The Thoracic Surgery and Thoracic Oncology groups of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) have backed the publication of a handbook on recommendations for the diagnosis and treatment of non-small cell lung cancer. Due to the high incidence and mortality of this disease, the best scientific evidence must be constantly updated and made available for consultation by healthcare professionals.
To draw up these recommendations, we called on a wide-ranging group of experts from the different specialties, who have prepared a comprehensive review, divided into 4 main sections. The first addresses disease prevention and screening, including risk factors, the role of smoking cessation, and screening programs for early diagnosis. The second section analyzes clinical presentation, imaging studies, and surgical risk, including cardiological risk and the evaluation of respiratory function. The third section addresses cytohistological confirmation and staging studies, and scrutinizes the TNM and histological classifications, non-invasive and minimally invasive sampling methods, and surgical techniques for diagnosis and staging. The fourth and final section looks at different therapeutic aspects, such as the role of surgery, chemotherapy, radiation therapy, a multidisciplinary approach according to disease stage, and other specifically targeted treatments, concluding with recommendations on the follow-up of lung cancer patients and surgical and endoscopic palliative interventions in advanced stages.
La Sociedad Española de Neumología y Cirugía Torácica (SEPAR), a través de las áreas de Cirugía Torácica y de Oncología Torácica, ha promovido la realización de un manual de recomendaciones para el diagnóstico y el tratamiento del cáncer de pulmón de células no pequeñas. Las elevadas incidencia y mortalidad de esta patología hacen necesaria una constante actualización de las mejores evidencias científicas para su consulta por parte de los profesionales de la salud.
Para su confección se ha contado con un amplio grupo de profesionales de distintas especialidades que han elaborado una revisión integral, que se ha concretado en 4 apartados principales. En el primero se ha estudiado la prevención y el cribado de la enfermedad, incluyendo los factores de riesgo, el papel de la deshabituación tabáquica y el diagnóstico precoz mediante programas de cribado. En un segundo apartado se ha analizado la presentación clínica, los estudios de imagen y el riesgo quirúrgico, incluyendo el cardiológico y la evaluación funcional respiratoria. Un tercero trata sobre los estudios de confirmación cito-histológica y de estadificación, con un análisis de las clasificaciones TNM e histológica, métodos no invasivos y mínimamente invasivos, así como las técnicas quirúrgicas para el diagnóstico y estadificación. En un cuarto y último capítulo se han abordado aspectos del tratamiento, como el papel de las técnicas quirúrgicas, la quimioterapia, la radioterapia, el abordaje multidisciplinar por estadios y otros tratamientos dirigidos frente a dianas específicas, terminando con recomendaciones acerca del seguimiento del cáncer de pulmón y los tratamientos paliativos quirúrgicos y endoscópicos en estadios avanzados.
The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) sponsored the publication of this document aimed at providing clinical practice guidelines, based on the best available evidence, for the diagnosis and treatment of patients with non-small cell lung cancer (NSCLC). The high incidence and poor prognosis of lung cancer (LC),1 the complexity of diagnostic techniques and the greater availability of treatments mean that clinical practice procedures in this disease must be constantly updated.
When drawing up this document, we searched various databases for the latest studies relating to each of the points under discussion, and evaluated and synthesized the published evidence. The American College of Chest Physicians-ACCP Grading System was used to formulate recommendations (Table 1).2
Grade of Recommendation According to the American College of Chest Physicians-ACCP Grading System.
