside for reuptake by the cell itself, or to be bound to membrane cytoplasmic receptors (A receptors).54–57 High adenosine concentrations have been detected in the bronchoalveolar lavage and exhaled breath condensate of patients with asthma and chronic bronchitis, compared with healthy individuals, and experimental models show that adenosine is released into the blood as a result of bronchial provocation with allergens.55,56
The bronchoconstrictive action of inhaled AMP is a result of the release of histamine, interleukin 8, leukotriene C4 and other mediators from the mast cells, platelets, neutrophils and epithelial cells, once their A2B receptors have been activated.54,55 Other mechanisms such as neurosensory mechanisms, the axon reflex and cholinergic stimulus also appear to be important.54,55,58
For the BPT, due to its higher solubility in saline solution, the disodium salt of AMP (adenosine 5'-monophosphate sodium salt, Sigma Chem., St. Louis, MO, USA) is used, which is dispensed as a dry crystalline powder. The purity grade of the product is 99%–100%.2 The powder preparation is stored in the refrigerator with a dry desiccant. Adenosine is a class C medicinal product for pregnancy.
Addresses of interest: Adenosine distributor: Sigma–Aldrich Química, S.A., Calle Ronda de Poniente 3, 2. Tres Cantos 28760, Madrid. Contact telephone number: 900101376. Fax: 900102028. Website: http://www.sigmaaldrich.com/spain/acerca.html. Email: esorders@sial.com.
Technical FoundationsPreparation of the DilutionsThe stock solution is prepared with the AMP powder and a sterile 0.9% sodium chloride solution, obtaining an AMP concentration of 800mg/ml.2,58 The different AMP concentrations are made up from this. Those recommended by the European Respiratory Society (ERS) are: 3.125, 6.25, 12.5, 25, 50, 100, 200 and 400mg/ml.16 Other authors use slightly different concentrations (between 0.03 and 256mg/ml).2 The dilutions should be protected from light using an amber flask. Their stability is at least 25 weeks if stored in the refrigerator (4°C) and less than 10 days if left at room temperature.59 Therefore, the dilutions should always be kept in the refrigerator until 30min before use.
EquipmentNebulisation SystemsAs with methacholine, the adenosine test can be carried out using the continuous generation system or the dosimetric technique, which is the ERS preferred method.16 For the general specifications for both methods, see Section “Nebulisation Systems” in under “Bronchial Provocation Tests with Direct Nonspecific Stimuli: Methacholine”.
Recording the ResponseThe response evoked by adenosine is usually recorded by analysing the changes in FEV1, and therefore the general comments made above explaining the recording of the response when methacholine is used as the bronchoprovocation agent are also applicable.
ProceduresThe procedure to be followed with the AMP BPT, either with spontaneous aerosol breathing or dosimetry, can be superimposed on the method described for methacholine. The first aerosol should be composed of 0.9% physiological saline and, if a greater than 20% fall in the FEV1 is not observed, nebulisation of AMP should be commenced, in incremental double concentrations, from the initial dose (3.125mg/ml) to the maximum dose (400mg/ml). The FEV1 should be measured after each dose and the highest measurement of 3 reproducible determinations, with a variation less than 100ml, should be recorded to construct the dose–response curve.2,16,58 The test should be interrupted when a fall in the FEV1 greater than or equal to 20% with respect to the diluent is observed, once the maximum permitted dose has been reached, or in case of symptoms or signs of bronchospasm.2,16,58
Although this protocol is the one that has been most successfully followed in children and adults, there is another abbreviated variant in which quadruple doses are administered, obtaining identical reproducibility while being less time-consuming (25–30min) with no additional adverse effects.60
Measurement and Interpretation of ResultsAs with methacholine, a dose–response curve should be constructed, considering the fall in FEV1 and AMP dose. The result of the test is expressed using PC20 or PD20 obtained by linear interpolation. If the FEV1 does not drop by at least 20% after the maximum concentration (400mg/ml), a PC20 or PD20 value >400mg/ml is given.16,58 However, the PC20 or PD20AMP cut-off points between healthy subjects and subjects with BH remain arbitrary (160–200mg/ml), since sufficiently large population data are still lacking.58 The coefficient of repeatability of the BPT with AMP ranges between 1 and 1.2 double doses,16,58 and studies published to date agree that this is a less potent bronchoconstrictor than methacholine or histamine, both in asthmatics and in patients with COPD or allergic rhinitis.61
Indications, Contraindications and General PrecautionsAlmost all asthmatics, atopic or not, respond to the inhalation of AMP concentrations =400mg/ml with bronchoconstriction, although this finding has also been observed in some healthy individuals and in COPD or allergic rhinitis.58 In other words, provocation with adenosine is much more specific than sensitive; it can be used to confirm asthma but not to discard other diagnostic possibilities. Beyond that, there are other settings in which its use may well be justified. For example, the bronchoconstriction observed after inhaling AMP has a close relationship with the magnitude of the eosinophilia in the respiratory tract of asthmatic patients, reflects the state of activation of the mast cells and is an indicator of the allergenic load and activity.58,61 It also seems clear that the response to adenosine is modified in an earlier, more sensitive and intense manner after inhaled corticosteroids than the response to methacholine.58,61
Under such premises, it is hardly surprising that the adenosine BPT has been proposed as a useful tool in two specific contexts58,61,62: (a) for clinical trials aimed at monitoring the response to corticosteroids, montelukast, omalizumab, allergen avoidance or specific immunotherapy; and (b) to identify which patients controlled with inhaled corticosteroids will tolerate reduction/withdrawal of treatment without destabilising or to determine their minimal effective dose.
