The use of uncontrolled donation after circulatory death (uDCD) for lung transplantations (LTx) has been limited to a few cases worldwide.1,2 With more than 100 uDCD LTxs performed so far, most of the experience is concentrated in Spain.3,4 Cases at our center accounted for more than half of these cases, i.e., 53. An assessment of our first ten years of experience revealed a higher incidence of grade 3 primary graft dysfunction (PGD) (34.2% vs. 24%) and a lower overall survival rate compared with donors after brain death (DBD).5 Since the beginning of our uDCD program, we have used in situ effluent gas analysis of the left atrium (ISLA) to assess oxygenation as our primary evaluation strategy. However, some uncertainties regarding the grafts’ hemodynamic behavior and ventilation dynamics, which ISLA cannot assess, may be linked to the reportedly higher incidence of PGD and poor prognosis. Thus, we believe that ex vivo lung perfusion (EVLP) may improve a potential donor's assessment.
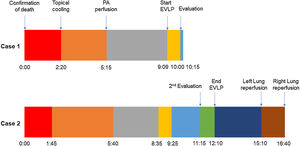
A 45-year-old man experienced chest pain and subsequent cardiac arrest at home. An emergency department declared him dead after attempting basic cardiopulmonary resuscitation per to our protocol. While authorizations from the family and the judge were being solicited, the donor was moved to the operating room. For topical cooling of the lungs, a chest drainage tube was inserted into each hemithorax, and Perfadex® solution (Medisan, Uppsala, Sweden) was introduced at 4°C while suspending mechanical ventilation. The warm ischemia time (WIT) was 140min, within our preferred time limit of 150min. We accepted the lungs for in situ appraisal. After obtaining consent, the preservation solution was drained, and 100% fraction of inspired oxygen with 5cm H2O of positive end-expiratory pressure lung ventilation was started.
Pulmonary artery (PA) perfusion with 60mL/kg of Perfadex® containing prostaglandin E1 and nitroglycerine was administered 175min after topical cooling, which was within our preferred time limit of 240min.
