The Glittre Activities of Daily Living Test (ADL-Test) is a reliable functional status measurement for stable Chronic Obstructive Pulmonary Disease (COPD) patients in a laboratory setting. We aimed to adapt the test to the home setting (mADL-Test) and to follow-up the functional status recovery of post-exacerbation COPD patients included in a home hospitalization (HH) program.
MethodWe assessed 17 exacerbated moderate-to-very-severe COPD patients in 3 home visits: at discharge to HH (V0), 10 days (V10post) and 1 month after discharge (V30post). Patients completed the mADL-Test (laps, VO2 and VE), COPD assessment test (CAT), London Chest ADL Test (LCADL), modified Medical Research Council (mMRC) and upper limb strength (handgrip).
ResultsThe number of laps of the mADL-Test (4, 5 and 5, P<.05), CAT (19, 12 and 12, P<.01), mMRC (2, 1.5 and 1, P<.01) and the self-care domain of the LCADL (6, 5 and 5, P<.01) improved during follow-up (V0, V10post and V30post, respectively). No significant changes were evidenced in VO2, VE or handgrip.
ConclusionOur results suggest that the mADL-Test can be performed in the home setting after a COPD exacerbation, and that functional status continues to improve 10 days after HH discharge.
La prueba de actividades de la vida diaria de Glittre (prueba ADL) es, en un entorno de laboratorio, una medida fiable del estado funcional de los pacientes con enfermedad pulmonar obstructiva crónica (EPOC) estable. Nos propusimos adaptar la prueba para poder llevarla a cabo en el entorno domiciliario (Test ADLm) y supervisar la recuperación del estado funcional de pacientes con EPOC después de una exacerbación atendida en hospitalización domiciliaria (HD).
MétodoEvaluamos a 17 pacientes con EPOC de moderada a muy intensa y exacerbación en tres visitas domiciliarias: el día del alta de la HD (V0), al cabo de 10 días (V10post) y un mes después del alta (V30post). Los pacientes realizaron la prueba ADLm (vueltas a un circuito, VO2 y VE), la prueba de evaluación de la EPOC (CAT), el Cuestionario de ADL London Chest (LCADL), la Escala del Medical Research Council modificada (MRCm) y una dinamometría de las extremidades superiores (fuerza de prensión).
Resultadosel número de vueltas al circuito en la prueba ADLm (4, 5 y 5, p<0,05), el CAT (19, 12 y 12, p<0,01), la MRCm (2, 1,5 y 1, p<0,01) y el dominio de cuidado personal del LCADL (6, 5 y 5, p<0,01) mejoraron durante el seguimiento (V0, V10post y V30post, respectivamente). No se constataron cambios significativos en el VO2, el VE o la fuerza de prensión.
ConclusiónNuestros resultados indican que, tras una exacerbación de la EPOC, es factible realizar la prueba ADLm en el entorno domiciliario, y que el estado funcional continúa mejorando diez días después del alta de la HD.
Functional status refers to the ability of patients to cope with their Activities of Daily Living (ADL). Chronic Obstructive Pulmonary Disease1 (COPD) affects the capacity of patients to perform their ADL.2 Moreover, a poor functional status is a risk factor for exacerbations.3 After an exacerbation, functional status may not return to the previous level, and this can cause patients to enter a negative cycle where the more exacerbations they suffer, the worse their functional status becomes.4 The result is an eventual increase in mortality and health care burden.5 Despite their importance, recovery patterns of functional status after a COPD exacerbation have been poorly studied.6
Home-based programs, such as home hospitalization (HH),7 are successful care services for COPD patients. However, functional capacity assessment outside the hospital or laboratory setting has been rarely studied.8 The home setting is unsuitable for most of the standard exercise field tests, such as the Six Minute Walking Test (6MWT)9; however, some performance tests for small settings have been suggested in recent years. Puhan et al.10 found that the results of the sit-to-stand test are associated with mortality in stable COPD patients. Jones et al.11 found the five-repetition sit-to-stand test to be a practical functional measurement, even at the bedside. And the Chester step-test may also be a suitable method.12 Even so, those tests could underestimate the daily functional limitations of patients, because they rely mostly on the use of lower limbs, whereas most of the common ADLs combine both extremeties.13 The ideal test would be one in which the patients have to reproduce the most common ADLs in their own environment.