There are no specific limitations for adenosine, except known hypersensitivity to the product itself. The list of absolute and relative contraindications already noted for the methacholine BPT applies here.2,58,61 Before performing the AMP test, anti-histamines, ß2-adrenergic agonists, anti-muscarinics, theophyllines, chromones and anti-leukotrienes should be suspended, while also bearing in mind that adenosine is extremely sensitive to the effect of inhaled glucocorticoids and that these should be withdrawn 12h previously. Technicians and physicians responsible for performing this test should be properly trained in bronchial provocation techniques and able to manage severe asthma attacks.
MannitolBasic FoundationsThis substance is a hexahydrate sugar alcohol (C6H14O6), with a molecular mass of 182.17g/mol, and is a stereoisomer of sorbitol.63,64 Up to the 1920s, it source was manna, a product derived from heating the bark of the Fraxinusornus tree. It is currently produced by high pressure hydrogenation, but manufacture using fermentation processes is already underway.63,65 It possesses osmotic properties and has been used for decades as a food additive (in diet foods and chewing gum). It was also introduced some years ago in the medical field to relieve intracranial hypertension, replace blood plasma and as a diuretic.63 When inhaled, mannitol dehydrates the bronchial mucosa, which is accompanied by an increase in osmolarity in the mucosa and release of inflammatory mediators from the mast cells, with a profile very similar to that evoked by adensosine.17,64,66,67 Its use as a bronchial provocation agent was approved in 2006 in Australia and later in the European Union (2007), Asia and the United States (2010). It was authorised in Spain in July 2008.
In the Spanish market, for the analysis of BH, mannitol is marketed in the form of dry powder capsules with preset doses (Osmohale™).
Data on the use of Osmohale™ during pregnancy are limited. Animal studies do not suggest direct or indirect harmful effects in terms of reproductive toxicity. However, the effects of a possible hyperreactivity reaction on the mother and/or foetus are unknown, so Osmohale™ should not be administered to pregnant women.68 With respect to lactation, no effects are expected in breastfeeding infants, since systemic exposure to mannitol in breastfeeding mothers is insignificant. Studies with oral mannitol do not suggest effects on fertility either.68
Addresses of interest: Osmohale™ distributor: Aldo-Unión, S.A. Baronesa de Maldá 73. Esplugues de Llobregat 08950, Barcelona. Contact telephone number: +34933727111. Fax: +34933716198. Website: http://www.aldo-union.com/. Email: info@aldo-union.com. General test information: http://www.aridol.info/spain.
Technical Foundations, Equipment and Recording the ResponseThe mannitol test requires less equipment and is much more simple than the other indirect tests (particularly those related with exercise), providing reasonably superimposable results.63 The Osmohale™ kit contains 19 capsules (one without active substance, three capsules containing 5, 10 and 20mg, respectively, and 15 capsules containing 40mg) together with a dry powder inhaler (Inhalator™, Boehringer Ingelheim, Germany). The FEV1 is generally used to record the response.68
ProceduresThe steps to follow are as follows68: (a) with the patient seated comfortably, apply a nose clip and instruct them to breathe through the mouth; (b) insert the 0mg capsule into the device. Puncture the capsule by depressing the buttons on the sides of the device carefully once only, since a second puncture may shatter it; (c) the patient should exhale completely away from the inhaler and then should bend their head slightly forwards, holding the device at 45°, to perform the inspiration manoeuvre which should be deep, rapid and short; (d) at the end of the inspiration, start a 60s timer; the patient should hold his or her breath for 5s and exhale through the mouth before removing the nose clip; (e) at the end of the 60s, perform spirometry, measuring the FEV1 at least in duplicate to obtain two reproducible measurements; the highest reading will be the baseline FEV1 value; (f) insert the 5mg capsule in the inhaler and proceed as above; and (g) the following doses to be administered will be: 10mg, 20mg, 40mg, 80mg (two consecutive 40mg capsules), 160mg (four 40mg capsules) and 160mg (four 40mg capsules); the test should be stopped when a total dose of 635mg is reached or the patient has a positive response.