| Grade of Recommendation | Benefit vs Risk | Methodological Strength of Supporting Evidence | Implications |
|---|---|---|---|
| 1A. Strong recommendation, high quality evidence | Benefits clearly exceed the risks or vice versa. | Consistent evidence from randomized clinical trials without significant limitations or exceptionally, strong evidence from observational studies. | Recommendation which can be applied in most patients and most circumstances. It is very unlikely that more research will change our confidence in the estimation of the effect. |
| 1B. Strong recommendation, moderate quality evidence | Benefits clearly exceed the risks or vice versa. | Evidence from randomized clinical trials with significant limitations (inconsistent results, methodological defects, indirect or inaccurate evidence) or very strong evidence from observational studies. | Recommendation which can be applied in most patients and most circumstances. High quality research may possibly have an impact on our confidence in the estimation of the effect and may change that estimation. |
| 1C. Strong recommendation, low quality evidence | Benefits clearly exceed the risks or vice versa. | Evidence of at least 1 critical result from observational studies, case series, or randomized clinical trials with serious defects or indirect evidence. | Recommendation which can be applied in most patients and in many circumstances. High quality research may probably have an impact on our confidence in the estimation of the effect and may change that estimation. |
| 2A. Weak recommendation, high quality evidence | Benefits are closely balanced with the risks | Consistent evidence from randomized clinical trials without significant limitations or exceptionally, strong evidence from observational studies. | The best action may differ, depending on the circumstances, the patients or social values. It is very unlikely that more research will change our confidence in the estimation of the effect. |
| 2B. Weak recommendation, moderate quality evidence | Benefits are closely balanced with the risks | Evidence from randomized clinical trials with significant limitations (inconsistent results, methodological defects, indirect or inaccurate evidence) or very strong evidence from observational studies. | The best action may differ, depending on the circumstances, the patients or social values. High quality research may possibly have an impact on our confidence in the estimation of the effect and may change that estimation. |
| 2C. Weak recommendation, high quality evidence | Uncertainty in the calculation of the benefits and risks, which may be closely balanced. | Evidence of at least 1 critical result from observational studies, case series, or randomized clinical trials with serious defects or indirect evidence. | Other alternatives may be equally reasonable. High quality research may probably have an impact on our confidence in the estimation of the effect and may change that estimation. |
Cigarette smoking is the main causative agent of LC (90% of cases). However, other factors have been identified which may act synergically with cigarette smoke to modify the prevalence of LC, such as3: diet, physical activity, occupational exposure in both domestic and industrial environments, radiation, environmental pollution, host-related factors, and acquired lung diseases, for example chronic obstructive pulmonary disease (COPD) and fibrotic diseases.
COPD is an independent risk factor for developing LC, and the highest incidence occurs with the emphysema phenotype4,5 (Grade 1A). Local molecular and cellular inflammatory events6 and oxidative stress contribute to the pathogenesis of LC in patients with chronic respiratory diseases (Grade 1A), while systemic oxidative stress has potentially predictive value for the development of LC in patients with COPD7 (Grade 1B).
Smoking CessationFirst line pharmacological treatment (nicotine replacement therapy, bupropion and varenicline), in monotherapy or combination, associated with psychological counseling has been shown to be cost-effective and should be offered to all smokers8,9 (Grade 1A).
In LC screening programs using low-dose computed tomography (CT), advice for quitting smoking should be provided, along with pharmacological treatment10 (Grade 1B).
Another important aspect is how to approach smoking in patients with LC who are about to receive treatment. Pharmacological therapy is recommended to improve cessation rates in patients who are scheduled to undergo surgery10 (Grade 1B). The use of chemotherapy (CT) can complicate the approach to smoking cessation. In these cases, both counseling and pharmacological treatment are recommended, in order to improve cessation rates (Grade 1B), and bupropion is recommended for patients with symptoms of depression11 (Grade 2B). We recommend that subjects undergoing radiation therapy (RT) should also receive counseling plus pharmacological treatment12 (Grade 1B).
ScreeningSeveral randomized studies have attempted to relate a yearly chest radiograph with reduced mortality.13,14 However, the results of these studies show that this practice does not reduce LC mortality, so it cannot be recommended as a screening tool (Grade 1A).
Data from the National Lung Screening Trial (NLST),15 together with evidence provided by other randomized studies, show a clear trend toward the use of low-dose CT in LC screening.15,16 The definition of high risk is not clearly established, but the NLST used the following inclusion criteria: age between 55 and 74 years, a smoking history of at least 30 pack-years, and a maximum period of smoking abstinence of 15 years (Grade 1B). The most significant finding in the low-dose CT screening group was a 20% reduction in LC mortality (Grade 1B). Evidence in several studies of the importance of emphysema as a risk factor has optimized subject selection, which in turn reduces costs, false positives, and anxiety associated with screening.4,17,18
According to the International Early Lung Cancer Action Program (iELCAP), the use of a protocol for evaluating and monitoring pulmonary nodules (PN) detected in LC screening ensures that over 90% of the biopsies performed for LC will be diagnostic.19 Thus, in screening programs, it is advisable to use follow-up protocols based on imaging techniques in order to reduce false positives and avoid unnecessary biopsies (Grade 1B). The risk of morbidity and mortality due to invasive procedures for the diagnosis of positive findings on screening CTs is very low (Grade 1B).
