In the last two decades, there has been a change in the natural history of neuromuscular diseases due, in large part, to improvements in the diagnosis and treatment of the respiratory complications that represent the leading cause of death.1 The increasingly widespread application of ventilatory support and assisted coughing, as well as the progressive change in the clinical approach to these patients, with early evaluation of respiratory function and management by multidisciplinary teams, has resulted in a considerable improvement in the quality and life expectancy of these patients.2,3
It was therefore considered timely to develop these SEPAR Guidelines, which are intended as a practical, simple and up-to-date guide, which may be a useful tool in clinical practice for all respiratory medicine specialists. The main recommendations of the Guidelines are shown in Table 1. In the most relevant issues, the GRADE4 system (Table 2) has been followed for grading the quality of evidence and strength of the recommendations available. Many of the recommendations have GRADE 1B/1C, i.e. although more scientific evidence is required in this respect, the ethical difficulty in conducting the pertinent studies means that these are questionable and, with the information available, the recommendation becomes final (Figs. 1 and 2).
Ten Basic Rules for Respiratory Care of the Neuromuscular Patient.
| 1. Assessment of lung function should be made in all neuromuscular patients, even in the absence of symptoms, and should be subsequently monitored |
| 2. For the choice of future treatments, it is very important to distinguish rapidly and slowly progressive diseases |
| 3. It is advisable to evaluate the possible existence of cardiovascular diseases and associated aspiration pathology |
| 4. Difficulty in draining respiratory secretions requires specific respiratory physiotherapy and occasionally mechanical assistance to achieve effective cough |
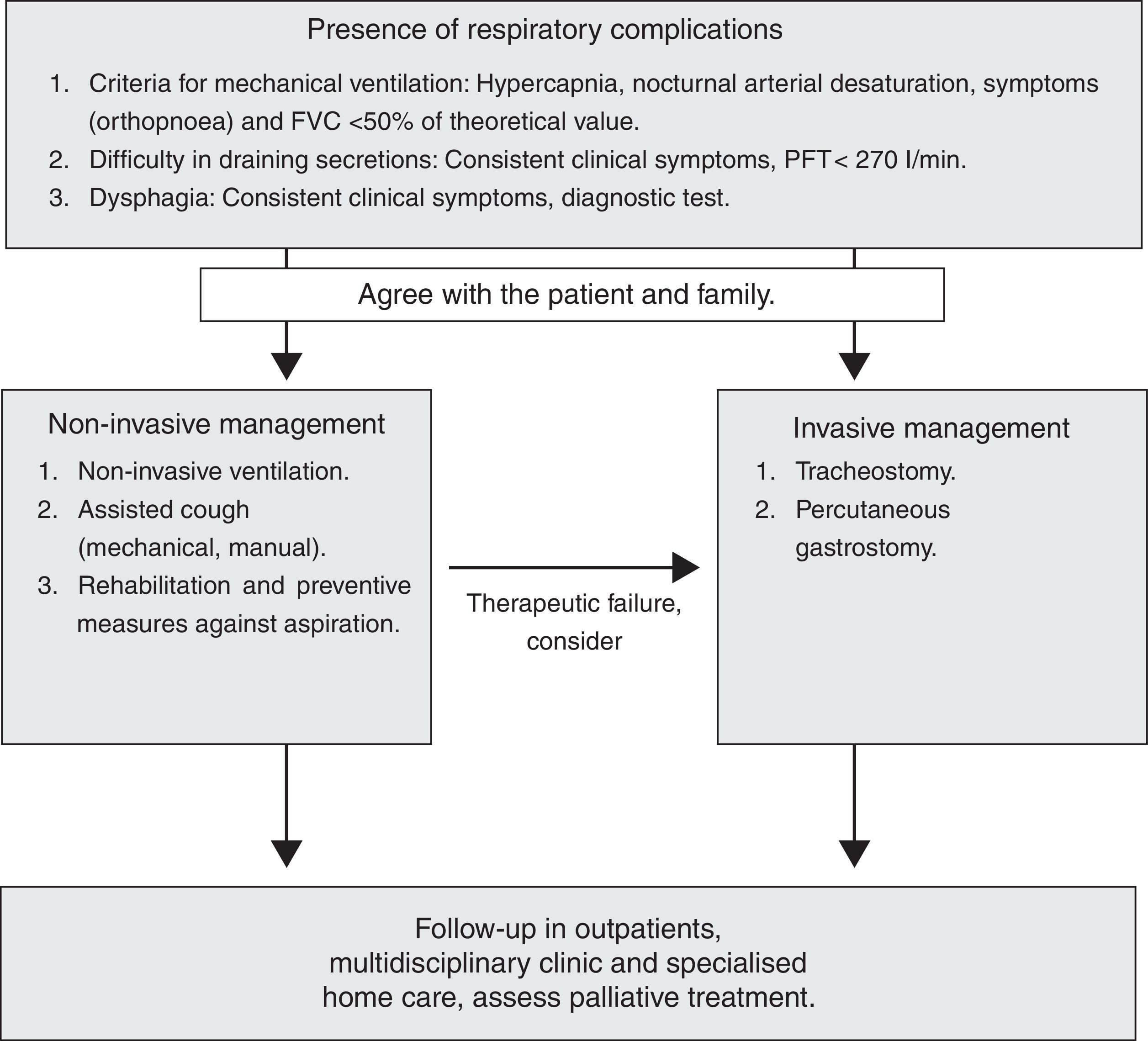
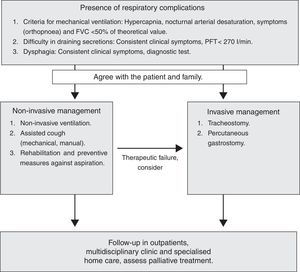
| 5. Ventilatory assistance is indicated when there is severe ventilatory impairment (FVC <50%), symptoms of diaphragmatic dysfunction (orthopnoea) and/or hypoventilation (hypercapnia) |
| 6. The correct choice of equipment and ventilation mode is fundamental. Portable respirators designed for life support are recommendable |
| 7. The indication for treatment with invasive mechanical ventilation by tracheostomy should be individualised and requires adequate care infrastructure |
| 8. Early, continuous communication with the patient and their family, and advance decision-making, is essential for choosing the best therapeutic measures, especially invasive mechanical ventilation |
| 9. Palliative treatment should not be delayed when indicated |
| 10. Multidisciplinary, coordinated care by all professionals involved in the management of these patients is desirable |
Grading of the Quality of Evidence and Strength of Recommendations According to the GRADE System.
| Quality of the evidence | Code | |
| High | Further research is unlikely to change our confidence in the estimate of effect | A |
| Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect | B |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect | C |
| Very low | Any estimate of effect is very uncertain | D |
| Strength of the Recommendation | |
| Strongly in favour of the intervention | 1 |
| Weakly in favour of the intervention | 2 |
| Weakly against the intervention | 2 |
| Strongly against the intervention | 1 |
Adapted from Schünemann et al.4
Respiratory muscle involvement occurs during the course of many neuromuscular diseases; in some cases it may appear in acute form (Guillain–Barré syndrome, myasthenic crisis, acute phase of poliomyelitis), although it presents progressively in most. For proper management of respiratory complications, it is important to distinguish rapidly progressive diseases, such as amyotrophic lateral sclerosis (ALS), from those that progress more slowly, such as Duchenne disease or myotonic dystrophy (Steinert's disease), as early respiratory action is more important in the former.
Three muscle groups are involved in the onset of respiratory complications: the inspiratory muscles, expiratory muscles and oropharyngeal muscles.
The pathophysiological mechanisms involved in the development of respiratory failure are various and complex,5,6 although they can be summarised in three:
Progressive weakness in the inspiratory muscles (mainly the diaphragm), secondary to the intrinsic cause of the muscle disease, leads to a respiratory pattern with low tidal volumes and increased frequency (superficial respiration). The muscle weakness also leads to changes in the mechanics of the respiratory system with reduced distensibility of the lungs and rib cage, resulting in a subsequent increase in workload and risk of muscle fatigue. This causes alveolar hypoventilation, which initially appears during the night in REM sleep phases in which muscle atonia occurs (except for the diaphragm). Alterations in the central control of ventilation must also be considered, either due to loss of sensitivity of the central and peripheral chemoreceptors or to direct damage of the respiratory centres, as occurs in myotonic dystrophy (Steinert's disease) or acid maltase deficiency. Sustained nocturnal hypoventilation, alterations in the control of ventilation and the change in the respiratory pattern will eventually lead to diurnal hypoventilation, which usually develops progressively, although it may occasionally appear acutely in the context of a respiratory infection secondary to the retention of secretions due to ineffective cough or bronchoaspiration.
Ineffective cough is secondary mainly to weakness of the expiratory muscles (internal intercostal and abdominal muscles), although the other muscle groups may also participate in this disorder. Producing an effective cough initially requires taking a deep breath (inspiratory muscles) followed by maximum contraction of the expiratory muscles, with glottis closure and subsequent opening (oropharyngeal muscles), generating an expiratory flow capable of eliminating secretions.
Impairment of the oropharyngeal muscles, in addition to contributing to ineffective cough, will cause phonation and swallowing disorders with a risk of bronchoaspiration that may lead to acute respiratory failure.