After inhalation of each dose, the capsule should be checked to ensure that it is empty. A second inhalation should be performed if it has not been completely emptied.68 After completion of the test, a standard dose of a fast-acting ß2-adrenergic agonist should be administered. The test duration is variable and depends, logically, on whether BH is detected (17±7min) or not (26±6min).69
Measurement and Interpretation of ResultsA response is considered to be positive when68: (a) there is a =15% fall in FEV1 with respect to the baseline value (using the FEV1 value after the administration of 0mg as a comparator) (PD15); or (b) there is a =10% incremental fall in FEV1 between two consecutive doses. Some studies recommend using the fall threshold of 10% (PD10) calculated by interpolation of the log-linear dose–response curve and thereby classifying BH in asthmatic subjects into severe (0–75mg), moderate (75–315mg) or mild (315–635mg).70
The repeatability of the mannitol test is similar or better than other BPTs that use drugs, both in adults67,71 and in children.72,73 However, after repeated tests within a short space of time, there is a refractory period (the same occurs with exercise-induced bronchoconstriction) due to a phenomenon of tachyphylaxis in the response of the ASM to contracting mediators released from the mast cells.74
Indications, Contraindications and General PrecautionsThe mannitol test translates into the intensity of asthmatic airway inflammation to a large extent, is modified by the action of inhaled corticosteroids and its PD15 maintains a close relationship with: (a) the responses evoked by other indirect stimuli (adenosine, exercise, eucapnic hyperpnoea and hypertonic saline); and (b) the amount of eosinophils in sputum and the FENO.63,66 The specificity of mannitol in the diagnosis of asthma is above 95%, its sensitivity is around 60% and its predictive values depend on the pretest probability of asthma.63,66,75 The sensitivity increases to almost 90% when patients on steroid treatment are excluded.69
Thus, the mannitol BPT is presented as a very interesting tool when evaluating the effectiveness of anti-asthmatic treatment and establishing the degree of control reached, and even for monitoring the evolution of occupational asthma.18,63,66,76 At the same time, the technique facilitates the collection of bronchial secretions (which increase after the test), providing a further source of analysis of airway inflammation at a given time, if desired.77
Finally, but no less importantly, the inhaled mannitol test is an alternative to consider for the diagnosis of exercise-induced asthma with an efficacy similar to that obtained with eucapnic hyperventilation.63,78,79
In this respect, the International Olympic Committee has approved its use for the study of BH in elite athletes, and to justify the use of ß2-adrenergic agonists before a sporting event.64
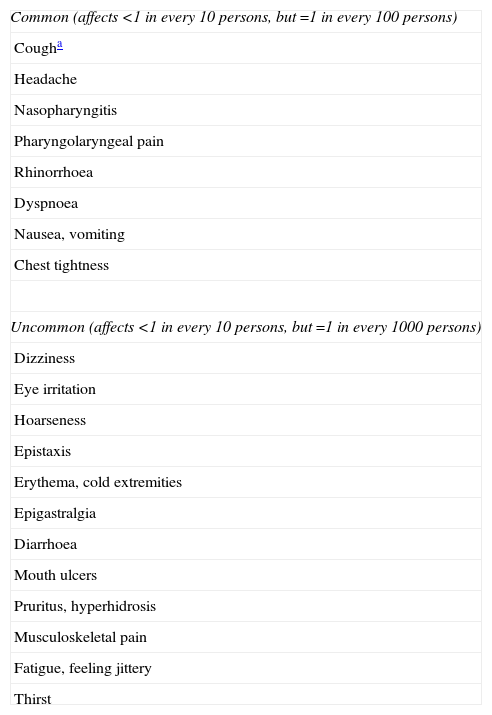
Mannitol is a safe compound; the most commonly observed side effects are cough (83%), which may sometimes cause interruption of the test, and headache.68,69 The tusigenic effect is associated with asthma, regardless of the bronchoconstriction, and tends to decrease after inhaled steroid treatment. Headache usually appears more often in subjects with a negative test, probably due to the high number of forced expiration manoeuvres performed.68,69 The complete list of its adverse effects is shown in Table 8.