The risk of the radiation received during screening is also probably very low. No reliable studies in this respect are available, as it is difficult to predict the risk of a dose of radiation lower than 50–100mSv, which may be inexistent20 (Grade 2C).
Clinical Presentation. Imaging Studies and Clinical Laboratory Tests. Surgical Risk and Functional EvaluationClinical Diagnosis. Serum MarkersClinical suspicion is based on clinical history and the ability to recognize the typical but sometimes non-specific signs and symptoms of the disease. Age, tobacco use, family history of LC and cancer of the oropharyngeal region, and exposure to asbestos increase the risk of LC.21,22 If LC is suspected clinically, the patient must be referred at once to a specialist for rapid diagnosis and evaluation by a multidisciplinary team (Grade 2C).
The role of serum markers in the early detection and diagnosis of LC has not been proven, and there are no clear recommendations regarding their determination and benefit in clinical practice. This is due, above all, to their low sensitivity and to the lack of specificity of exclusive markers for pulmonary neoplasms. Various studies have shown that in the initial evaluation, serum tumor markers, particularly CEA and CYFRA 21-1 may be useful for diagnosis and estimating prognosis23,24 (Grade 2C).
Imaging TechniquesChest radiograph is still the most widely used technique for ruling out possible LC and for studying the effect of certain treatments.25 If an undetermined PN is observed on a radiograph and/or CT, the patient's complete record of imaging studies should be reviewed (Grade 1C). If the nodule was diagnosed by radiography, a CT should be performed to improve characterization of the lesion26,27 (Grade 1C).
CT is the gold standard test for the diagnosis and staging of LC, and is mandatory if lesions suspected to be malignant are observed on radiography.26,27 Clinical and radiological parameters (size, shape, density, speed of growth) can be used to determine the likelihood of malignancy when a solitary PN is detected.28 If the PN is solid and has been stable for at least 2 years, it is recommended that follow-up is curtailed (Grade 2C). An important classification has been developed that categorizes PNs as solid, non-solid or part-solid.29 Particular interest lies in non-solid lesions which develop toward a part-solid pattern, as this may indicate the development of a malignant component.
The combined use of CT with positron emission tomography (PET) is an important tool in the diagnosis and treatment of LC, and a method for characterizing PNs.29 In clinical stage IA with peripheral tumor and PET-CT without enlarged lymph nodes or mediastinal uptake, invasive clinical staging is not necessary (Grade 2B). In contrast, invasive staging is recommended in patients with enlarged lymph nodes and no distant metastasis with or without PET-CT uptake, and in those with normal-sized lymph nodes, without distant metastasis but who show uptake on PET-CT,30,31 (Grade 1C).
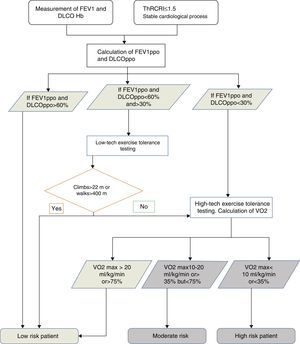
Preoperative Evaluation of Surgical RiskAccording to published guidelines32,33 the first step in preoperative evaluation is quantification of cardiovascular risk. Lung function is then assessed, followed by aerobic exercise tolerance, if necessary (Fig. 1).
The cardiology evaluation must include the Thoracic Revised Cardiac Risk Index (ThRCRI).34,35 All patients with a stable heart status and ThRCRI ≤1.5 can undergo surgery without undergoing a specific cardiac evaluation35 (Grade 1C). Moreover, lung resection should be avoided in patients with recent acute myocardial infarction (within the following 30 days) (Grade 1B).