After half of the solution was perfused, 300mL of the donor's stored blood was added to the remaining Perfadex®, and ISLA was performed by infusing it through the PA while the lungs were ventilated. Five samples were taken, one from each pulmonary vein and one from the left atrium. The partial pressure of oxygen/fraction of inspired oxygen (P/F) ratio of the donor's lower lobe samples was just under 400mmHg, which is acceptable. Therefore, due to the marginal P/F ratio and WIT, otherwise acceptable organs were subjected to EVLP for further evaluation.
We used the Scandinavian protocol, which is a cellular, full flow, open atrium protocol, for EVLP. Peak airway pressure, plateau pressure, dynamic lung compliance, pulmonary vascular resistance, PA pressure trends, and blood samples were evaluated before progressing to an evaluation phase.
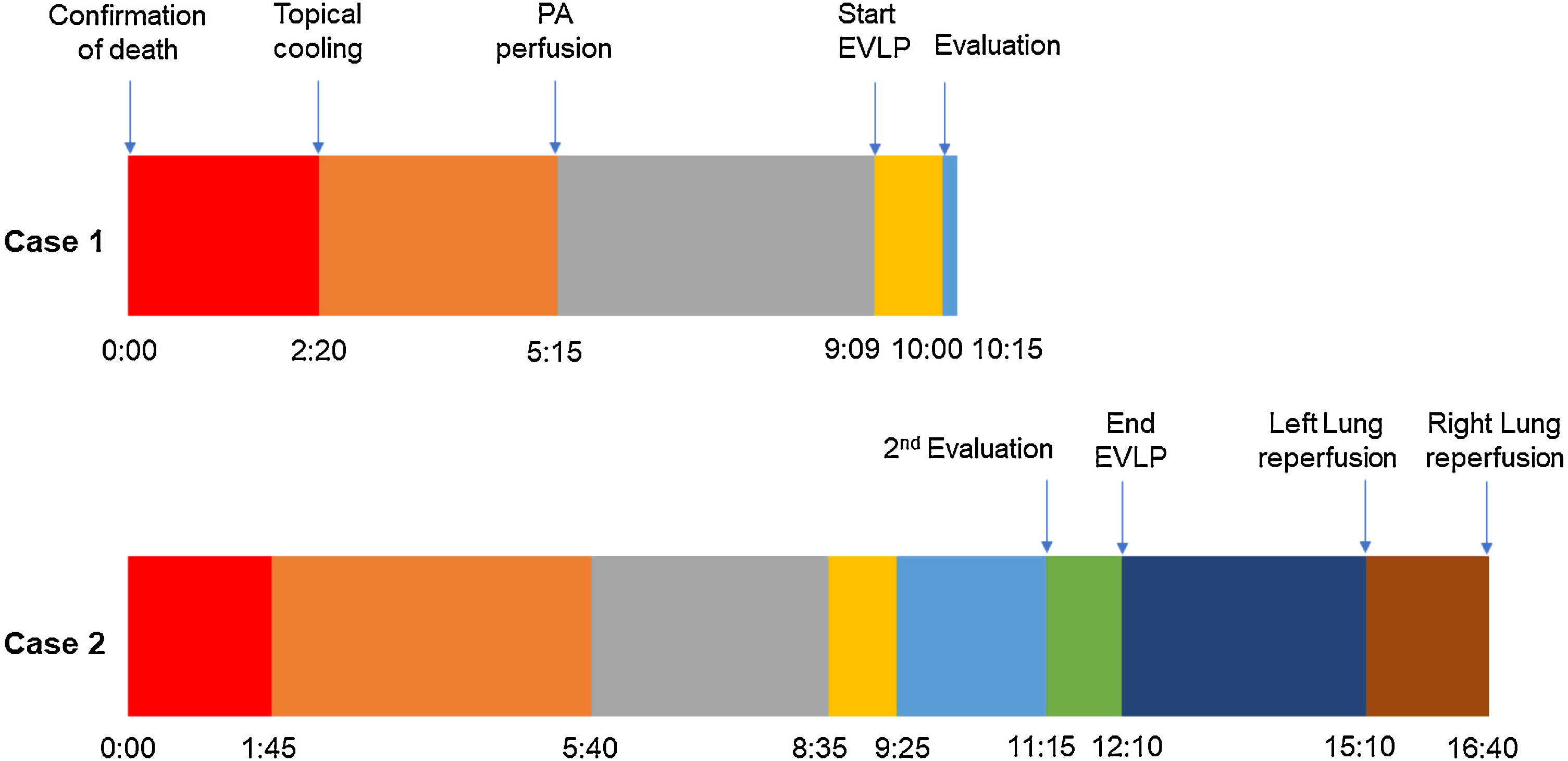
In this case, the full flow was attained after 60min of perfusion. However, we observed a high PA pressure, pulmonary vascular resistance, and low dynamic lung compliance. The grafts were discharged due to a copious amount of frothy pink sputum and an impaired collapse test (Figs. 1 and 2).
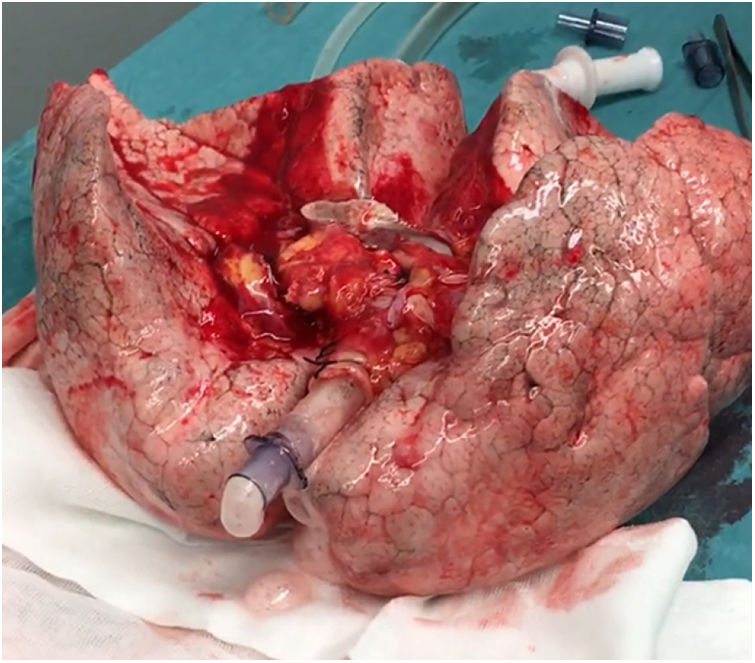
A 20-year-old woman with a history of obesity and oral contraceptive use experienced sudden death at home. The uDCD protocol was followed with 105min of WIT and 235min of perfusion. Numerous red clots were observed in the PA during retrograde perfusion, which disappeared at the end of the perfusion. An EVLP evaluation was performed due to a moderate suspicion of pulmonary embolism (Fig. 1). Within 60min of lung perfusion, the full flow was achieved without any alterations in hemodynamic and pulmonary dynamics. At this point, a gas analysis revealed a P/F ratio of over 400mmHg, and the final inspection and collapse tests yielded optimal results. Hence, previously rejected grafts were considered suitable for LTx.
