The effectiveness of home high flow nasal cannula (HFNC) for the treatment of chronic respiratory failure in patients with chronic respiratory diseases (CRDs) has not been summarized. We aimed to conduct a systematic review of the effectiveness, adherence, and safety of HFNC in the long-term treatment of patients with chronic respiratory diseases and respiratory failure.
MethodsA systematic review was conducted. PubMed, Web of science, and SCOPUS were search up to August 2023. Long-term HFNC studies (≥4 weeks) reporting dyspnea; exacerbations, hospitalizations; peripheral oxygen saturation (SpO2), comfort; patient experience, health-related quality of life or partial pressure of carbon dioxide (paCO2) were included.
ResultsThirteen articles (701 patients) based on 10 studies were selected: randomized control trials (n=3), randomized crossover trials (n=2), crossover (n=3) and retrospective (n=2) studies. COPD (n=6), bronchiectasis (n=2), COPD/bronchiectasis (n=1) and ILD (n=1) were the underlined CRDs. HFNC reduced exacerbations when compared to usual care/home respiratory therapies (n=6). Quality of life outcomes were also in favor of HFNC in patients with COPD and bronchiectasis (n=6). HFNC had significant effects on hospitalizations, paCO2, and lung function. Adherence ranged from 5.2 to 8.6h/day (n=5). Three studies reported no events, 3 non-serious events and 2 no differences compared with other home respiratory therapies.
ConclusionsHFNC seems more effective than usual care or other home respiratory therapies in reducing exacerbations and improving quality of life in patients with COPD and bronchiectasis, while presenting good adherence and being safe. Its apparently superior effectiveness needs to be better studied in future real-world pragmatic trials.
In 2019, respiratory diseases accounted for three of the top 10 causes of death, resulting in more than 8 million deaths annually, with chronic obstructive respiratory disease (COPD) being the third leading cause of death.1 However, other chronic respiratory diseases also contribute to this high burden. The global incidence of non–cystic fibrosis bronchiectasis ranges from 67 to 566 per 100,000 inhabitants in Europe and North America.2 Between 1990 and 2013, Interstitial Lung Disease (ILD) was among the top 50 causes of global years of life lost worldwide.3 In addition, regardless of the underlying chronic respiratory disease, patients experience frequent exacerbations, respiratory failure and a decrease in their quality of life.4 Various forms of treatment are available to improve physiological parameters, symptoms and patient-centered outcomes, including non-invasive ventilation and oxygen therapy. In recent years, high flow nasal cannula therapy has been introduced as another innovative approach to treat some groups of respiratory patients.
High flow nasal cannula (HFNC) has emerged as a home treatment for patients with chronic respiratory diseases to increase the carbon dioxide (CO2) washout, while improving the mucociliary clearance.5 HFNC provides heated and humidified gas admixture at a high flow rate (up to 60L/min) via a wide-bore nasal cannula. This therapy was widely studied in acute respiratory failure, including COVID-19 and has been shown to reduce intubation and mortality in comparison with conventional oxygen therapy.6–10 In addition, HFNC seems probably better than non-invasive ventilation in terms of dyspnea, comfort, and decreasing of respiratory rate in patients either post-extubation or during acute respiratory failure.11
In the home setting, evidence has been pooled in patients with COPD, with HFNC shown to improve health-related quality of life12 and reduce the rate of exacerbations.13 Other systematic reviews or meta-analysis of HFNC have shown inconsistent and conflicting results.14–16 These may be due to the fact that these syntheses included heterogeneous studies, mixing acute and chronic patients, short-term and long-term treatment. To our knowledge, there is only one systematic review focusing exclusively on stable patients with COPD, but unfortunately it was based on randomized control trials only.13 Adding information from real-world observational studies and other chronic respiratory diseases, such bronchiectasis and ILD, may help in clinical reasoning.17 Furthermore, the adherence and safety of long-term HFNC treatment in patients with chronic respiratory diseases remains unclear.
Therefore, we aimed to conduct a systematic review of the effectiveness, adherence, and safety of HFNC in the long-term treatment of patients with chronic respiratory diseases and respiratory failure.
Material and methodsStudy designA systematic review was conducted and reported according to PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.18 The protocol was registered at PROSPERO (CRD42023461837).