Sufficient evidence is currently available32,33 to support the measurement of forced expiratory volume in 1 second (FEV1) and carbon monoxide diffusing capacity (DLCO) in LC patients under evaluation for lung resection (Grade 1B). If the calculated predicted postoperative (ppo) FEV1 and DLCO are greater than 60%, no further studies are required (Grade 1C). If they are below 60% but greater than 30%, evaluation of aerobic exercise tolerance with a low-tech test is recommended, for example, the symptom-limited stair-climbing test or the Shuttle Walking Test (Grade 1C): if a subject does not achieve a height of 22m36 or a distance of 400m, they should be referred for cardiopulmonary exercise testing with direct measurement of oxygen uptake (Grade 1C). If, however, ppo FEV1 and DLCO are less than 30%, the patient should be referred directly for high-tech cardiopulmonary exercise testing32 (Grade 1B). If oxygen uptake is less than 10ml/kg/min or 35%32 on these tests, the recommendation is not to perform anatomical resection, and to opt for a minimally invasive technique or non-surgical treatment (Grade 1C).
Cytohistological Confirmation and StagingStagesThe TNM classification is of unquestionable prognostic value and critical importance in guiding the choice of the best possible treatment.37 However, the grade of tumor extension is not the only factor guiding the choice of treatment. This also depends on the histological features of the tumor and the clinical and functional circumstances of the patient.38 Thus, in patients with suspected or confirmed diagnosis of LC, a careful clinical evaluation is recommended to establish an initial TNM stage39 (Grade 1B).
The greatest advantage of this classification lies not only in its consistency, but also its universal applicability in terms of geography, therapy and histology.40–42 Moreover, stage grouping is more in keeping with surgical possibilities.
Classification and Pathology StudyThe pathological diagnosis of LC must be performed in accordance with the classification of the World Health Organization (WHO) and the International Association for the Study of Lung Cancer (IASLC) for adenocarcinoma.43,44 It is essential that the specific subtype of all NSCLCs is determined to facilitate therapeutic decision-making and to reduce as far as possible the rate of not-otherwise-specified NSCLCs. In NSCLC, we recommend differentiating between adenocarcinoma and squamous cell carcinoma, even in small biopsies or scant cytology material45 (Grade 1B). Moreover, in the case of glandular tumors (adenocarcinomas), in situ and minimally invasive adenocarcinomas should be distinguished from invasive tumors45 (Grade 1C).
Currently, EGFR gene mutations and ALK rearrangements must be determined in all patients with stage IV non-squamous NSCLC, regardless of their smoking habit, and in all non-smokers, regardless of their tumor histology.46,47
Non-invasive MethodsSputum cytological testing has the advantage of being inexpensive and simple, and is performed on material from the respiratory tract that can be obtained non-invasively.48 The quality and storage of the sputum are very important if an accurate result is to be obtained, and the recommendation is to take 3 samples on different days. On the other hand, the sensitivity of the test varies depending on the sampling and processing conditions and the characteristics of the tumor.49,50 A negative result must be followed up with other invasive diagnostic tests (Grade 1C).
Minimally Invasive TechniquesThe development of new techniques in bronchoscopic interventionism means that lesions even in the peripheral lung51 and mediastinal lymphatic metastasis52 can be accessed. If LC with mediastinal involvement is suspected on imaging techniques, in the absence of evidence of extrathoracic metastatic disease (PET negative), we recommend establishing LC diagnosis using a safer, less invasive method: conventional bronchoscopy with blind transbronchial needle aspiration (TBNA), endobronchial ultrasound (EBUS) with fine-needle aspiration (FNA), endoscopic ultrasound (EUS) with BTP, transthoracic FNA or mediastinoscopy (Grade 1C).
Blind TBNA can be used to obtain cytology and histology samples from hilar and mediastinal lymph nodes. The diagnostic yield is lower than that of EBUS,53 but increases if it is combined with EBUS or EUS.54
FNA is a technique which, when combined with EBUS/EUS, can be used to evaluate and stage mediastinal lymph nodes,55 while reducing the need for mediastinoscopy or thoracotomy (more costly, more aggressive methods).