Adverse Effects of Inhaled Mannitol (Adapted From Reference68).
| Common (affects <1 in every 10 persons, but =1 in every 100 persons) |
| Cougha |
| Headache |
| Nasopharyngitis |
| Pharyngolaryngeal pain |
| Rhinorrhoea |
| Dyspnoea |
| Nausea, vomiting |
| Chest tightness |
| Uncommon (affects <1 in every 10 persons, but =1 in every 1000 persons) |
| Dizziness |
| Eye irritation |
| Hoarseness |
| Epistaxis |
| Erythema, cold extremities |
| Epigastralgia |
| Diarrhoea |
| Mouth ulcers |
| Pruritus, hyperhidrosis |
| Musculoskeletal pain |
| Fatigue, feeling jittery |
| Thirst |
General precautions relative to performing spirometries and BPTs as listed in extenso in previous sections should be observed for the mannitol test, including extra precautions in patients with haemoptysis of unknown origin, pneumothorax and recent abdominal or thoracic surgery.68
ExerciseBasic FoundationsPhysical exercise provokes bronchospasm in a significant proportion of asthmatic patients.80 It is also the only trigger in a group of patients who play sports or have an occupation that requires a high level of physical effort, thus defining the entity known as exercise-induced asthma (EIA). The prevalence of EIA has been estimated at 40%–90% in asthmatics, 10%–50% in elite athletes, 20%–35% in ice skaters, 30%–50% in Nordic ski competitors, 15% in long distance runners and 11%–28% in competitive swimmers.81 Particularly relevant predisposing factors are atopy (especially in long distance runners), a high concentration of pollutants from the combustion of fossil fuels in the inhaled air and the inhalation of chloramines in swimming pools. If the presence of a reversible obstruction has not been documented, EIA must be diagnosed using an exercise bronchial provocation test (EBPT) or other properly accredited alternative bronchial provocation test.28,81,82
The development of exercise-induced bronchospasm (EIB) has been justified by three pathogenic hypotheses: the heat, osmolar and capillary theories. The heat theory attributes bronchospasm to an adrenergic reflex triggered by temperature loss in the airways.83 The hyperventilation caused by exercise also increases the periciliary osmolarity, due to dehydration of the airway surface liquid, which could favour the degranulation of mast cells and release of inflammatory mediators.84 Finally, the hyperaemic or capillary theory proposes that microvascular vasoconstriction due to cooling of the airway wall during exercise is followed by rapid reheating in the recovery period, causing reactive hyperaemia and oedema of the airways.85 Since none of these physiopathological theories fully explains the phenomenon, it has been suggested that the pathogenic mechanism may be different for each individual, type of exercise and environmental condition.
In half of patients, EIB has a refractory period consisting of a decrease in the bronchoconstrictive response when the exercise is repeated within an interval of under 2h.80 This phenomenon, which may be crossed between various indirect stimuli,16,71 was attributed to the depletion of inflammatory mediator deposits by the mastocytes.16 However, controversy remains over its origin, and more recent hypotheses consider that it may be due to the absence of a response to the hyperosmolar stimulus in repeated exercise.86
Technical FoundationsIndications, Contraindications and General PrecautionsThe main indications for the EBPT are82: (a) to confirm the diagnosis of EIA, especially in subjects with normal spirometry and a negative bronchodilator test but with high clinical suspicion, due to its greater sensitivity than isocapnic hyperventilation with cold air; (b) to stratify asthma severity; (c) prevention in professions and athletes in which an EIB could endanger the life of the individual or others; (d) monitoring anti-inflammatory therapy; and (e) epidemiological studies. Table 9 shows the main contraindications of the test. In addition to the usual recommendations for nonspecific bronchial provocation,27,28 an EBPT should not be carried out in patients with unstable cardiac ischaemia, malignant arrhythmias, or orthopaedic limitations that impede performing high intensity exercise.87
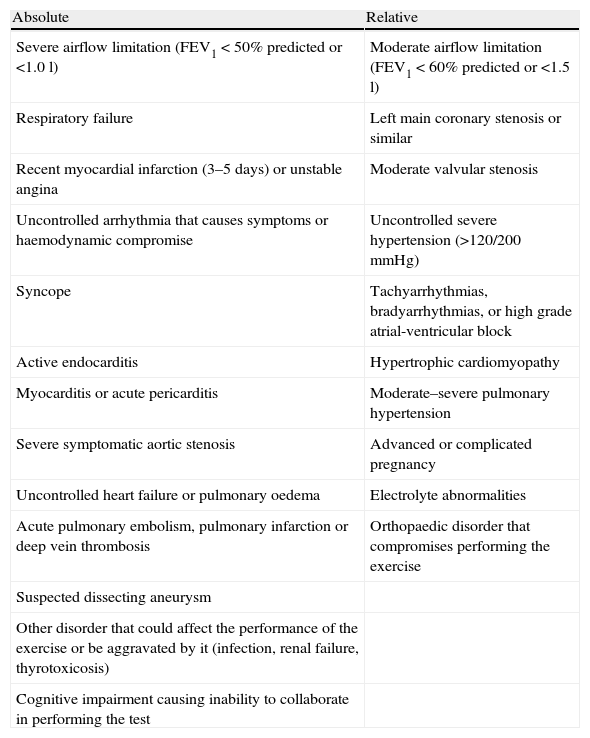
Contraindications for the Exercise Bronchial Provocation Test.