The Glittre ADL-Test14 (ADL-Test) was specifically developed for valid and reliable functional status assessment of COPD patients in terms of both performance and capacity.8 It reproduces the 5 most common ADLs in a 10-m long corridor, and requires the use of both extremities.14 In stable COPD patients, the ADL-Test induces a sub-maximal steady-state physiological response,15,16 it discriminates the functional capacity of COPD patients from healthy people,17 and it is also reproducible16 and responsive to pulmonary rehabilitation.14 However, the ADL-Test has not been tested in COPD patients recovering from an exacerbation or in the home setting.
Our research group had already tested the ADL-Test in stable COPD patients in a hospital setting.18 In this study, we first aimed to determine whether it was also suitable for the home setting, and then attempted to follow-up ADL-Test performance during the early recovery phase of a COPD exacerbation. We achieved these objectives by studying post-exacerbation COPD patients included in an HH program.
MethodsWe conducted a prospective observational feasibility study. Subjects were consecutively recruited in the HH unit of the Hospital Clinic in Barcelona (Spain) between March and June 2011. The study protocol was approved by the independent Hospital's Ethics Committee, and all participating patients signed the consent form.
PopulationDuring the study period, all COPD patients admitted to the HH19 program due to an exacerbation were invited to take part in the study. We were not able to calculate a sample size due to the exploratory nature of the research.
No changes were made to the existing HH care protocol.19 Briefly, patients were admitted to the HH program if they did not meet criteria for imperative hospitalization (such as need for mechanical ventilation) or had been admitted to the hospital for less than 48 hours. HH exclusion criteria included: not domiciled in the healthcare area or admitted from a nursing home; lung cancer and other advanced neoplasm; extremely poor social conditions; severe neurological or cardiac comorbidities; and no phone at home. During HH, patients were visited daily by a skilled respiratory nurse. Standard pharmacological treatment was given, following national guidelines20 in force at the time of the study, and the comprehensive therapeutic approach was adapted to the needs of each patient. Discharge visit (V0) was scheduled and carried out by both the nurse and the medical staff.
Specific inclusion criteria for this study were: (1) COPD diagnosis following GOLD criteria1 and (2) COPD exacerbation as the sole admission diagnosis. We excluded patients with muscular, skeletal, cardiac or cognitive conditions that could impede performance of the ADL-Test or compromise the safety of the test.
ProtocolPatients were assessed by a respiratory physiotherapist during 3 home visits: at the time of discharge to HH (V0), 10 days post-discharge (V10post) and 1 month post-discharge (V30post). To ensure the well-being of the patients, V0 measurements were obtained over 2 consecutive days. The day before the planned discharge, we performed the clinical assessment (questionnaires). We explained the test, and the patients were invited to simulate 1 lap of the test to minimize the learning effect. On the day of discharge, patients performed the functional status assessment under supervision of the medical staff, to ensure their safety. The following V10post and V30post visits were carried out fully in one day each one.
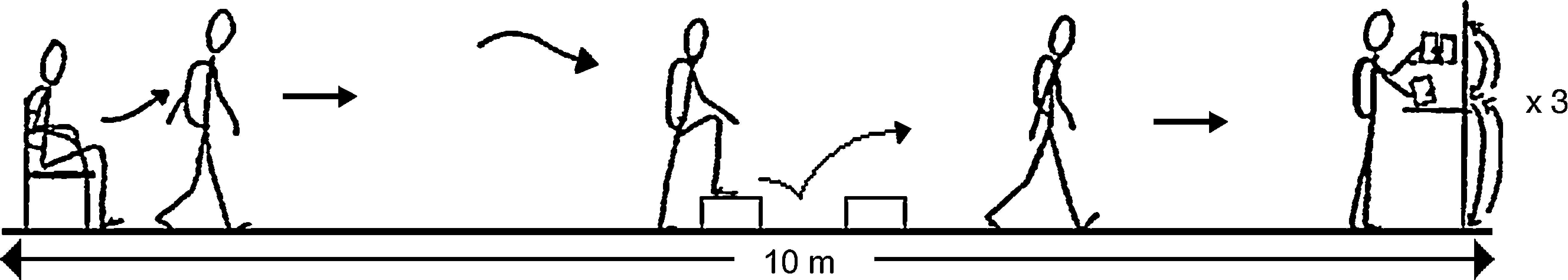
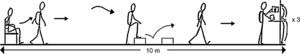
Functional Status AssessmentFunctional status was assessed using a modified version of the original Glittre ADL-Test14 (mADL-Test) (Fig. 1). We introduced 2 changes: first, the original outcome goal of 5 laps14 was replaced by a self-paced time-limited test, so for this study the main outcome variable was the number of laps that the patient could cover in 6min on the first, the second and the third visits. Secondly, the original 2-step staircase was replaced by 2 steps placed separately, one in front of the other. This improved the portability of the equipment without affecting the work-load of the original test (i.e., the new steps had the same height and width as the original ones).