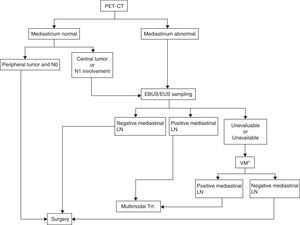
Evaluation of hilar and mediastinal lymphadenopathy samples using real-time ultrasound-guided techniques, whether endobronchial or endoscopic, provides more accurate staging. EBUS is superior to surgical staging in patients with a high suspicion of mediastinal tumor involvement56,57 (Grade 1C). The EBUS/EUS combination also has a greater yield as it can be used to obtain samples of both lung lesions and mediastinal lymphadenopathies58,59 (Grade 1C). In patients with no distant metastasis and a high suspicion of N2-N3, either on CT or PET, EUS, EBUS or a combination of both are recommended56 (Grade 1B) (Fig. 2).
Algorithm for mediastinal lymph node staging. EBUS/EUS unevaluable: an EBUS sample is considered unevaluable when it does not contain lymphocytes and only contains blood or bronchial cells or fibrosis, and it cannot be confirmed to be a lymph node puncture. If it contains tumor cells but nodal origin cannot be confirmed by the pathology study, this can be assumed as the puncture is guided by ultrasonography.
LN, lymph nodes; Trt., treatment; VM, video-assisted mediastinoscopy.
*This also includes other surgical techniques such as extended cervical mediastinoscopy, anterior mediastinoscopy, or video-assisted thoracoscopy when suspected lymph nodes are inaccessible by video-assisted mediastinoscopy, as is the case for lymph node stations 5 and 6 in left upper lobe carcinomas.
Radial EBUS is recommended as a diagnostic modality in patients with a peripheral pulmonary nodule suspected of malignancy who are not candidates for surgery (Grade 1C). It is more accurate (75%) than CT (51%) for detecting intrathoracic central tumors. It can also be used to differentiate between the tumor infiltrating the wall and extrinsic compression without infiltration.60
Electromagnetic navigation bronchoscopy (ENB) is recommended in patients with peripheral lung lesions that are difficult to access with conventional bronchoscopy61 (Grade 1C). It can also be used to sample mediastinal lymph nodes.
Surgical TechniquesUntil recently, mediastinoscopy was the technique of choice for staging mediastinal tumors. However, the generalized use of PET-CT as a staging method in LC and the recent introduction of lymph node biopsies using EBUS and EUS have led to a reduction in the indications of mediastinoscopy,62 and it no longer occupies the first step in mediastinal staging algorithms31,30,63 (Fig. 2).
In patients with NSCLC of the upper left lobe and lymph node stations 5 and/or 6 positive on PET-CT, surgical biopsy is indicated (by anterior mediastinotomy, extended cervical mediastinoscopy or video-assisted thoracoscopy) when no other mediastinal stations are involved31,64 (Grade 2B). If lymph nodes in other stations show pathological uptake, they should be biopsied using EBUS/EUS or mediastinoscopy.31
Compared with mediastinoscopy, video-assisted thoracoscopy is useful for evaluating the pleural cavity and the lung and for accessing the lower mediastinal lymph node stations.65 Video-assisted thoracoscopy is also useful in the diagnosis and treatment of peripheral PN.66
TreatmentSurgeryIn stage I and II NSCLC, radical surgery offers the best possibility of cure (Grade 1B), and the higher the stage, the poorer the survival.67,68 In patients with stage IIIA and discrete N2 involvement, identified pre-operatively, surgery as the first therapeutic option is not valid outside the clinical trial setting (Grade 1C). Nevertheless, following induction therapy and subsequent diagnosis of resectable residual disease, it can be useful in the context of a multidisciplinary therapeutic setting in carefully selected patients, (Grade 1A), since it offers better long-term survival than other treatments.69 After induction therapy, pneumonectomy should be avoided, particularly on the right side,70,71 given the high post-operative mortality, except in highly experienced centers (Grade 2C), where mortality is nearly same as when not preceded by induction.72 In the case of occult mediastinal disease, resection of the tumor and lymphadenopathies is indicated if it can be complete (Grade 2C).