Search strategyA comprehensive search of PubMed, Web of science, and SCOPUS databases was performed until August 8, 2023. The search strategy included 2 concept sets: ((“chronic obstructive lung disease” OR COPD OR “chronic obstructive airway disease” OR “chronic obstructive lung disease” OR “interstitial lung disease” OR “ILD” OR “bronchiectasis” OR “hypercapnic” OR “normocapnic” OR “pulmonary disease” OR “chronic respiratory insufficiency”) AND (“high-flow oxygen” OR “high flow nasal cannula” OR “high flow nasal oxygen” OR “high flow oxygen therapy” OR “nasal high flow” OR “HFNC” OR “HFNO” OR “HFOT” OR “NHF” OR “NHFT” OR “short-term nasal high-flow” OR “domiciliary high-flow nasal cannula oxygen therapy” OR “domiciliary nasal high flow therapy”). Additional searches were performed using weekly automatic updates retrieved from these databases. We also searched for relevant references in the list of references of the included studies. In addition, we manually searched published meta-analyses and systematic reviews, and the references of the included studies to identify other potentially relevant studies. No language restrictions were applied.
Eligibility criteriaInclusion criteria were studies that included patients with chronic respiratory diseases such as COPD, bronchiectasis, ILD, and others and chronic respiratory failure (Population) and provided long-term HFNC, defined as at least 4 weeks with a flow of at least 20L/min (Intervention), which could be or not compared with other forms of respiratory support (Comparators, not mandatory). To be included, primary articles had to report outcomes such dyspnea; exacerbations, hospitalizations; peripheral oxygen saturation (SpO2), comfort; patient experience, health-related quality of life or partial pressure of carbon dioxide (paCO2). Randomized controlled trials (RCTs), randomized crossover studies, quasi-experimental studies, case-control studies and retrospective studies were included. Study protocols, book chapters, reviews, editorials/commentaries to articles, case reports and abstracts were excluded.
Screening, selection process and data extractionAfter removal of duplicate studies, the articles were screened independently by 2 reviewers (CJ and MJ) to identify relevant articles by the title and abstract using the Rayyan software.19 In case of disagreement, a third researcher (CC) was consulted. The 2 reviewers (MJ and CJ) used a standardized form to independently extract data from each article, including the author's surname and year of publication, study design, sample size, participants and condition, HTF protocol, outcomes, and results. The third author (CC) was consulted in case of discrepancies.