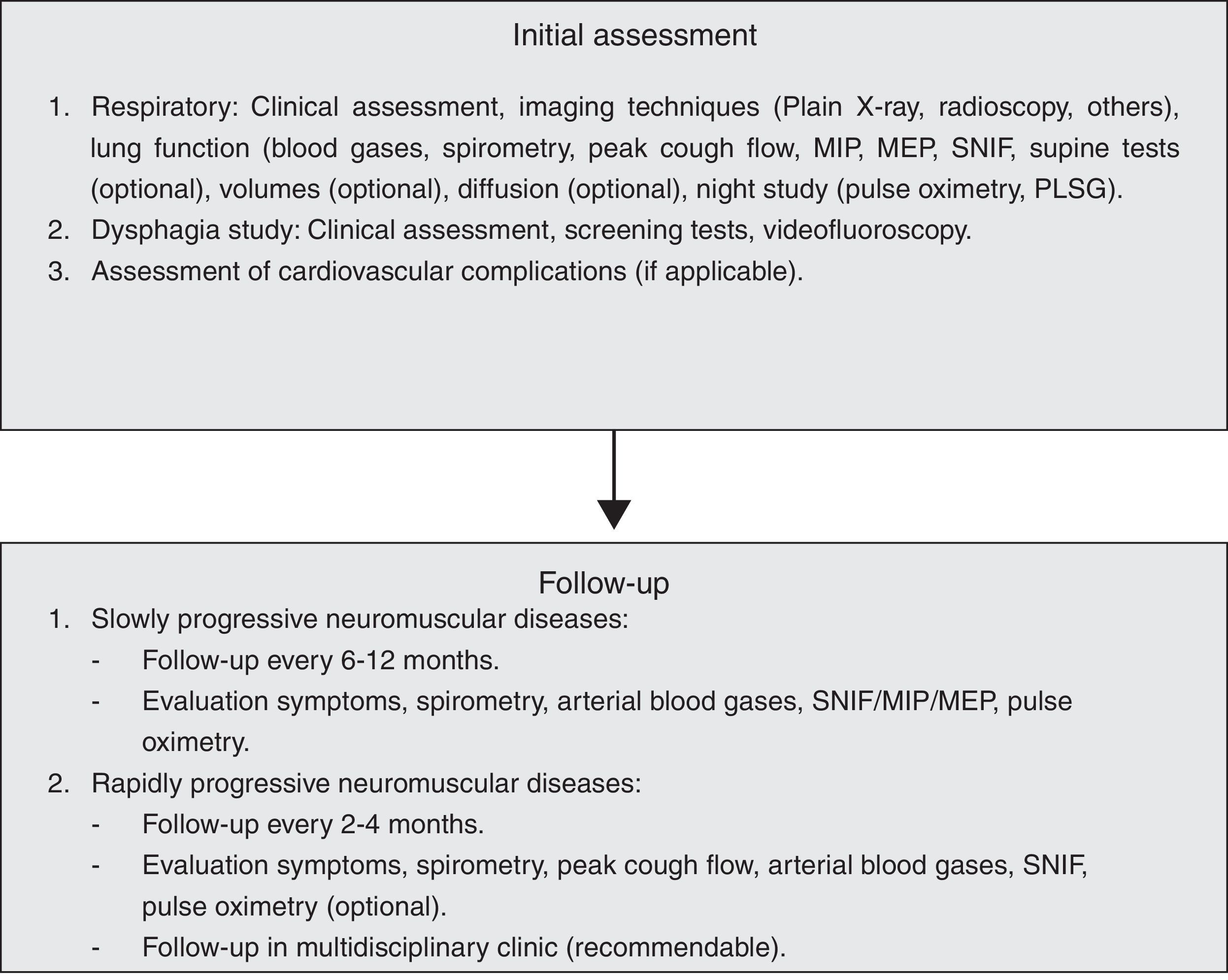
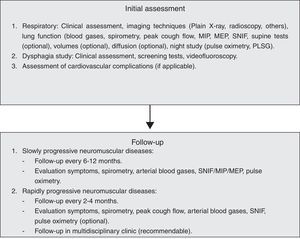
Respiratory Evaluation of the Neuromuscular PatientObjective tests enable us to estimate the prognosis and monitor the evolution, start mechanical ventilation (MV) and anticipate complications.7
Symptoms and signs of muscle failure will be sought in the clinical assessment. Malaise, lethargy and difficulty concentrating appear early on, while dyspnoea and orthopnoea appear later. In bulbar (VII, IX, X and XII pairs), masticatory (V pair) and laryngeal (C1 root) involvement, dysarthria, dysphagia, weak mastication, choking and ineffective cough are found. Hypersomnia and tiredness on waking, as well as malaise, drowsiness and morning headache, suggest sleep hypoventilation.