In stage IIIA due to T3N1, surgery within a multidisciplinary therapeutic setting is recommended in patients with potentially resectable NSCLC (Grade 1B). En bloc resection should be performed in patients with parietal pleural involvement73 (Grade 2C). In tumors of the apex of the lung, surgery with en bloc parietal lobectomy should be preceded by induction therapy (Grade 2B)—this procedure also offers better survival.74,75
In a multidisciplinary therapeutic setting, surgery is recommended in NSCLC with T4 involvement with ipsilateral tumor nodules in different lobes (Grade 1B), giving 5-year survival figures of almost 15%,75 and in potentially resectable NSCLC, with no mediastinal involvement and with eradicable adrenal or brain metastasis76,77 (Grade 1C).
With regard to mediastinal lymphadenectomy, systemic lymphadenectomy is recommended to improve staging accuracy, and eventually to increase disease-free and overall survival75,78 (Grade 2C).
There may be a survival advantage after incomplete resection if the residual disease is microscopic or restricted to the lymph nodes, but not when it is gross79 (Grade 1C).
With regard to surgical technique, anatomical lung resection using thoracoscopy, which reduces morbidity and mortality and length of hospital stay, is preferable to open thoracotomy when feasible (Grade 2B).80,81 In terms of resection type, we conclude that:
- a.
In stage I NSCLC patients (tumor<2cm) with a high risk of intra-operative mortality, anatomical segmentectomy or wedge resection with negative margins are preferable to lobectomy82 (Grade 2C). The latter technique is the procedure of choice in stages I and II83,84 (Grade 1B).
- b.
Pneumonectomy should only be performed when the NSCLC cannot be completely resected by lobectomy, including bronchoangioplastic techniques85 (Grade 1B).
- c.
In a multidisciplinary treatment setting, surgery is indicated in carefully selected patients with T4N0-186 (Grade 2B).
Treatment of NSCLC is constantly evolving. Currently, most patients are candidates for multimodal management, with CT being an essential component of the treatment.87,88 Thus, post-operative or adjuvant CT is recommended in patients operated for stage II or III disease, provided the resection is complete. If it is incomplete, CT and RT are recommended.
Stage III has conventionally been managed with neoadjuvant CT followed by surgery, and stage IIIB with sequential or concurrent chemo and radiotherapy (CT/RT). However, the borders are becoming increasingly blurred, since data are available to support the role of surgery in stage IIIB patients, and the use of concurrent CT/RT before surgery in stage IIIA.89,90
In resectable stage III disease, combined treatment with adjuvant CT and surgery provides a greater benefit than other treatments (Grade 1A). The foreseeable objective response rate with a 2-drug CT regimen containing cisplatin is around 60%–70%. In contrast, no survival benefit has been reported for the combination of pre-operative RT and CT in potentially resectable N2 patients.90 In IIIB patients in good general condition (ECOG 0-1), combined CT and RT is recommended89 (Grade 1A).
In patients with stage IV NSCLC, in good general condition (ECOG 0-1) and who are not candidates for targeted therapy, the current recommendation is to use a platinum-based 2-drug combination (Grade 1A) to prolong survival and improve quality of life. CT must be started immediately on diagnosis. No more than 4–6 cycles of first-line CT are recommended (Grade 2B). In the absence of molecular changes, histology is a determinant factor for the choice of treatment (Grade 1A); best results for non-squamous tumores are obtained with cisplatin-pemetrexed. Moreover, bevacizumab combined with CT is only recommended in patients with non-squamous carcinomas, who show less toxicity than patients with squamous tumors.91,92
In advanced NSCLC which has not progressed after first-line treatment, maintenance therapy has been shown to improve survival93,94 (Grade 1A). After completing first-line therapy, the patient will continue monotherapy until progression or toxicity.
Second-line CT has shown benefits in survival and symptom control in advanced NSCLC and ECOG 0-2, irrespective of histology95 (Grade 1A). Monotherapy is recommended in second-line treatment (Grade 1A), and should be continued until progression.