Assessment of methodological quality and risk of biasRisk of bias and methodological quality of the included studies were independently assessed by 2 authors (MJ and CJ). The risk of bias for randomized studies was assessed using the Cochrane Collaboration's tool.20 Risk of bias for observational studies was assessed using the Newcastle–Ottawa Scale (NOS).21 Disagreements were resolved by discussion or by a third reviewer (CC).
Data synthesis and analysisAs the included studies were clinically heterogeneous, narrative synthesis was used to report the findings. This was considered the most appropriate approach given the heterogeneity of data between the included studies. Findings were initially draft by one researcher (CJ), then reviewed by a second researcher (MJ).
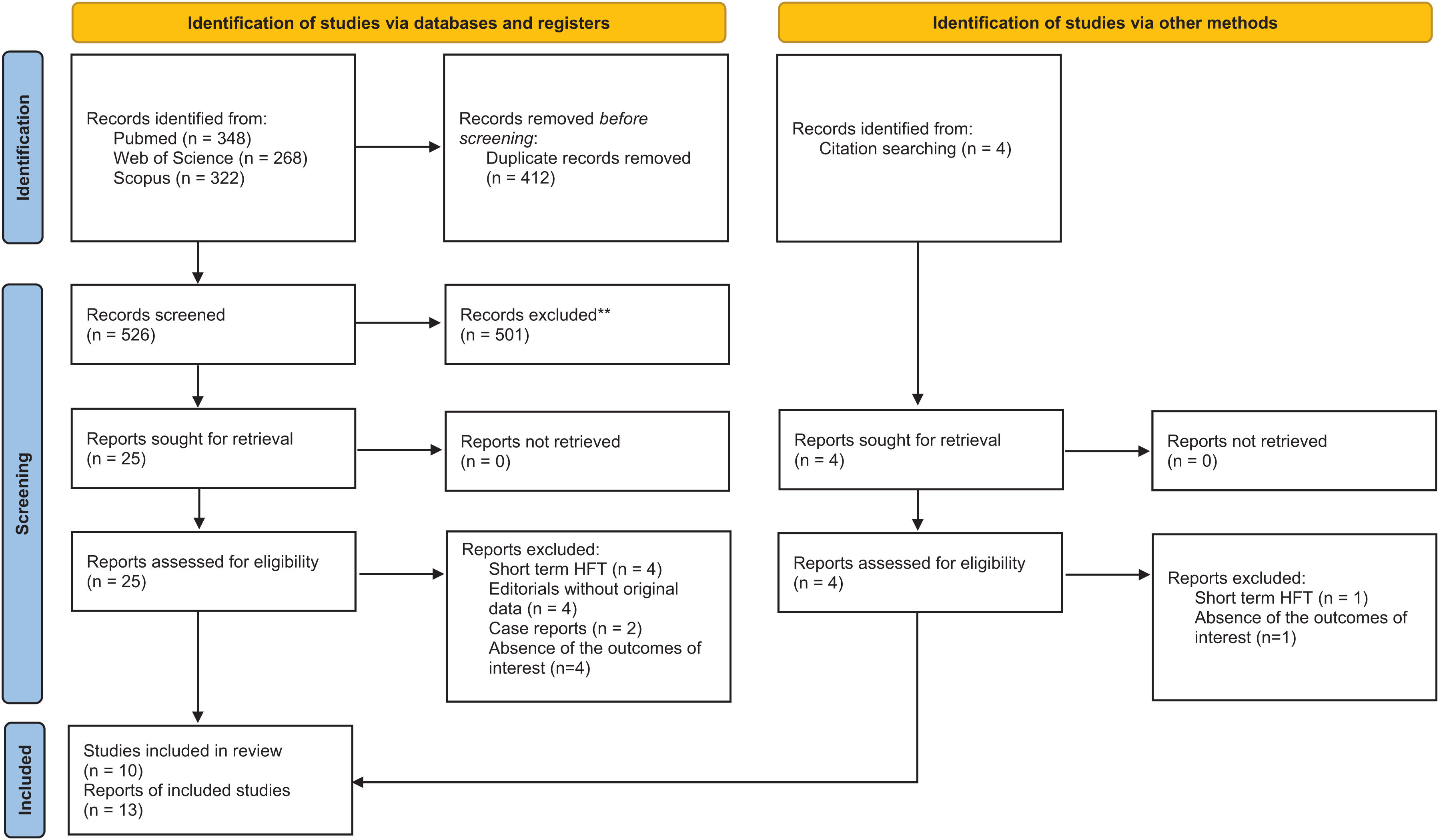
ResultsStudy selectionThe database search yielded 938 studies. After removing duplicate results, 526 articles were screened for relevant content. During title and abstract screening, 501 articles were excluded. Finally, 24 articles were selected for full-text screening. Two additional papers were included through manual search and screening of the reference lists of full-text articles. After excluding 8 articles, 13 articles from 10 studies were selected for qualitative analysis (Fig. 1).
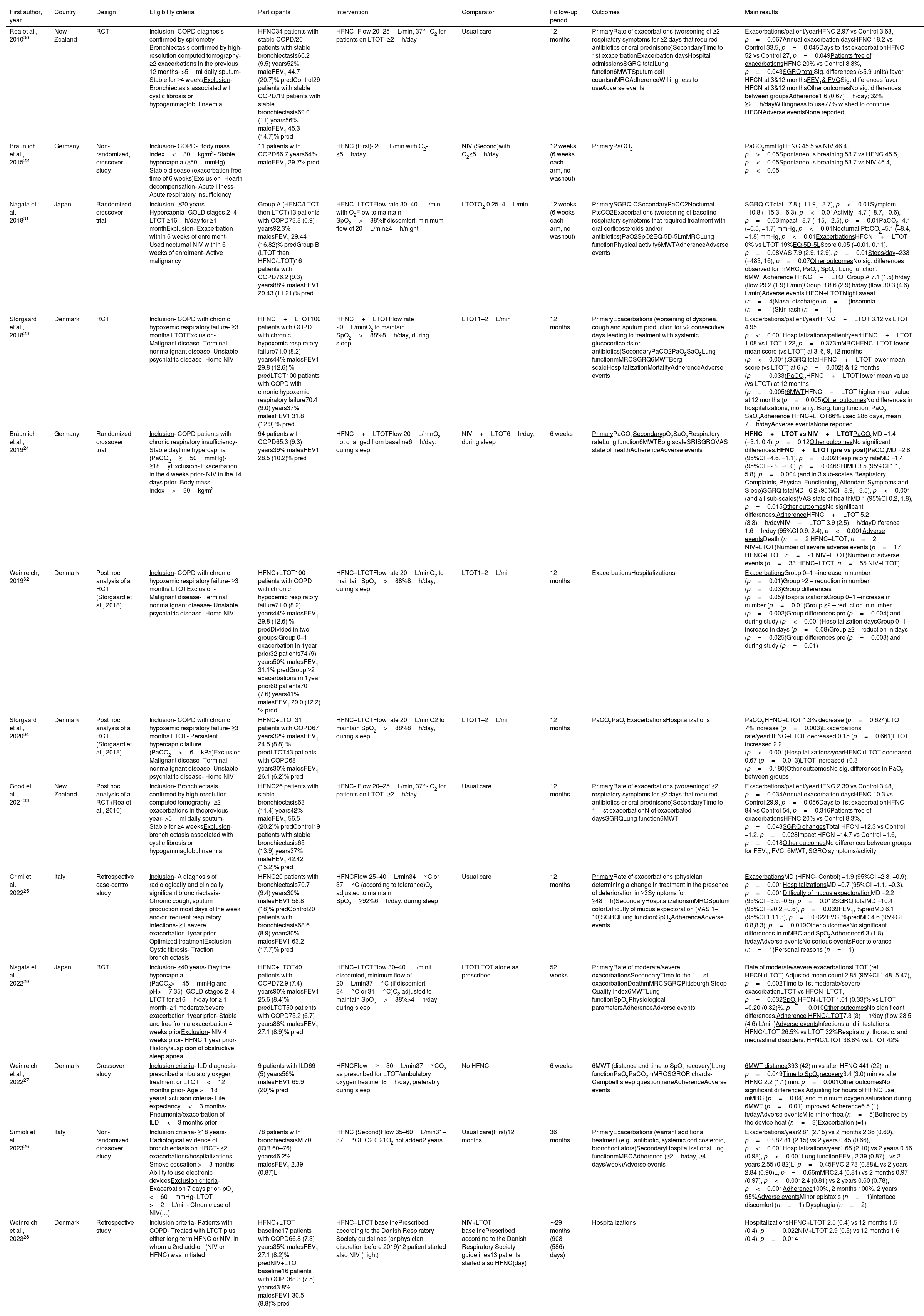
Methodological quality and risk of biasDetails of the included articles are shown in Table 1. The 13 articles were published between 2010 and 2023, mainly in European countries (n=7).22–28 The articles had different designs, namely RCTs (n=3),23,29,30 randomized crossover trials (n=2),24,31 non-randomized crossover studies (n=3)22,26,27 and retrospective studies (n=2).25,28 Three articles were secondary analysis of RCTs.32–34 Risk of bias in RCTs was mainly related to blinding of participants/staff, blinding of outcome assessor and incomplete data (Supplementary Fig. 1), whereas in non-randomized studies it was related to the outcome assessment and groups comparability (Supplementary Table 2).
Details of the included articles.
| First author, year | Country | Design | Eligibility criteria | Participants | Intervention | Comparator | Follow-up period | Outcomes | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Rea et al., 201030 | New Zealand | RCT | Inclusion- COPD diagnosis confirmed by spirometry- Bronchiectasis confirmed by high-resolution computed tomography- ≥2 exacerbations in the previous 12 months- >5ml daily sputum- Stable for ≥4 weeksExclusion- Bronchiectasis associated with cystic fibrosis or hypogammaglobulinaemia | HFNC34 patients with stable COPD/26 patients with stable bronchiectasis66.2 (9.5) years52% maleFEV1 44.7 (20.7)% predControl29 patients with stable COPD/19 patients with stable bronchiectasis69.0 (11) years56% maleFEV1 45.3 (14.7)% pred | HFNC- Flow 20–25L/min, 37°- O2 for patients on LTOT- ≥2h/day | Usual care | 12 months | PrimaryRate of exacerbations (worsening of ≥2 respiratory symptoms for ≥2 days that required antibiotics or oral prednisone)SecondaryTime to 1st exacerbationExacerbation daysHospital admissionsSGRQ totalLung function6MWTSputum cell countsmMRCAdherenceWillingness to useAdverse events | Exacerbations/patient/yearHFNC 2.97 vs Control 3.63, p=0.067Annual exacerbation daysHFNC 18.2 vs Control 33.5, p=0.045Days to 1st exacerbationHFNC 52 vs Control 27, p=0.049Patients free of exacerbationsHFNC 20% vs Control 8.3%, p=0.043SGRQ totalSig. differences (>5.9 units) favor HFCN at 3&12 monthsFEV1& FVCSig. differences favor HFCN at 3&12 monthsOther outcomesNo sig. differences between groupsAdherence1.6 (0.67)h/day; 32% ≥2h/dayWillingness to use77% wished to continue HFCNAdverse eventsNone reported |
| Bräunlich et al., 201522 | Germany | Non-randomized, crossover study | Inclusion- COPD- Body mass index<30kg/m2- Stable hypercapnia (≥50mmHg)- Stable disease (exacerbation-free time of 6 weeks)Exclusion- Hearth decompensation- Acute illness- Acute respiratory insufficiency | 11 patients with COPD66.7 years64% maleFEV1 29.7% pred | HFNC (First)- 20L/min with O2- ≥5h/day | NIV (Second)with O2≥5h/day | 12 weeks (6 weeks each arm, no washout) | PrimaryPaCO2 | PaCO2mmHgHFNC 45.5 vs NIV 46.4, p>0.05Spontaneous breathing 53.7 vs HFNC 45.5, p<0.05Spontaneous breathing 53.7 vs NIV 46.4, p<0.05 |
| Nagata et al., 201831 | Japan | Randomized crossover trial | Inclusion- ≥20 years- Hypercapnia- GOLD stages 2–4- LTOT ≥16h/day for ≥1 monthExclusion- Exacerbation within 6 weeks of enrolment- Used nocturnal NIV within 6 weeks of enrolment- Active malignancy | Group A (HFNC/LTOT then LTOT)13 patients with COPD73.8 (6.9) years92.3% malesFEV1 29.44 (16.82)% predGroup B (LTOT then HFNC/LTOT)16 patients with COPD76.2 (9.3) years88% malesFEV1 29.43 (11.21)% pred | HFNC+LTOTFlow rate 30–40L/min with O2Flow to maintain SpO2>88%If discomfort, minimum flow of 20L/min≥4h/night | LTOTO2 0.25–4L/min | 12 weeks (6 weeks each arm, no washout) | PrimarySGRQ-CSecondaryPaCO2Nocturnal PtcCO2Exacerbations (worsening of baseline respiratory symptoms that required treatment with oral corticosteroids and/or antibiotics)PaO2SpO2EQ-5D-5LmMRCLung functionPhysical activity6MWTAdherenceAdverse events | SGRQ-CTotal −7.8 (−11.9, −3.7), p<0.01Symptom −10.8 (−15.3, −6.3), p<0.01Activity −4.7 (−8.7, −0.6), p=0.03Impact −8.7 (−15, −2.5), p=0.01PaCO2−4.1 (−6.5, −1.7) mmHg, p<0.01Nocturnal PtcCO2−5.1 (−8.4, −1.8) mmHg, p<0.01ExacerbationsHFCN+LTOT 0% vs LTOT 19%EQ-5D-5LScore 0.05 (−0.01, 0.11), p=0.08VAS 7.9 (2.9, 12.9), p=0.01Steps/day−233 (−483, 16), p=0.07Other outcomesNo sig. differences observed for mMRC, PaO2, SpO2, Lung function, 6MWTAdherence HFNC+LTOTGroup A 7.1 (1.5) h/day (flow 29.2 (1.9) L/min)Group B 8.6 (2.9) h/day (flow 30.3 (4.6) L/min)Adverse events HFCN+LTOTNight sweat (n=4)Nasal discharge (n=1)Insomnia (n=1)Skin rash (n=1) |