On the physical examination, an increase in the respiratory rate, thoracoabdominal incoordination, recruitment of the accessory muscles of the neck and weakness of the trapezium can be noted. The technique of counting words after maximum inspiration is associated with hypoventilation (50 is normal and less than 15 is severe), but has not been validated.
Functional examination of the neuromuscular patient includes mainly the following additional tests:
Arterial Blood GasesAlthough hypoxia with hypocapnia can be detected in an incipient phase, the typical finding is hypercapnia with a normal alveolar–arterial gradient, bicarbonate retention and hypochloraemia (in the blood test). If there is previous lung disease or complications (infections and atelectasis), the gradient will be high.
Spirometry and VolumesThe presence of a restrictive ventilatory defect is characteristic, with reduced forced vital capacity (FVC) and total lung capacity (TLC), normal or low functional residual capacity (FRC) and increased residual volume (RV), due to weakness of the expiratory muscles.8 Possible leaks though the nozzle should be monitored during the manoeuvres (common in bulbar patients), and it should be replaced by an oronasal mask when necessary. The flow/volume loop trace shows slow expiration with reduced peak flow, ending abruptly. The inspiratory flow is also reduced. Oscillations in the expiratory flow are typical in Parkinson's disease. The FVC in decubitus may be abnormally low with respect to the FVC in sitting position (>25%), indicating significant diaphragmatic weakness and probable nocturnal hypoxaemia.9 Sequential FVC measurements enable the evolution of the process to be monitored, and a value <55% of the theoretical value predicts the possibility of hypercapnia. The deterioration is severe when FVC <30ml/kg (normal, 60–70ml/kg). In patients with Guillain–Barré syndrome, a decrease to 15ml/kg suggests ventilatory support.10 This measure is less useful in monitoring myasthenic crisis, as its behaviour is erratic.
Maximal Muscle PressuresThis is a more sensitive parameter than FVC. The maximal inspiratory pressure (MIP) is measured with an open glottis against an occlusion, with maximum effort that begins at FRC or VR (Muller's manoeuvre).11 The maximal expiratory pressure (MEP) is measured after a Valsalva manoeuvre at TLC or FRC level. A MIP less than −80cmH2O, or MEP greater than +90cmH2O excludes significant muscle weakness. A MIP less than 30% of the theoretical is associated with a significant deterioration in blood gas values.10
Peak Cough Expiratory FlowThis is measured with a peak flow metre or pneumotachograph after a vigorous cough effort (the best of 4–7 attempts is chosen) and enables the efficacy of the expiratory muscles to be checked. In adults it is greater than 350l/min. Lower figures, especially <270l/min, indicate a deterioration in the ability to clear secretions (1B).12 An inability to generate effective expiratory “peaks” in the expiratory flow trace indicates a poor prognosis (1B).
Sniff Nasal Inspiratory Force (SNIF Test)The recording is made in the nasal choanae during the sniff manoeuvre13 at FRC. It is very useful when there is facial weakness or dental malocclusion. It is measured by plugging one nostril, while the other is occluded and freed in each manoeuvre. About ten measurements are taken; a pressure greater than 60cmH2O in women and 70 in men excludes significant respiratory muscle weakness (1B).
Transdiaphragmatic PressureThis is the invasive measurement of inspiratory muscle strength. It is predictive of nocturnal hypoventilation and is usually used for research, since it requires placement of a probe to calculate the transdiaphragmatic pressure. Transdiaphragmatic pressure is >80cmH2O in women and >100 in men.
Phrenic Nerve StimulationThe phrenic nerve is accessible along the length of the neck and can be stimulated by an electric current or magnetic field. This technique is usually reserved for patients who do not cooperate in volitional manoeuvres or for research. Magnetic stimulation is easier to perform and better tolerated, but also excites the roots of the muscles of the rib cage, so it is less specific for the phrenic nerve. In both cases, the response in the diaphragm is recorded using a gastric-oesophageal probe, although they have also been used to measure the pressure in the mouth. Its interest lies in that it is a non-volitional technique, but it is not always easy to obtain reproducible recordings. Abdominal wall impedance, potentiation with voluntary contraction (the patient must be breathing quietly for 10min) and the interference of other muscles add difficulties to the measurement. To avoid these biases, electromyographic recording by oesophageal catheter has been proposed. The phrenic nerve conduction time can also be measured with electrostimulation, recording the diaphragmatic action potential on an electromyogram (normal, less than 9ms). However, the technique that offers most clinical advantages is magnetic stimulation.14
Imaging TechniquesChest X-ray shows elevated diaphragms, but although useful for the diagnosis of unilateral paralyses, it is of little value in neuromuscular patients. Inspiratory/expiratory chest X-rays and fluoroscopy are not very sensitive or specific, and are complex to perform if the patient is bedridden. Ultrasonography allows the diaphragm thickness in the zone of apposition to the rib cage to be measured, and its movements to be evaluated.15 The thickness is proportional to the strength. In diaphragmatic paralysis, it is less than 2mm and does not increase in inspiration. It is non-invasive, portable and requires little patient cooperation.