mADL-Test. Patients were instructed to do as many laps as they could in 6min. They were not verbally encouraged during the test. Two separate steps (17cm high and 27cm deep) were placed one in front the other to mimic the climbing movements required by the original test.
Patients were shown how to perform the mADL-Test correctly and safely: subjects had to complete the mADL-Test laps as fast as they could in 6min, they could stop if they were in pain, too exhausted, or for any other reason. If the research team detected any alarm signs, the test would be stopped.
All patients were connected through a face mask to a portable gas analyzer (Fitmate, Cosmed; Rome, Italy) to assess whether their physiological response during the test was similar to the original test.15 All patients had to complete the tests without supplementary oxygen, or if they were already receiving oxygen therapy, after 20min of wash-out. Oxygen uptake (VO2) and ventilation (VE) were measured breath by breath. The equipment was calibrated before each assessment and carried by the patient inside the same backpack used for the test. However, the final weight was adjusted according to the device (1.5kg) and the gender of the patient, as stated in the original test. Finally, heart rate and oxygen saturation were continuously monitored with a hand-held pulse oximeter (3Xi Konica Minolta; Osaka, Japan).
Clinical AssessmentOn each assessment visit the following patient-reported outcomes were obtained, always in the same order: (1) dyspnea level: modified Medical Research Council dyspnea scale21 (mMRC), (2) dyspnea related to ADL: London Chest Activities of Daily Living questionnaire22 (LCADL), (3) health status: COPD Assessment Test23 (CAT) and (4) physical activity: modified Baecke questionnaire for older people24 (mBaek) administered only on V0 and V30post visits.
In addition, the patients performed a handgrip dynamometry10 to explore upper limb muscle strength. The test was performed first with the dominant hand, and the best measurement (1 out of 3, for each hand) was used for statistical analysis.
Secondary variables such as socio-demographical and other clinical data were obtained from the clinical history.
Statistical AnalysisStatistical analysis was carried out using PASW (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0, Chicago, IL, USA). Initially, the normality of the data was assessed through the Shapiro–Wilk test. Due to small sample size, non-parametric tests were used. Wilcoxon signed-rank tests were used for comparisons between visits. Results are expressed as median (Mdn) and Interquartile range (IQR), otherwise indicated. All tests were two-tailed, and the level of significance was set at P<.05. For metabolic response analysis (VO2 and VE), last minute values of the mADL-Test were used. Physiological VO2 profile of the mADL-Test was analyzed with Friedman test.
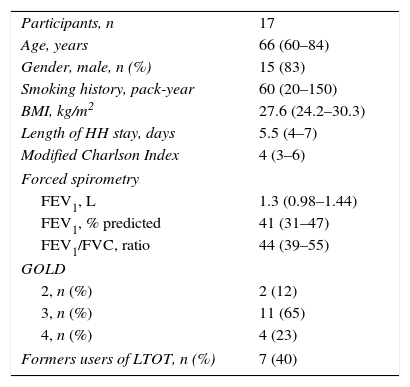
ResultsDuring 2011, 71 COPD patients were admitted to the HH program due to exacerbation. However, only 29 patients (41%) were admitted during the recruitment phase of this study and invited to participate. Unfortunately, 10 patients were excluded: 5 with neoplasia, 4 with movement disturbances, 1 with cardiac instability (New York Heart Association25 class IV), and 2 declined the invitation. Consequently, 17 patients were included. The general characteristics of the sample were (Mdn and IQR): 66 (60–84) years, 15 were men, and FEV1 was 38% (29%–44%) of predicted. More information about the study population at the time of inclusion is shown in Table 1.