EGFR mutations and ALK translocations are currently used as prognostic factors for therapeutic efficacy in advanced NSCLC, so patients with these characteristics should receive targeted treatments in the form of tyrosine kinase96 and ALK inhibitors,97 respectively.
In stages I and II, hypofractionated stereotactic body radiation therapy (SBRT) is indicated in inoperable patients, patients with high surgical risk, or patients who refuse surgery and have stage I (T1, or T2≤5cm) disease located more than 2cm from the main bronchial tree and at least 1.5cm from aorta and main pulmonary artery.98 Conventional RT is indicated in inoperable patients who do not meet criteria for SBRT, and post-operative RT is not indicated, unless there is involvement of the margin. In these cases, concurrent CT/RT may be applied. In potentially resectable stage III disease, RT may form part of the treatment, both before surgery, in combination with CT (this is the first choice in tumors of the superior sulcus)74 and after. In unresectable stage III disease, standard treatment is concurrent CT/RT.99 In stage IV, RT is indicated to relieve symptoms, both loco-regional and in brain and/or bone metastases.
Initial Treatment by StagesIn view of the increasingly common multidisciplinary approach, we have listed and grouped the basic aspects of initial treatment in the various clinical stages (Table 2). This information, together with the above sections, gives a more comprehensive explanation of the characteristics of the available treatments and evidence.
Summary of Treatment Recommendations According to Clinical Stage.
| Recommendation | Grade |
|---|---|
| Stages I and II | |
| In patients in stages IA, IB, IIA and IIB with no medical contraindication, treatment of choice is surgical resection. | 1B |
| Sublobar resection is recommended in inoperable patients or those with high comorbidity burden. | 1B |
| Therapeutic alternatives in patients who refuse surgery or who are inoperable are RT or radiofrequency ablation. | 2C |
| Adjuvant RT is not recommended in stage I. | 1A |
| Adjuvant CT is recommended in stage II (N1). | 1A |
| Stage III | |
| In resectable stage III without N2 involvement, proposed treatment is surgical resection with adjuvant CT/RT. | 1A |
| In potentially resectable stage IIIA without N2 lymph node involvement, proposed treatment is neoadjuvant CT/RT, surgical resection, and adjuvant treatment. | 2C |
| In unresectable stage IIIA, treatment should be radical CT/RT. | 1A |
| In stage IIIA with intra-operative (occult) N2 involvement, treatment is surgical resection and adjuvant CT/RT. | 1A/2C |
| In preoperative stage IIIA-N2, treatment may be definitive CT/RT or induction therapy and surgery. | 1A |
| In preoperative stage IIIA-N2, initial surgical resection and adjuvant therapy is NOT recommended (with exceptions). | 1C |
| In patients in stage IIIB and good general condition, recommended treatment is concurrent CT/RT with curative intent. | 1A |
| Rescue surgery is not recommended in patients in stage IIIB, except in very exceptional cases. | 1C |
| Stage IV | |
| Recommended treatment is polychemotherapy | 1A |
| In patients with EGFR mutation, the first line of treatment is TKI. | 1A |
| In carefully selected patients with single brain and/or adrenal metastases and resectable tumors without mediastinal lymphadenopathies, multidisciplinary treatment of both lesions is recommended. | 1B/2B |
CT, chemotherapy; CT/RT, chemotherapy/radiation therapy; RT, radiation therapy; TKI, tyrosine kinase inhibitors.
With the exception of 1 systematic review,100 no randomized prospective trials have defined the duration, frequency and type of examinations which must be performed during the follow-up of LC. Nevertheless, treatment of therapy-related complications must be taken into account, and tumor relapse and/or the appearance of any second primary tumor must be detected. To achieve this, we recommend that decisions during follow-up should be taken by a multidisciplinary team. Since most relapses occur during the first 2–3 months after treatment, monitoring is recommended every 3 or 6 months, and then once a year. A 5-year period is thought to be insufficient to consider a patient cured, particularly in the case of lymph node or vascular involvement.101
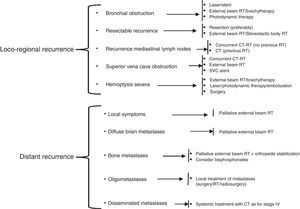
In the treatment of NSCLC relapse, it is important to first identify whether the primary tumor has recurred, or whether a second primary pulmonary tumor has developed. Recurrences can be locoregional or distant, the latter being more common. The treatment of relapses follows the recommendations of the National Comprehensive Cancer Network (NCCN),102 and is summarized in Fig. 3.