Respiratory Pattern and Sleep DisordersDuring sleep, patients with neuromuscular disease can develop various respiratory conditions. The type of condition depends on the muscle group predominantly affected. When the inspiratory muscles (diaphragm, intercostal and accessory muscles) are affected, hypoventilation with hypoxia and hypercapnia appears, not only due to the muscle weakness but also because the control of the respiratory centre is reduced during the REM phase. One indicative sign is retention of bicarbonate in the blood in the absence of diuretics. If the diaphragm is not affected very much, but the muscles of the upper airways are, obstructive apnoeas and hypopnoeas arise. These conditions must be known before choosing the appropriate ventilation mode. A nocturnal recording of the O2 saturation at home is recommended and, depending on the results, the study should be completed with a polygraph or polysomnograph.
Other Complications: Aspiration and Cardiovascular PathologyAspiration PathologyThe objective is proper assessment of the patient's ability to swallow all requirements (water and nutrients), as well as the safety of the ingestion without respiratory aspiration or complications occurring. Clinical methods, imaging techniques and specific complementary studies are available for this.16,17
Clinical ExaminationThis should consist of the medical history and a general neurological examination that includes oral and pharyngeal motor function with examination of gag and swallowing reflexes and presence and efficacy of voluntary cough. The water test should be applied together with measurement of the degree of desaturation (considering desaturation of more than 2% as a sign of aspiration) or the texture test.18 These will enable us to assess swallowing apraxia, oral debris, cough or hoarseness, reduced laryngeal elevation, “wet voice” or repeated swallows for the same bolus (indirect dysphagia data with high risk of aspiration) (1C).
PharyngolaryngoscopyThis is a validated method for the assessment of pharyngeal dysphagia and estimation of aspiration risk (1C).
VideofluoroscopyThis technique is considered as the gold standard in the assessment of dysphagia, as it provides direct information on the oral, pharyngeal and oesophageal phases of swallowing, as well the safety of the oral route (presence of aspiration). Videofluoroscopy also enables the possibilities of rehabilitation to be assessed and to indicate gastrostomy placement if aspiration is detected (1C).
Pharyngo-oesophageal ManometryThis enables the quantification of pharyngeal contractility, complete relaxation of the upper oesophageal sphincter and synchrony between both events (1C). It can be used together with videofluoroscopy (manofluoroscopy), although placement of the manometer probe is uncomfortable and may alter the configuration of the oropharynx and the hyoid movement.
Prevention of AspirationThe enteral route can continue to be used providing that the patient maintains their nutritional status with no evidence of aspiration in the swallowing assessment techniques. In patients with neurogenic dysphagia, reducing the bolus volume and increasing the viscosity produce a significant therapeutic effect on the efficacy and safety of swallowing (1C). Swallowing techniques can be used (facilitative and postural manoeuvres) in patients who retain minimal cognitive and motor functions, together with modifications in texture and volume, providing that the efficacy and safety of swallowing is observed (2C). In patients in whom dysphagia does not permit these manoeuvres, endoscopic or radiological placement of a gastrostomy tube should be indicated (in patients with a high risk of respiratory complications).17 In patients with severe respiratory involvement (FVC<50%), radiological gastrostomy placement is advisable, as sedation is thus avoided and non-invasive ventilation (NIV) can be carried out during the procedure (1C).
Cardiovascular PathologyMany neuromuscular diseases are associated with heart disease.19–21 On many occasions, its severity may indicate survival, hence the importance of early diagnosis, often hindered by the lack or absence of symptoms. Diagnostic management should include ECG (at the time of disease diagnosis and annually), echocardiogram and continuous 24h electrocardiographic monitoring (Holter) in pathologies with a risk of associated arrhythmias. Electrophysiological study will be indicated only in pathologies in which exact determination of the location and severity of the conduction disorder is necessary. In the case of arrhythmogenic heart disease, the placement of implantable defibrillators should be considered (1C).
Respiratory PhysiotherapyRespiratory physiotherapy (RP) plays an essential role in the treatment of respiratory complications in neuromuscular diseases. Basically we can describe two types of action: preventive RP and active RP.