| Absolute | Relative |
| Severe airflow limitation (FEV1<50% predicted or <1.0l) | Moderate airflow limitation (FEV1<60% predicted or <1.5l) |
| Respiratory failure | Left main coronary stenosis or similar |
| Recent myocardial infarction (3–5 days) or unstable angina | Moderate valvular stenosis |
| Uncontrolled arrhythmia that causes symptoms or haemodynamic compromise | Uncontrolled severe hypertension (>120/200mmHg) |
| Syncope | Tachyarrhythmias, bradyarrhythmias, or high grade atrial-ventricular block |
| Active endocarditis | Hypertrophic cardiomyopathy |
| Myocarditis or acute pericarditis | Moderate–severe pulmonary hypertension |
| Severe symptomatic aortic stenosis | Advanced or complicated pregnancy |
| Uncontrolled heart failure or pulmonary oedema | Electrolyte abnormalities |
| Acute pulmonary embolism, pulmonary infarction or deep vein thrombosis | Orthopaedic disorder that compromises performing the exercise |
| Suspected dissecting aneurysm | |
| Other disorder that could affect the performance of the exercise or be aggravated by it (infection, renal failure, thyrotoxicosis) | |
| Cognitive impairment causing inability to collaborate in performing the test |
The EBPT should be directly supervised, both during the exercise and in the recovery period, by a doctor or experienced technician who has been sufficiently trained to detect exercise-related respiratory or cardiac problems. In the latter case, the rapid availability of a doctor must be guaranteed for treatment of any complications. It is recommended that a 12-lead electrocardiogram be performed previously in patients over 60 years of age or with any known cardiovascular disorder.28 A sufficiently equipped crash trolley should also be available in the exercise test area.
Patient Preparation and Environmental ConditionsThe patient must not have done any strenuous physical exercise in the 4h prior to the study, should wear proper clothing and footwear, have eaten a light meal 2h before the study and must have completed the asthma treatment-free interval. It should be remembered that inhaled and intranasal corticosteroids reduce the intensity and prevalence of EIB, especially in children.88 Although it is not a formal contraindication, it is recommendable that the patient has an FEV1 >75% of the predicted or >80% of the previous at baseline, with variability less than 10% and oxyhaemoglobin saturation (SpO2) >94%.80 It is also recommended that it has been more than 6 weeks since the last respiratory tract infection and, in subjects who are sensitised to pollens, not to perform the test in the pollen season.