The recipient was a 60-year-old man with idiopathic pulmonary fibrosis who underwent a double LTx. Postoperative venovenous extracorporeal membrane oxygenation was required due to PGD; however, the patient was weaned off it within 72h and exhibited excellent respiratory recovery after withdrawal. He was discharged from the intensive care unit after 11 days. At 17 months postoperatively, the patient was alive and in good health.
DCD is significantly different from DBD with regards to donation and recovery techniques. In DBD, additional evaluation of the thoracic cavity before cross-clamping the aorta is feasible since improved atelectasis, repetitive blood gas analyses, and a collapse test are easy to complete. Besides, it is unnecessary to consider warm ischemic damage. However, in uDCD, it is impossible to proceed with such strategies since the lungs are preserved at low temperatures before undergoing physical evaluation. No opportunities for further ventilatory or mechanical evaluation are likely. Furthermore, it is almost impossible to determine the etiology of the lesion that may have occurred in the vascular or alveolar-capillary barrier during the arrest, warm ischemia, or topical cooling.
EVLP has been proven to be safe and efficient for evaluating lung grafts. It was repeatedly successful in high-risk DBD lungs, presenting outcomes comparable to those from standard criteria donors.6 Moreover, since EVLP provides an excellent environment for the functional and hemodynamic evaluation of organs, it has been integrated into the uDCD's assessment armamentarium.1
Initially, ISLA was our strategy to evaluate uDCD donors. However, there were some concerns regarding its accuracy, as evidenced in the first case. It has now been superseded by EVLP, used as a supplementary assessment for uDCD. While our first case was considered suitable per the ISLA criteria, clinical experience based on the WIT and borderline P/F ratio led us to perform EVLP, which revealed severe reperfusion edema. According to the earlier criteria, this lung might have been implanted, resulting in severe PGD. Interestingly, according to our experience, the second case was otherwise a conventional uDCD donor. Although a large thrombus in the PA made it unacceptable for transplantation, it was recovered and utilized after a thorough EVLP evaluation.
We have now implemented several EVLP protocols (Toronto and Organ Care System Lung protocol) to evaluate uDCD donors. Although the Toronto group reported favorable outcomes using their protective ex vivo protocol,2 we experienced a high incidence of PGD. We believe this may be due to compromised lung integrity from ischemic damage, which was not thoroughly tested for by this protocol.6 Since 2017, we have adopted the Scandinavian protocol, which we believe simulates the clinical physiological environment more accurately. Nonetheless, further research is required to determine an effective method for a thorough assessment of uDCD lungs.
ConclusionsIn conclusion, we believe uDCD donors can be an invaluable source of organs. However, a balanced, well-established harvesting protocol is needed to increase yield and reduce dysfunction. It is crucial not to rely on a single strategy but to consider a more flexible approach that employs clinical experience and an in situ evaluation amalgam with a physiological setup, such as the Scandinavian EVLP protocol.
FundingNone.
Conflict of interestsNone.
We acknowledge the Departments of Pneumology, Anaesthesiology, Pathology, Microbiology, Rehabilitation, ICU and all the local coordinators that were involved in our Lung Transplant Program.