Preventive Respiratory PhysiotherapyPreventive RP is intended to maintain thoracic and lung compliance, and to prevent the appearance of microatelectases. Chest expansion or hyperinflation techniques should be applied, and may be manual or mechanical. It is recommended that these techniques be initiated with vital capacity values less than 1500ml or 70% of the theoretical value.22,23 Manual hyperinflation consists of insufflating air using an Ambu® manual resuscitator, instructing the patient in coordination of the insufflation with closure of the glottis to prevent the exit of air. It is generally recommended to perform 2–3 hyperinflations at least 2 or 3 times a day (2B). Hyperinflation can be performed mechanically using intermittent positive-pressure breathing24–26 through a mouthpiece or nasal/facial mask, providing that there is no major bulbar involvement, or using a tracheostomy tube (2C). The parameters that can be adjusted are the end-inspiratory pressure (5–40cmH2O), inspiratory flow rate (20–60l/min) and expiratory resistance.
Active Respiratory PhysiotherapyActive RP is aimed at maintaining adequate drainage of respiratory secretions. It is initiated when ineffective cough is observed through clinical symptoms and functional examination (peak cough flow [PCF]). PCF values less than 270l/min indicate a high risk of ineffective cough during acute respiratory processes,12 so learning assisted coughing techniques is recommended; these may be manual or mechanical, and are generally performed at home27 (1B). Manually assisted coughing begins with maximal inhalation followed by retention of air by closing the glottis. It is common for patients to also have inspiratory muscle weakness, so in many cases it will be necessary to combine previous hyperinflation to achieve maximal inspiration (1B). At the start of expiration, hand compressions are performed on the chest, abdomen or both. The result obtained from adding the different forces increases the intrathoracic pressure, increases the PCF and the effectiveness of the cough. It is essential that the patient retains normal bulbar function that allows closure of the glottis at the end of insufflation.
Mechanically assisted coughing is indicated when effective cough is not achieved with manual techniques, and is performed with a mechanical insufflation–exsufflation device. The device generates a positive pressure (insufflation) followed by a negative pressure (exsufflation), resulting in a flow of air that removes the secretions. It can be applied via a nasobuccal mask or tracheal tube. In order for the technique to be effective, pressures >30cmH2O for both insufflation and exsufflation are recommended.28 The recommended time of application of each phase is 2–3s in the insufflation phase and 3 s in the exsufflation, with a short pause between cycles. Daily sessions are advised, applying 5–6 cycles in each session (1B). In any case, when the bulbar involvement is very severe, the usefulness of this treatment is very limited.28
Non-invasive Treatment of Respiratory ComplicationsIn recent years, the evolution and development of NIV has had a major impact on the natural history of neuromuscular diseases, where respiratory failure is one of the most common causes of premature death. In these patients, treatment with ventilatory support has considerably increased survival and improved the quality of life (1B).
In patients with slowly progressive diseases, NIV stabilises the vital capacity, increasing the PaO2, decreasing the PaCO2 and improving the quality of sleep.29 This benefit can be significantly observed in patients who use NIV>4h.30 NIV should be indicated in all neuromuscular patients with symptoms of respiratory fatigue (orthopnoea) associated with functional respiratory dysfunction (drop in FVC/MIP) or symptoms of hypoventilation in the presence of hypercapnia or nocturnal desaturation. Occasionally, in slowly progressive neuromuscular diseases, symptoms occur during exacerbations, requiring NIV promptly. Likewise, exacerbations and respiratory infection may necessitate more time on ventilation in patients already on home MV.
Details of this treatment are described in the SEPAR procedure manual (no. 16, E. Barrot Cortés and E. Sánchez, 2008). However, some specific aspects of the treatment of these patients must be mentioned.2,5,31 The respirator selected must be life support and include all clinical ventilation modes. We can initially use a spontaneous ventilation mode if the patient's ventilatory autonomy allows, since it facilitates adaptation to the ventilation. As the patient loses ventilatory autonomy, it may be necessary to increase the number of hours of ventilation, or to assess switching to a controlled ventilation mode (by pressure or volume) if NIV has not been initiated with this ventilation mode. The interface selected is as important as the respirator itself. In patients with severe bulbar involvement, NIV can be started with a nasal mask, using a facial mask if there is excessive leakage through the mouth. When the patient is on more than 12h of ventilation, it is essential that, in addition to having two respirators and extra batteries, mouthpieces or masks without support on the nasal dorsum are used, either nasal or nasal–buccal, to prevent pressure sores. In this case, some patients use different ventilatory parameters depending on the interface selected.
Invasive Treatment of Respiratory ComplicationsInvasive treatment may be used in neuromuscular diseases that present with progressive weakness of the respiratory muscles (especially in ALS patients), when bulbar involvement reaches a critical point, non-invasive aids to the respiratory muscles fail and it is essential to perform a tracheotomy (TM) or intensify palliative care.8,32–35
Home MV by TM may prolong survival in some neuromuscular diseases, and is the procedure of choice for patients who wish to continue living when non-invasive aids are inadequate (1C).