An increase in the ambient humidity reduces EIB,89 while cold increases it.90 It is therefore recommended to record the humidity and temperature of the inspired air when performing the EBPT. It should be carried out in ambient temperature conditions of between 29 and 25°C, with relative humidity less than 50% (10mg H2O/l). Performing the test in an air-conditioned room, programmed for the aforementioned temperature conditions, may be sufficient to reach these requirements. If these conditions cannot be achieved, it is recommended to breathe from a tank of dry medicinal air through a valve on demand or from a regulator.91
ProceduresThe exercise should last 6–8min, or up to 6min in children under 12 years.27 It is also recommended that patients use a nose clip, since nasal respiration reduces water loss from the airways.28 Ventilation or heart rate can be used for monitoring exercise intensity in children. It is recommended that the ventilation be recorded in adults, since, in addition to being a trigger for EIB, it is less variable than the heart rate and is not as dependent on the fitness level.92
It is preferable to record the ventilation using a mouthpiece and nose clip, although if a naso-bucal mask is used, it should include a nasal occluder. The exercise intensity must guarantee that the patient maintains ventilation greater than 40%–60% of their maximum voluntary ventilation (calculated as FEV1 [l]×35) for the last 4–6min.27 The heart rate should be monitored systematically with a 3-lead ECG, or 12-lead ECG if there is a risk of coronary syndrome.27 In the case of monitoring by heart rate, the exercise intensity should be modulated to achieve, in less than 4min, a target heart rate between 80% and 90% (around 95% is recommended in high level athletes) of the estimated maximum heart rate (calculated as 208-age in years×0.7). The SpO2 should also be monitored during the entire study, or at least at the start and end of the test.28
Either a cycle ergometer or treadmill may be used for the EBPT. The performance of both systems is equivalent for similar exercise intensity levels.93 If both are available, the one that adapts best to the sports played by the individual studied should be used. The slope and speed should be adapted on the treadmill until the target ventilation or heart rate is achieved. The test should be commenced by increasing the speed until the patient is walking fast but comfortably, gradually increasing the slope for the first 2 or 3min until the required exercise intensity is reached. A typical protocol is to start with a speed of 4.5km/h with a slope of 2.5%.80 The following is proposed for calculating the workload on the cycle ergometer: 53.76×FEV1 [l]-11.07.28 Sixty percent (60%) of the power (W) thus calculated should be applied in the first minute, 75% in the second, 90% in the third and 100% in the fourth.28 When the subject reaches the required ventilation level, the power should be maintained for the next 4min, although it may need to be reduced slightly so that the target ventilation can be sustained for 4–6min.
The safety directives and reasons for stopping the EBPT are those normally recommended in exercise tests.87 Although some epidemiological studies have used free running as another exercise provocation modality, it has low sensitivity and specificity for detecting EIB (60%–67% and 47%–67%, respectively),94 so there is insufficient evidence to recommend it as a diagnostic procedure. Furthermore, it does not allow the exercise intensity to be monitored nor patient safety to be controlled during the provocation.
Assessment of the ResponseA reference spirometry should be performed immediately before the exercise and repeated 5, 10, 15, 20, and 30min after its completion.28 If the FEV1 has returned to its baseline value (±5%) after 20min, the 30min post-exercise spirometry can be skipped.28 At each stage of the recording, two reproducible manoeuvres should be obtained for the measurement variable (difference FEV1<0.15l) and the one with the highest FEV1 selected.28 The spirometric manoeuvres must meet the acceptability criteria for the start of the manoeuvre,34 although the expiratory time does not need to be prolonged beyond the first 2–3s.28 Suspected vocal cord dysfunction is an exception for this recommendation. When this occurs, complete flow-volume curves should be obtained as soon as possible after the exercise, to demonstrate the existence of inspiratory flow limitation.27
Serial post-exercise spirometries should not be halted unless the patient has a fall in FEV1>50%, in which case inhaled bronchodilators must be administered immediately. If a fall in the FEV1 persists after the measurements have been concluded, a fast-acting ß2-adrenergic agonist must be administered and the patient should remain under observation until he or she recovers 95% of their baseline value.27,28 Recording the potential delayed response is not recommended, since the interpretation of a fall in lung function 3–8h after the end of the exercise is unclear.27
Measurement and Interpretation of ResultsThe result of the EBPT is expressed using a time–response curve, where the response is the percentage fall in the FEV1 with respect to the value prior to the exercise. An EBPT is considered to be positive when the FEV1 falls by more than 10% with respect to baseline.28 In turn, the EIB is classified as mild when the fall in FEV1 is between 10% and 25%, moderate between 25% and 50% and severe if it is greater than 50%.95 It may be useful in epidemiological studies to express the intensity of the response by determining the area under the time–response curve, using a trapezoidal method on the graph.95