Characteristics of the Study Group.
| Participants, n | 17 |
| Age, years | 66 (60–84) |
| Gender, male, n (%) | 15 (83) |
| Smoking history, pack-year | 60 (20–150) |
| BMI, kg/m2 | 27.6 (24.2–30.3) |
| Length of HH stay, days | 5.5 (4–7) |
| Modified Charlson Index | 4 (3–6) |
| Forced spirometry | |
| FEV1, L | 1.3 (0.98–1.44) |
| FEV1, % predicted | 41 (31–47) |
| FEV1/FVC, ratio | 44 (39–55) |
| GOLD | |
| 2, n (%) | 2 (12) |
| 3, n (%) | 11 (65) |
| 4, n (%) | 4 (23) |
| Formers users of LTOT, n (%) | 7 (40) |
BMI, Body Mass Index; HH, home hospitalization: FEV1, force expiratory volume during the first second; FVC, forced vital capacity; LTOT, long term oxygen therapy.
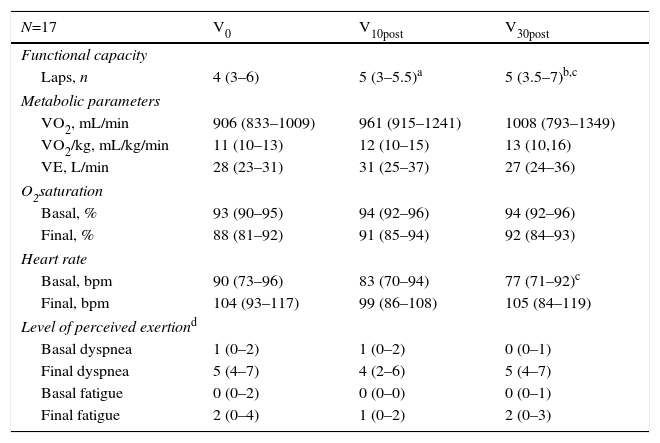
The mADL-Tests performance is shown in Table 2. The majority of the patients increased, gradually and significantly, the number of laps (4, 5 and 5, P<.05) during follow up (L0, L10post and L30post, respectively). Overall, 12 out of 17 patients increased ≥1 lap between V0 and V30post. Nevertheless, whereas the number of laps increased, the final values of VO2 and VE did not improve significantly between the first and the last assessment (P=.331 and P=.244, respectively). However, exercise performance improvement, represented by an increment of the peak VO2, was observed. Moreover, patients presented lower basal HR and higher basal saturation, indicating improved physical status.
Cardiopulmonary and Physiological Response of the mADL-Test.
| N=17 | V0 | V10post | V30post |
|---|---|---|---|
| Functional capacity | |||
| Laps, n | 4 (3–6) | 5 (3–5.5)a | 5 (3.5–7)b,c |
| Metabolic parameters | |||
| VO2, mL/min | 906 (833–1009) | 961 (915–1241) | 1008 (793–1349) |
| VO2/kg, mL/kg/min | 11 (10–13) | 12 (10–15) | 13 (10,16) |
| VE, L/min | 28 (23–31) | 31 (25–37) | 27 (24–36) |
| O2saturation | |||
| Basal, % | 93 (90–95) | 94 (92–96) | 94 (92–96) |
| Final, % | 88 (81–92) | 91 (85–94) | 92 (84–93) |
| Heart rate | |||
| Basal, bpm | 90 (73–96) | 83 (70–94) | 77 (71–92)c |
| Final, bpm | 104 (93–117) | 99 (86–108) | 105 (84–119) |
| Level of perceived exertiond | |||
| Basal dyspnea | 1 (0–2) | 1 (0–2) | 0 (0–1) |
| Final dyspnea | 5 (4–7) | 4 (2–6) | 5 (4–7) |
| Basal fatigue | 0 (0–2) | 0 (0–0) | 0 (0–1) |
| Final fatigue | 2 (0–4) | 1 (0–2) | 2 (0–3) |
VO2, oxygen uptake; VO2/kg, oxygen uptake per kilogram per minute; VE, ventilation; bpm, beats per minute.