When to Perform the TracheotomyIn neuromuscular diseases, TM may be necessary due to both failure of NIV and ineffectiveness of coughing aids, either during acute lung injury (generally an infection), or as a result of severe progressive weakening of the patient's respiratory muscles. Therefore, to avoid rushing decision-making, the patient's informed desires with respect to TM should be obtained before bulbar involvement becomes severe (1C).
Cannulae and VentilatorsFor safety, cannulae should always have an inner cannula that can be extracted immediately in case of obstruction.33 In many cases, non-balloon cannulae allow adequate alveolar ventilation to be maintained, and proper management of secretions until the bulbar involvement reaches a critical point in which the leaks become excessive or saliva aspirations are uncomfortable or cause hypoxaemia. The use of non-balloon cannulae may facilitate phonation in patients on 24h MV if proper bulbar function is maintained. In patients on MV <24h, the use of fenestrated cannulae may be useful for facilitating phonation during break times. When balloon cannulae are used, the fill pressure should be less than 25mmHg, and in patients without gastrostomy the balloon should not be filled up for eating, as doing so increases mechanical interferences in swallowing and favours aspiration.33
When MV is not full time, and provided that fibroscopy confirms that the fenestration is well positioned, fenestrated cannulae decrease the ventilatory work and enable talking during break times33 (1A).
In previous guidelines, volume controlled ventilators were proposed for MV by TM in neuromuscular diseases.32 The technical characteristics of current ventilators enable the most comfortable and effective mode to be tailored for each patient. Ventilators should have a battery included and spare equipment is necessary.32,33
Management of SecretionsTo ensure adequate ventilatory support, the airways must be kept free from secretions. Clearing the airways is an essential procedure33,34 (1C). To perform tracheal suction, it is advisable to introduce the probe minimally and to avoid deep suction (2C). The use of mechanically assisted coughing systems has been suggested to reduce the risk of complications associated with tracheal suction (1C). In any case, clean suction techniques should be used, although a sterile environment is not required (1C). There are no studies designed to determine the right time to perform suction. Experts suggest being guided by the patients’ sensations, increase in ventilator peak pressure and drops in SpO2. Routine saline instillation through the TM should be avoided. Guidelines33,34 recommend filters that retain heat and humidity as opposed to humidifiers–heaters during MV by TM (1A).
Main Complications of Mechanical Ventilation by TracheotomyIf tracheal lesions (caused mainly by traumatic suctioning, overinflation of the balloon or contact with the distal end of the cannula) can be avoided with good practice, respiratory infections are the most common complications in these patients. Protocols that facilitate early therapeutic responses should be used to manage them properly (1C). Due to the possibility of accidental disconnections or breakdowns in the system, it is essential to have effective alarms to avoid serious or fatal episodes32–34 (1C).
Ethical Aspects and Palliative TreatmentNIV can be considered, in part, a palliative treatment, as it reduces the respiratory symptomatology associated with neuromuscular diseases36 and improves quality of life.1,8 While NIV is a widely accepted therapeutic option, there is controversy with respect to invasive ventilation through the tracheal route, which improves survival but does not change the progression of the disease. This ambivalence, especially in the case of rapidly progressive diseases, is due to the possibility that undesirable situations will arise, such as “locked in” syndrome, not to mention the huge burden for both the family and healthcare system. The choice of non-invasive or invasive MV should be discussed early on with the patient and those around them, in order for the decision to conform to the patient's wishes.36 Therefore, it is essential to perform a respiratory assessment that enables respiratory involvement to be detected in early phases, and to establish communication about the patient's wishes among all persons involved: family and professionals. We should encourage making decisions in advance at all times, informing the patient of the advantages and disadvantages of treatment in a realistic manner, while also trying to ease the emotional impact. An enormously helpful element in making difficult decisions that should always be recommended is to document advance decisions or directives. This has already been in force in several autonomous regions since 2002, and allows the patient, when their condition prevents them from doing so personally, to express at least how they would not want to die. This document reduces the family's anguish upon having to authorise a certain treatment or not.
The major ethical problem arises when there is acute decompensation that requires invasive MV for its treatment and the patient's wishes in this respect are unknown. A single action strategy cannot be established in this situation, so only individualised patient evaluation appears to be legitimate.