Good reproducibility in the FEV1 fall, with a coefficient of variation of 15% in one week and 21% in one month can generally be assured by following a strict protocol.95 The EBPT is more specific than direct stimuli for the diagnosis of EIA, although is notably less sensitive.16,58,96 It has high specificity for the detection of EIB in children, although its sensitivity is more modest.97 In adults, the sensitivity does not increase and the specificity is lower than that obtained in children.98
Eucapnic Voluntary HyperventilationBasic FoundationsThe aforementioned mechanisms that trigger bronchoconstriction in response to intense exercise can be simulated with eucapnic hyperventilation.99
Technical FoundationsEquipmentThis test requires the subject to voluntarily hyperventilate dry air at ambient temperature (or cold) in a low resistance circuit that contains 5% CO2 to maintain a pCO2 similar to that in exercise. The inspired air should be dry and contain 21% O2, 4.9%–5.1% CO2 and the rest made up of N2. The most simple technique is to have a bottle with this mixture of gases connected to a reservoir (120l meteorological balloon or similar), from which the patient breathes through a low resistance one-way valve. CO2 can be added to the inspired air, but this requires measuring the CO2end-tidal fraction to maintain the eucapnia, which makes the test more complex. The inhaled air may also be cooled. In asthmatic patients, the response to 4min of hyperventilation with cold air is equivalent to that obtained after hyperventilation with room air for 8min.100
During the test, the minute volume should be measured, preferably with a turbine spirometer, to verify that the minute ventilation marked as a target is reached. As in other BPTs, serial spirometries are performed to evaluate the response. The equipment required should be similar to that proposed for performing standard spirometry.34,35
ProcedureThis test is likely to make the patient uncomfortable, so the reasons for its indication should be explained, what it is hoped to achieve, as well as asking for their cooperation in maintaining maximum ventilation for the time required and in performing the spirometries. The existence of known factors that can modify the response to the hyperventilation should be assessed (Table 5).28 It is particularly important to tell the patient that they must not do any strenuous physical exercise in the 4h prior to the test (or better, on the day of the test), as refractoriness may appear.101 Short or long-acting ß2-adrenergic bronchodilators and anti-cholinergics can prevent the response to hyperventilation.102 There is also evidence that the administration of chromones may inhibit the response to isocapnic hyperventilation.103 Studies with small patient numbers have also demonstrated that the administration of montelukast 6h before the test has a protective effect against hyperventilation.104 With respect to inhaled corticosteroids, there are data that show that the response to hyperventilation is decreased, both with chronic (6 months) and acute use (4h).99,105
ProtocolsSingle and multi-step protocols are available.
Single-step ProtocolThis is the most widely used protocol, especially in elite athletes with suspected EIA.106 It requires the subject to perform voluntary hyperventilation for 6min, aiming to maintain a minute ventilation greater than 30×FEV1 (measured before the start of the test), which is equivalent to 85% of the maximal voluntary ventilation (MVV). In some cases, the test may be positive even without reaching this level of hyperventilation. The meteorological balloon is initially filled with 90l of gas and is then kept filled at a rhythm close to the minute ventilation that the subject must maintain. The subject breathes through a one-way valve, inhaling from the bag used as a reservoir and exhaling the air into the room. Once the subject starts hyperventilating, he or she should be encouraged to keep the ventilation rhythm using the inflation and deflation of the balloon as a reference.
Multi-step ProtocolThis protocol is safer and should be used when the baseline FEV1 is less than 75% or moderate–severe asthma is suspected.107 The ventilation rate is increased over 3 steps:
- -
Step 1: 3min at 30% of the MVV.
- -
Step 2: 3min at 60% of the MVV.
- -
Step 3: 3min at maximal ventilation.
After each step, the response is evaluated by measuring the FEV1 and, if negative, the following step is then performed.
The response to bronchoprovocation with identical hyperventilation is reproducible when performed on separate days.108
Measurement and Interpretation of ResultsBronchoconstriction in response to hyperventilation is detected by the percentage fall in the FEV1. In the single-step protocol, the test is considered positive if there is a =10% fall in the FEV1.109 The stimulus is reported as the mean of the minute ventilation during the 6min of the test, expressed as a percentage of the theoretical MVV (which is calculated by multiplying the FEV1 measured by 35). The maximal ventilation reached by a healthy person during the exercise rarely exceeds 60% of the MVV, or 90% in the case of elite athletes. The response provoked by hyperventilation is considered mild if the FEV1 falls by 10%–19.9% with ventilation equal to or greater than 60% of the MVV, moderate if it falls between 20% and 29.9% and severe if the fall is =30% for any grade of ventilation or if the fall is >10% with ventilation less than 30% of the MVV.109
In the multi-step protocol, the response is evaluated at minutes 1, 3, 5, 7, and 10 after each step. If the FEV1 falls by more than 15%, the test is stopped. If it does not fall, or reaches a plateau with values less than 15% after minute 5, it proceeds to the next level. After the third step, serial spirometries are performed at 5, 10, 20, and 30min, or until the subject's FEV1 has returned to its baseline value. The minute ventilation that causes a 10% fall in the FEV1 (PVE10) is calculated by linear interpolation, using the response at each ventilation level, and is expressed as a percentage of the subject's theoretical MMV.