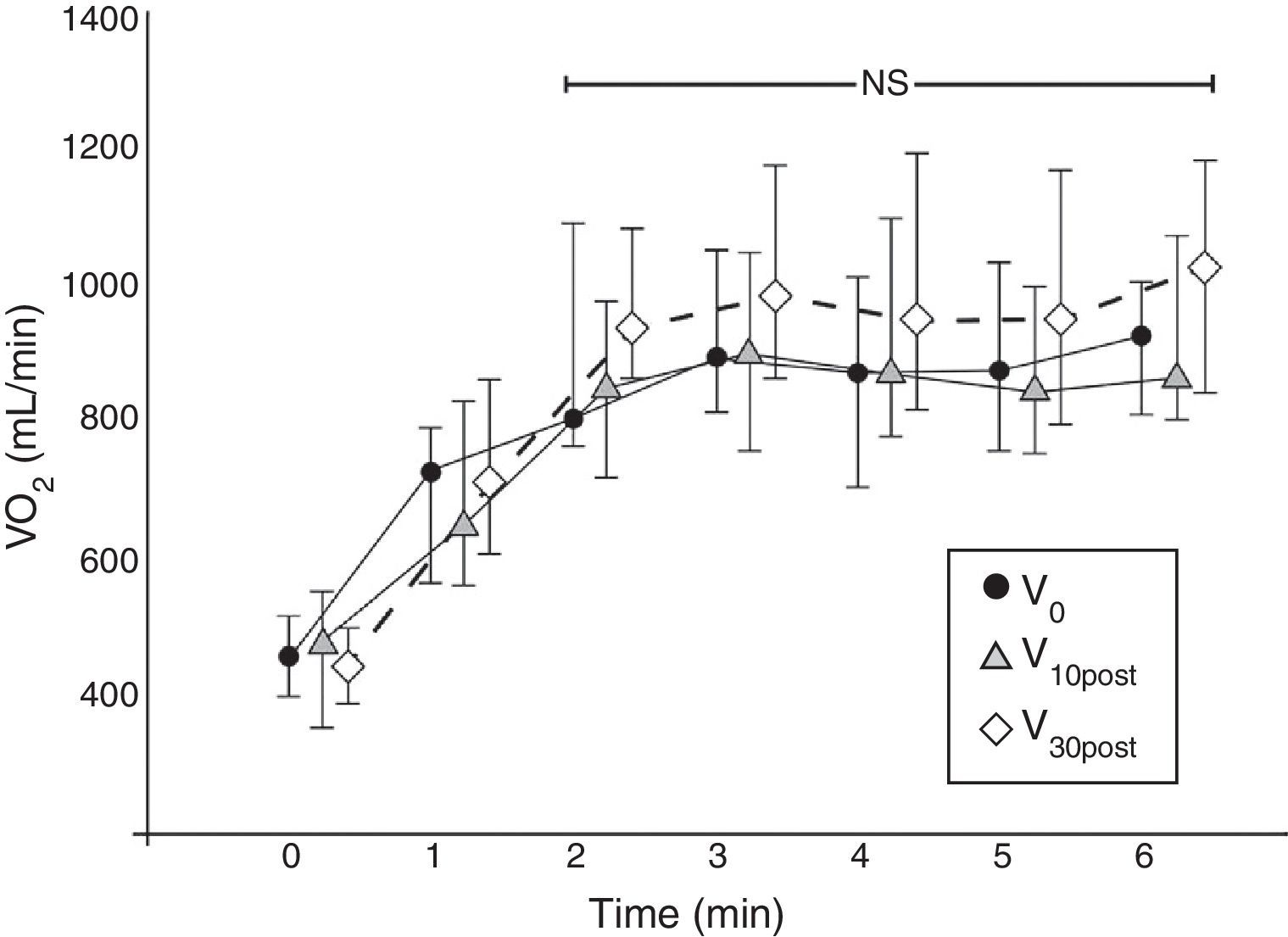
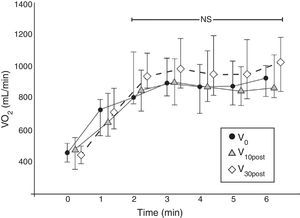
The physiological response of the test showed a steady-state VO2 profile from the second minute up to the end of the test (Fig. 2), which is representative of sub-maximal tests such as the 6MWT.26
VO2 physiological profile in the three follow-up visits. Each line represents the mADL-Test VO2 values (mean values with standard deviation) of the 17 patients together and for each assessment visit. The VO2 values are plotted minute by minute. From the second minute the VO2 profile reaches a plateau (P=NS). NS, non-significant.