Medicolegal AspectsThere are five relevant scenarios in relation to end-of-life decision making. These are: (a) euthanasia and assisted suicide; (b) limitation of therapeutic effort; (c) rejection of treatment; (d) palliative sedation, and (e) suspension of medical care due to death. It is important to clarify these concepts when considering the different situations that may lead to legal and ethical problems.36,37 Both euthanasia and assisted suicide are illegal in Spain, according to article 143 of the 1995 penal code.36
For optimal planning, the following objectives should be considered: (a) to help prepare the dying process; (b) to exercise the principle of patient autonomy and put it into practice; (c) to seek resources to confront this situation; (d) to ease the emotional impact, and (e) to seek the best communication about the patient's wishes among all persons involved: family and professionals.
The following steps are recommended to put this into practice: (a) introduce this point in conversations with the patient; (b) start a structured dialogue about it; (c) document the patient's preferences; (d) periodically review and update the advance directives and (e) apply the directives in real clinical situations.
In the final stage of life, we often resort to the limitation of therapeutic effort, which includes both the withdrawal of life support measures as well as non-initiation of same, but ensuring that the level of general care is always optimal. Procedures such as invasive ventilation may be disproportionate, and in these cases maintaining this measure would cause suffering to be prolonged without providing comfort benefits. It should be considered that the limitation of therapeutic effort “permits” death in the sense that it does not prevent it, but in no case does it “produce” or “cause” it; it is the disease that causes death of the patient, so it is not considered euthanasia. The desire of a patient diagnosed with a neuromuscular disease on a MV programme to discontinue this treatment would form part of this scenario. It is important that the withdrawal of ventilation is performed in the proper environment. Family surrogacy for the decision to withdraw MV is accepted as valid in the absence of explicit advance directives.
With respect to the rejection of treatments, the current ethical model for decision-making, which in Spain has full legal backing in current law 41/2002 regulating patient autonomy, argues that patients can always exercise their moral autonomy and take decisions that they believe are best for their body or health.
Palliative TreatmentIn patients with an inadequate clinical response to MV or who cannot tolerate it, palliative treatment38 (such as oxygen therapy and morphine) should be considered. Palliative sedation consists of administering drugs to a terminally ill patient, in the dosages and combinations required to reduce their awareness as much as necessary to relieve a refractory symptom. If carried out according to the clinical indications and technical specifications, and with the informed consent of the patient or their representative, it should not be considered very different from any other medical procedure. In the final stage of patients with neuromuscular disease and ventilatory failure who have rejected MV, its application must be guaranteed if necessary to relieve symptoms of dyspnoea, anxiety, distress and malaise, and thus to ensure a death with the least suffering possible.
Outpatient CareMultidisciplinary ClinicThe multidisciplinary clinic answers the multiple needs of patients with neuromuscular diseases, by enabling and facilitating the coordination of all procedures, reinforcing good practices, with early and complete care of their needs.39–44
Multidisciplinary clinics have been shown to improve the survival and quality of life of patients with neuromuscular diseases (1C), as well as offering a considerable saving in healthcare costs compared to the traditional care system model, as they optimise resources and foster expertise with the result of a better patient approach.
The basic objective is comprehensive, individualised, multidisciplinary treatment of the disease. It is a priority to reduce the schedule of visits, speed up the diagnosis, prevent through genetic counselling and not defer the start of treatment of respiratory complications when they present.
The neurologist, respiratory medicine specialist, rehabilitation physician, physiotherapist, social worker and nutritionist should participate in this clinic, to a greater or lesser extent. Within the possibilities of each centre, the occupational therapist, geneticist, psychologist, cardiologist, ENT specialist and ophthalmologist should also be included. It is advisable that the visits be integrated into a single schedule to prevent repeated journeys and to coordinate information. The frequency of the visits will depend on the progression of the disease. For rapidly progressive diseases, one visit every 2–4 months is recommended; these can be spaced out in slowly progressive diseases.
Home CareAlthough there are no studies designed to specifically assess the essential resources required by these patients, experts recommend that they depend on experienced hospital units and that they have the proper technical and human (caregivers) material (1C).32–35 When the medical and social support is sufficient, in patients treated with TM who were previously treated with NIV, the level of suffering does not increase but it adds a huge burden of physical and emotional work to caregivers (GRADE 1C). After TM, experts32–35 suggest not discharging the patient until they reach clinical stability, the caregivers are properly trained and the home meets the proper conditions (1C).
Home care of patients with heavy dependence on ventilation by a medical and nursing care team, ideally integrated into the hospital team, is highly recommended (1C). The duties of the home care team should be: to supervise patient transfer to the home, to verify the correct installation and functioning of the ventilation equipment and accessories, to ensure correct management of treatment by the caregivers, to attend future incidents and to work together with the hospital team and primary care services.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Farrero E, et al. Normativa sobre el manejo de las complicaciones respiratorias de los pacientes con enfermedad neuromuscular. Arch Bronconeumol. 2013;49:306–13.