| Storgaard et al., 201823 | Denmark | RCT | Inclusion- COPD with chronic hypoxemic respiratory failure- ≥3 months LTOTExclusion- Malignant disease- Terminal nonmalignant disease- Unstable psychiatric disease- Home NIV | HFNC+LTOT100 patients with COPD with chronic hypoxemic respiratory failure71.0 (8.2) years44% malesFEV1 29.8 (12.6) % predLTOT100 patients with COPD with chronic hypoxemic respiratory failure70.4 (9.0) years37% malesFEV1 31.8 (12.9) % pred | HFNC+LTOTFlow rate 20L/minO2 to maintain SpO2>88%8h/day, during sleep | LTOT1–2L/min | 12 months | PrimaryExacerbations (worsening of dyspnea, cough and sputum production for >2 consecutive days leading to treatment with systemic glucocorticoids or antibiotics)SecondaryPaCO2PaO2SaO2Lung functionmMRCSGRQ6MWTBorg scaleHospitalizationMortalityAdherenceAdverse events | Exacerbations/patient/yearHFNC+LTOT 3.12 vs LTOT 4.95, p<0.001Hospitalizations/patient/yearHFNC+LTOT 1.08 vs LTOT 1.22, p=0.373mMRCHFNC+LTOT lower mean score (vs LTOT) at 3, 6, 9, 12 months (p<0.001).SGRQ totalHFNC+LTOT lower mean score (vs LTOT) at 6 (p=0.002) & 12 months (p=0.033)PaCO2HFNC+LTOT lower mean value (vs LTOT) at 12 months (p=0.005)6MWTHFNC+LTOT higher mean value at 12 months (p=0.005)Other outcomesNo differences in hospitalizations, mortality, Borg, lung function, PaO2, SaO2Adherence HFNC+LTOT86% used 286 days, mean 7h/dayAdverse eventsNone reported |
| Bräunlich et al., 201924 | Germany | Randomized crossover trial | Inclusion- COPD patients with chronic respiratory insufficiency- Stable daytime hypercapnia (PaCO2≥50mmHg)- ≥18yExclusion- Exacerbation in the 4 weeks prior- NIV in the 14 days prior- Body mass index>30kg/m2 | 94 patients with COPD65.3 (9.3) years39% malesFEV1 28.5 (10.2)% pred | HFNC+LTOTFlow 20L/minO2 not changed from baseline6h/day, during sleep | NIV+LTOT6h/day, during sleep | 6 weeks | PrimaryPaCO2SecondarypO2SaO2Respiratory rateLung function6MWTBorg scaleSRISGRQVAS state of healthAdherenceAdverse events | HFNC+LTOT vs NIV+LTOTPaCO2MD −1.4 (−3.1, 0.4), p=0.12Other outcomesNo significant differences.HFNC+LTOT (pre vs post)PaCO2MD −2.8 (95%CI −4.6, −1.1), p=0.002Respiratory rateMD −1.4 (95%CI −2.9, −0.0), p=0.046SRIMD 3.5 (95%CI 1.1, 5.8), p=0.004 (and in 3 sub-scales Respiratory Complaints, Physical Functioning, Attendant Symptoms and Sleep)SGRQ totalMD −6.2 (95%CI −8.9, −3.5), p<0.001 (and all sub-scales)VAS state of healthMD 1 (95%CI 0.2, 1.8), p=0.015Other outcomesNo significant differences.AdherenceHFNC+LTOT 5.2 (3.3)h/dayNIV+LTOT 3.9 (2.5)h/dayDifference 1.6h/day (95%CI 0.9, 2.4), p<0.001Adverse eventsDeath (n=2 HFNC+LTOT; n=2 NIV+LTOT)Number of severe adverse events (n=17 HFNC+LTOT, n=21 NIV+LTOT)Number of adverse events (n=33 HFNC+LTOT, n=55 NIV+LTOT) |