Treatment schedule for NSCLC recurrence, adapted from the NCCN clinical practice guidelines in oncology. CT, chemotherapy; CT–RT, chemotherapy/radiation therapy; RT, radiation therapy; SVC, superior vena cava.
Pleural effusion can be managed with repeated thoracocentesis if patients show clinical improvement, particularly in patients with very advanced disease and short-term life expectancy103 (Grade 1C). VATS talc pleurodesis is a minimally invasive surgical procedure with low morbidity and mortality, during which the whole pleural cavity can be examined and talc can be administered under direct vision103 (Grade 1C). If pleurodesis cannot be performed due to the patient's poor general condition, short life expectancy or lack of post-evacuation pulmonary expansion, a permanent catheter can be introduced, and the patient can be managed in an outpatient setting103 (Grade 1C).
The indications and complications of the main palliative endoscopic treatments are summarized in Table 3. In advanced stage LC with symptomatic airway obstruction, bronchoscopic treatment with mechanical debridement, tumor ablation or stent placement is recommended104 (Grade 1C). In the case of tracheo-esophageal fistula, placement of self-expanding metallic stents in the esophagus and the airway, or in the esophagus only, is recommended (Grade 1B).
Summary of Palliative Endoscopic Treatments in Lung Cancer.
| Procedure | Indications | Complications |
|---|---|---|
| Tracheobronchial prosthesis or stent | Stenosis due to extrinsic compression Tracheobronchial fistulas Tracheoesophageal fistulas | Obstruction Local inflammation Migration Perforated airway Airway infection Hemoptysis |
| Argon plasma electrocoagulation | Stenosis due to exophytic lesions Hemostasis | Burns Perforated airway Air embolism |
| Laser resection | Stenosis due to exophytic lesions | Hemorrhage Perforated airway Airway necrosis Fistulas Air embolism |
| Cryotherapy | Stenosis due to exophytic lesions Hemoptysis due to tumors | Very safe method Massive hemorrhage |
| Brachytherapy | Tumor relapse after full doses of radiation therapy (RT) RT intolerance | Massive hemoptysis Fistulas Post-radiation bronchitis Bronchostenosis |
| Electrocogaulation | Stenosis due to exophytic lesions Less expensive than laser | Intrabronchial fire Hemoptysis Perforation Pneumonia |
| Photodynamic therapy | Stenosis due to exophytic lesions in the distal bronchi | Photosensitivity Perforation Hemoptysis Fever Dyspnea due to edema |
In the case of massive hemoptysis, the source of bleeding can be identified on bronchoscopy, and if the lesions are visible, additional techniques, such as argon plasma coagulation, Nd:YAG laser and electrocautery can be applied (Grade 1C). Endobronchial bronchoscopic treatment is indicated for mild or moderate hemoptysis due to visible lesions in the central airways. In distal or parenchymatous lesions, external beam radiotherapy is recommended (Grade 1C).
Conflict of InterestDr. Segismundo Solano Reina has collaborated with GSK and Pfizer pharmaceutical industries with an interest in the field of treatment of smoking. The other authors refer having no conflict of interest.
The following are the supplementary data to this article:
The full, electronic version of this document can be consulted online at http://www.archbronconeumol.org/carcinomapulmon/.
Please cite this article as: Villar Álvarez F, Muguruza Trueba I, Belda Sanchis J, Molins López-Rodó L, Rodríguez Suárez PM, Sánchez de Cos Escuín J, et al. Sumario ejecutivo de las recomendaciones SEPAR de diagnóstico y tratamiento del cáncer de pulmón de células no pequeñas. Arch Bronconeumol. 2016;52:378–388.
These authors are coauthors as first signatories and so should appear in the manuscript, says so for appropriate action.