The mADL-T was suitable for any location; it was well tolerated by all patients, and no adverse events were reported. None of the patients were excluded for logistical issues (such as the size of the home), and there were no significant difficulties in carrying the mADL-Test equipment or setting up the test in each patient's home.
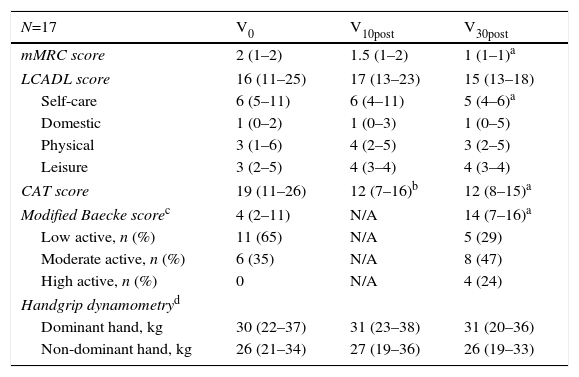
Clinical AssessmentThe clinical evolution of the patients after HH discharge is shown in Table 3. Overall, patients continued to improve their clinical status during follow-up. The CAT score improved significantly in the first 10 days, and the mMRC level also showed a tendency to improve (P<.05 and P=.058, respectively). Both variables had improved by the end of the study.
Clinical Evolution of Patients After HH Discharge.
| N=17 | V0 | V10post | V30post |
|---|---|---|---|
| mMRC score | 2 (1–2) | 1.5 (1–2) | 1 (1–1)a |
| LCADL score | 16 (11–25) | 17 (13–23) | 15 (13–18) |
| Self-care | 6 (5–11) | 6 (4–11) | 5 (4–6)a |
| Domestic | 1 (0–2) | 1 (0–3) | 1 (0–5) |
| Physical | 3 (1–6) | 4 (2–5) | 3 (2–5) |
| Leisure | 3 (2–5) | 4 (3–4) | 4 (3–4) |
| CAT score | 19 (11–26) | 12 (7–16)b | 12 (8–15)a |
| Modified Baecke scorec | 4 (2–11) | N/A | 14 (7–16)a |
| Low active, n (%) | 11 (65) | N/A | 5 (29) |
| Moderate active, n (%) | 6 (35) | N/A | 8 (47) |
| High active, n (%) | 0 | N/A | 4 (24) |
| Handgrip dynamometryd | |||
| Dominant hand, kg | 30 (22–37) | 31 (23–38) | 31 (20–36) |
| Non-dominant hand, kg | 26 (21–34) | 27 (19–36) | 26 (19–33) |
Abbreviations: mMRC, Modified Medical Research Council dyspnea scale; LCADL, London Chest Activities of Daily Living questionnaire; CAT, COPD Assessment Test questionnaire; N/A, not available.
The self-care domain of the LCADL questionnaire showed significant improvement during follow-up (P=.017), as well as the mBaek (P=.004). Specifically, according to mBaek questionnaire criteria24 4 patients became highly active by 1 month post-discharge, while only 5 patients remained inactive (29% of the total population).
DiscussionThis study has shown the mADL-Test to be a suitable tool for measuring the functional status of moderate-to-very-severe post-exacerbation COPD patients in the home setting. We have also shown that functional status, measured by the mADL-Test, continues to improve in the first 10 first days after HH discharge.
Functional status is related to COPD clinical outcomes, and therefore its assessment is relevant to patient management, although there is no gold standard test8 and difficulties are sometime encountered. The clinical status of the patient – stable and exacerbated phase – and the care setting – hospital and outpatient environments – can make this task difficult. Specifically, exercise field tests can be challenging in home-based programs due to space limitations. After previous evaluation of the Glittre test,18 we aimed to explore the feasibility of using it to assess functional status in the home setting. In this study, the mADL-Test was found to be suitable for home use, even in post-exacerbation COPD patients with only one evaluator. However, for the purpose of this study, we modified the original Glittre test to facilitate set-up and performance of the test in different home settings. The work-load of the original test remained unchanged, although the lay-out was modified. The mADL-Test physiological profile is analogous to the original test.15 We also established the number of laps completed by the patient in 6min as the main outcome. The aim was to obtain comparable physiological records from all the subjects, and avoid the possible ‘floor’ effect that has been described when patients are instructed to complete a fixed number of laps.11 In addition, recent studies have shown that the Glittre test is reproducible16 with suitable instructions. However, we did not validate our modifications against a gold standard test or the original one, and therefore our results should be treated with caution.