| Weinreich, 201932 | Denmark | Post hoc analysis of a RCT (Storgaard et al., 2018) | Inclusion- COPD with chronic hypoxemic respiratory failure- ≥3 months LTOTExclusion- Malignant disease- Terminal nonmalignant disease- Unstable psychiatric disease- Home NIV | HFNC+LTOT100 patients with COPD with chronic hypoxemic respiratory failure71.0 (8.2) years44% malesFEV1 29.8 (12.6) % predDivided in two groups:Group 0–1 exacerbation in 1year prior32 patients74 (9) years50% malesFEV1 31.1% predGroup ≥2 exacerbations in 1year prior68 patients70 (7.6) years41% malesFEV1 29.0 (12.2) % pred | HFNC+LTOTFlow rate 20L/minO2 to maintain SpO2>88%8h/day, during sleep | LTOT1–2L/min | 12 months | ExacerbationsHospitalizations | ExacerbationsGroup 0–1 –increase in number (p=0.01)Group ≥2 – reduction in number (p=0.03)Group differences (p=0.05)HospitalizationsGroup 0–1 –increase in number (p=0.01)Group ≥2 – reduction in number (p=0.002)Group differences pre (p=0.004) and during study (p<0.001)Hospitalization daysGroup 0–1 –increase in days (p=0.08)Group ≥2 – reduction in days (p=0.025)Group differences pre (p=0.003) and during study (p=0.01) |
| Storgaard et al., 202034 | Denmark | Post hoc analysis of a RCT (Storgaard et al., 2018) | Inclusion- COPD with chronic hypoxemic respiratory failure- ≥3 months LTOT- Persistent hypercapnic failure (PaCO2>6kPa)Exclusion- Malignant disease- Terminal nonmalignant disease- Unstable psychiatric disease- Home NIV | HFNC+LTOT31 patients with COPD67 years32% malesFEV1 24.5 (8.8) % predLTOT43 patients with COPD68 years30% malesFEV1 26.1 (6.2)% pred | HFNC+LTOTFlow rate 20L/minO2 to maintain SpO2>88%8h/day, during sleep | LTOT1–2L/min | 12 months | PaCO2PaO2ExacerbationsHospitalizations | PaCO2HFNC+LTOT 1.3% decrease (p=0.624)LTOT 7% increase (p=0.003)Exacerbations rate/yearHFNC+LTOT decreased 0.15 (p=0.661)LTOT increased 2.2 (p<0.001)Hospitalizations/yearHFNC+LTOT decreased 0.67 (p=0.013)LTOT increased +0.3 (p=0.180)Other outcomesNo sig. differences in PaO2 between groups |
| Good et al., 202133 | New Zealand | Post hoc analysis of a RCT (Rea et al., 2010) | Inclusion- Bronchiectasis confirmed by high-resolution computed tomography- ≥2 exacerbations in theprevious year- >5ml daily sputum- Stable for ≥4 weeksExclusion- bronchiectasis associated with cystic fibrosis or hypogammaglobulinaemia | HFNC26 patients with stable bronchiectasis63 (11.4) years42% maleFEV1 56.5 (20.2)% predControl19 patients with stable bronchiectasis65 (13.9) years37% maleFEV1 