Indications, Contraindications and General PrecautionsThe BPT with eucapnic voluntary hyperventilation is recommended by the International Olympic Committee for the diagnosis of EIA in elite athletes. It is an alternative to the EBPT, previously described, which has false negatives due to the difficulty in controlling the ambient conditions in the laboratory. Eucapnic hyperventilation offers the possibility of performing the evaluation in the laboratory, with controlled environmental conditions (dry air at ambient temperature or cold). In field studies, it has been shown to be better than the exercise provocation test for identifying EIA.110 The contraindications for eucapnic voluntary hyperventilation are the same as for the methacholine BPT.
As with other bronchoprovocation tests with indirect stimuli, eucapnic hyperventilation correlates well with airway inflammation and has high specificity for the diagnosis of asthma. Hyperresponsiveness to indirect stimuli, which requires the presence of inflammatory cells in the airways, is attenuated with inhaled corticosteroid treatment and, therefore, can be used to measure the control of inflammation.111
Eucapnic voluntary hyperventilation performs better than methacholine in identifying athletes with EIA.112 In asthmatic non-athletes with symptoms of EIA, there is poor agreement between both techniques, with more positive results when provoked by hyperventilation.113
Hypertonic Saline Bronchial Provocation TestBasic FoundationsInhalation of non-isotonic saline aerosols can cause bronchoconstriction due to the release of endogenous mediators that cause contraction of the ASM and oedema.17 Changes in the airway osmolarity caused by these types of aerosols promote the release of mediators, such as histamine, leukotrienes and prostaglandins,114,115 as well as causing neural alteration, with release of neuropeptides and an increase in the parasympathetic tone.116 In fact, drugs that reduce the release of mast cell mediators or that modify non-cholinergic neural activity, such as nedocromil or furosemide, play a protective role against hypertonic saline BH,27 as occurs with inhaled corticosteroids.117
Technical Foundations, Equipment, Procedure and Measurement and Interpretation of ResultsFor the hypertonic saline BPT, it is recommended to use 4.5% saline solution, which should be administered using ultrasonic nebulisers, as they generate a more dense aerosol than jet nebulisers.27 It also requires a two-way non-rebreathing valve and a connection tube with the nebuliser, which must have smooth inner walls and constant length and diameter. The valve should have a saliva collector, since the test usually causes profuse salivation. The ultrasonic nebuliser used should generate an aerosol flow of at least 1.2ml/min and have an easily disassembled cup with a capacity for 100–200ml of solution. The most commonly used nebulisers (Mistogen and De Vilbis Ultraneb) provide a flow of 1.5–3ml/min. In any case, it should be determined by weighing the cup and tubing before and after a nebulisation. Once the nebuliser flow has been established, the dose administered can be determined in an operating period.
The increase in the hypertonic saline dose is obtained by gradually increasing the nebulisation times. In the first step, the subject is nebulised for 30s, then 1min, 2, 4 and 8min in the following steps. The bronchoconstrictive response is measured at 30–90min using spirometry, and if the fall in FEV1 exceeds 10% with respect to the baseline value, the last exposure time is repeated instead of proceeding to the next step.27 The test is considered to be positive when there is a >15% fall in the FEV1,118,119 at which point it should be discontinued. A bronchodilator should be administered both in this case and if the test is ended after reaching the maximum dose.
The bronchial response to hypertonic saline solution is expressed using a dose–response curve, which shows the change in the FEV1 (percentage fall with respect to the baseline value) against the cumulative dose of aerosol administered (in ml). The PD15 is determined by linear interpolation, enabling the severity of the BH to be classified into mild (PD15>6ml), moderate (PD15: 2.1–6.0ml) and severe (PD15<2.0ml).120 There is still insufficient information to recommend the systematic determination of bronchial reactivity indices.117,121
The hypertonic saline BPT may be useful for the diagnosis of asthma, reaching a sensitivity of 85% in adults and 75% in children, with a specificity of almost 100%.122,123 The response to hypertonic saline has a notable relationship with the presence of respiratory symptoms and the use of rescue medication.117,124 Moreover, in the detection of exercise-induced bronchospasm, it may reach results similar to exercise provocation,125 and even slightly higher in children.7 Nevertheless, in many cases, its clinical use may be compromised with the introduction of the mannitol BPT.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Perpiñá Tordera M, et al. Normativa sobre el estudio de la hiperrespuesta bronquial inespecífica en el asma. Arch Bronconeumol. 2013;49:432–46.