The originality of our study also lies in the contribution of new data on the natural functional recovery of post-exacerbation COPD patients: the mADL-Test performance improves in the first 10 days following HH discharge. In our study, despite its limited sample size, performance of the mADL-test improved in parallel with the clinical improvement shown by patients. Symptoms improved by the end of the study (significant improvement in dyspnea level, health status and physical activity) along with an improvement in cardiopulmonary variables (lower HR at the begging of the test, higher SpO2 at the end of the test, and a tendency toward peak V02 improvement). We acknowledge that the metabolic response of the test did not significantly differ between visits, even though median VO2 values would seem to increase during follow-up. One possible explanation, together with the small sample size, is that clinical recovery, such a reduction in mMRC, might have allowed patients to increase the number of laps without a significant increase in oxygen uptake. It should be noted that our patients did not participate in any pulmonary rehabilitation program after discharge, so we speculate that the slight recovery might be a result of the previous treatment received (within an integrated care unit) and the small margin of improvement without supplementary treatment (such as exercise training). In addition, sub-maximal exercise tests have been shown to be better than incremental test in detecting functional changes in COPD patients,27 which would explain the response to the mADL-Test during the recovery phase after an exacerbation. The 6MWT has already been shown to achieve significant improvement 1 month after discharge in untrained post-exacerbation COPD patients,6,28 whereas other studies failed to detect any improvement at 6-week follow-up using the incremental shuttle walking test.29,30 In addition, there were no safety concerns during performance of any of the tests, so we believe that the mADL-Test is a good option for functional status assessment of COPD patients, even during the recovery phase of an exacerbation.
Finally, some other limitations of this study should be mentioned. We could not calculate the sample size in advance, since this was a feasibility study with no previously recorded data. This may have contributed to the insufficient statistical power obtained in some comparisons, such as establishing a correlation between mADL-Test results and clinical variables. Also, the vast majority of our patients were male, and therefore, results cannot be generalized to both genders. Lastly, it is important to note that 7 out of the 17 patients had to perform the mADL-Test without their supplementary oxygen therapy in order to record physiological parameters, and we believe this could explain some of the variability seen in our results.
In conclusion, the mADL-Test is effective in measuring overall functional status in the home setting. It can be particularly useful in home care units and as a means of assessing other non-COPD patients with potentially altered functional status.31 More research into the mADL-Test properties is needed, as well as more studies to fully clarify the recovery pattern after a COPD exacerbation.
ConclusionsThe findings of this study suggest that the mADL-Test is a feasible and safe tool for assessing the functional status of moderate-to-very-severe COPD patients recovering from an exacerbation at home. Post-exacerbation functional status treated in a HH program improves 10 days after discharge. Undoubtedly, larger studies are warranted to confirm and expand these results.
Conflict of InterestWe have no conflict of interest to declare.
FundingThis work was partially supported by NEXES UE-FP7 (CIP-ICT-PSP-2007-1) 225,025 and SEPAR0123/09.
The authors would like to acknowledge the patients who participated in this study and also thank the strong collaboration of the Integrated Care team of Hospital Clinic for their help on patients’ recruitment and organizational efforts. They would also recognize Cosmed (Italy) and Sonmedica (Spain) for providing the equipment to do the physiologic measurements at home. The authors would also like to thank Bill De Felice for his altruistic collaboration in the revision of the text.
Please cite this article as: Valeiro B, Hernández C, Barberàn-Garcia A, Rodríguez DA, Aibar J, Llop L, et al. Viabilidad de la evaluación domiciliaria del estado funcional de pacientes con enfermedad pulmonar obstructiva crónica en fase de recuperación de una exacerbación. Arch Bronconeumol. 2016;52:256–261.