42.42 (15.2)% pred | HFNC- Flow 20–25L/min, 37°- O2 for patients on LTOT- ≥2h/day | Usual care | 12 months | PrimaryRate of exacerbations (worseningof ≥2 respiratory symptoms for ≥2 days that required antibiotics or oral prednisone)SecondaryTime to 1st exacerbationN of exacerbated daysSGRQLung function6MWT | Exacerbations/patient/yearHFNC 2.39 vs Control 3.48, p=0.034Annual exacerbation daysHFNC 10.3 vs Control 29.9, p=0.056Days to 1st exacerbationHFNC 84 vs Control 54, p=0.316Patients free of exacerbationsHFNC 20% vs Control 8.3%, p=0.043SGRQ changesTotal HFCN −12.3 vs Control −1.2, p=0.028Impact HFCN −14.7 vs Control −1.6, p=0.018Other outcomesNo differences between groups for FEV1, FVC, 6MWT, SGRQ symptoms/activity |
| Crimi et al., 202225 | Italy | Retrospective case-control study | Inclusion- A diagnosis of radiologically and clinically significant bronchiectasis- Chronic cough, sputum production most days of the week and/or frequent respiratory infections- ≥1 severe exacerbation 1year prior- Optimized treatmentExclusion- Cystic fibrosis- Traction bronchiectasis | HFNC20 patients with bronchiectasis70.7 (9.4) years30% malesFEV1 58.8 (18)% predControl20 patients with bronchiectasis68.6 (8.9) years30% malesFEV1 63.2 (17.7)% pred | HFNCFlow 25–40L/min34°C or 37°C (according to tolerance)O2 adjusted to maintain SpO2≥92%6h/day, during sleep | Usual care | 12 months | PrimaryRate of exacerbations (physician determining a change in treatment in the presence of deterioration in ≥3Symptoms for ≥48h)SecondaryHospitalizationsmMRCSputum colorDifficulty of mucus expectoration (VAS 1–10)SGRQLung functionSpO2AdherenceAdverse events | ExacerbationsMD (HFNC- Control) −1.9 (95%CI −2.8, −0.9), p=0.001HospitalizationsMD −0.7 (95%CI −1.1, −0.3), p=0.001Difficulty of mucus expectorationMD −2.2 (95%CI −3.9,−0.5), p=0.012SGRQ totalMD −10.4 (95%CI −20.2,−0.6), p=0.039FEV1, %predMD 6.1 (95%CI 1,11.3), p=0.022FVC, %predMD 4.6 (95%CI 0.8,8.3), p=0.019Other outcomesNo significant differences in mMRC and SpO2Adherence6.3 (1.8) h/dayAdverse eventsNo serious eventsPoor tolerance (n=1)Personal reasons (n=1) |
| Nagata et al., 202229 | Japan | RCT | Inclusion- ≥40 years- Daytime hypercapnia (PaCO2>45mmHg and pH>7.35)- GOLD stages 2–4- LTOT for ≥16h/day for ≥ 1 month- ≥1 moderate/severe exacerbation 1year prior- Stable and free from a exacerbation 4 weeks priorExclusion- NIV 4 weeks prior- HFNC 1 year prior- History/suspicion of obstructive sleep apnea | HFNC+LTOT49 patients with COPD72.9 (7.4) years90% malesFEV1 25.6 (8.4)% predLTOT50 patients with COPD75.2 (6.7) years88% malesFEV1 27.1 (8.9)% pred | HFNC+LTOTFlow 30–40L/minIf discomfort, minimum flow of 20L/min37°C (if discomfort 34°C or 31°C)O2 adjusted to maintain SpO2>88%>4h/day during sleep | LTOTLTOT alone as prescribed | 52 weeks | PrimaryRate of moderate/severe exacerbationsSecondaryTime to the 1st exacerbationDeathmMRCSGRQPittsburgh Sleep Quality Index6MWTLung functionSpO2Physiological parametersAdherenceAdverse events | Rate of moderate/severe exacerbationsLTOT (ref HFCN+LTOT) Adjusted mean count 2.85 (95%CI 1.48–5.47), p=0.002Time to 1st moderate/severe exacerbationLTOT vs HFCN+LTOT, p=0.032SpO2HFCN+LTOT 1.01 (0.33)% vs LTOT −0.20 (0.32)%, p=0.010Other outcomesNo significant differences.Adherence HFNC/LTOT7.3 (3)h/day (flow 28.5 (4.6) L/min)Adverse eventsInfections and infestations: HFNC/LTOT 26.5% vs LTOT 32%Respiratory, thoracic, and mediastinal disorders: HFNC/LTOT 38.8% vs LTOT 42% |
| Weinreich et al., 202227 | Denmark | Crossover study | Inclusion criteria- ILD diagnosis- prescribed ambulatory oxygen treatment or LTOT<12 months prior- Age >18 yearsExclusion criteria- Life expectancy<3 months- Pneumonia/exacerbation of ILD<3 months prior | 9 patients with ILD69 (5) years56% malesFEV1 69.9 (20)% pred | HFNCFlow≥30L/min37°CO2 as prescribed for LTOT/ambulatory oxygen treatment8h/day, preferably during sleep | No HFNC | 6 weeks | 6MWT (distance and time to SpO2 recovery)Lung functionPaO2PaCO2mMRCSGRQRichards-Campbell sleep questionnaireAdherenceAdverse events | 6MWT distance393 (42) m vs after HFNC 441 (22) m, p=0.049Time to SpO2recovery3.4 (3.0) min vs after HFNC 2.2 (1.1) min, p=0.001Other outcomesNo significant differences.Adjusting for hours of HFNC use, mMRC (p=0.04) and minimum oxygen saturation during 6MWT (p=0.01) improved.Adherence6.5 (1) h/dayAdverse eventsMild rhinorrhea (n=5)Bothered by the device heat (n=3)Exacerbation (=1) |
| Simioli et al., 202326 | Italy | Non-randomized crossover study | Inclusion criteria- ≥18 years- Radiological evidence of bronchiectasis on HRCT- ≥2 exacerbations/hospitalizations- Smoke cessation >3 months- Ability to use electronic devicesExclusion criteria- Exacerbation 7 days prior- pO2 <60mmHg- LTOT >2L/min- Chronic use of NIV(…) | 78 patients with bronchiectasisM 70 (IQR 60–76) years46.2% malesFEV1 2.39 (0.87)L | HFNC (Second)Flow 35–60L/min31–37°CFiO2 0.21O2 not added2 years | Usual care(First)12 months | 36 months | PrimaryExacerbations (warrant additional treatment (e.g., antibiotic, systemic corticosteroid, bronchodilators)SecondaryHospitalizationsLung functionmMRCAdherence (≥2h/day, ≥4 days/week)Adverse events | Exacerbations/year2.81 (2.15) vs 2 months 2.36 (0.69), p=0.982.81 (2.15) vs 2 years 0.45 (0.66), p<0.001Hospitalizations/year1.65 (2.10) vs 2 years 0.56 (0.98), p<0.001Lung functionFEV1 2.39 (0.87)L vs 2 years 2.55 (0.82)L, p=0.45FVC 2.73 (0.88)L vs 2 years 2.84 (0.90)L, p=0.66mMRC2.4 (0.81) vs 2 months 0.97 (0.97), p<0.0012.4 (0.81) vs 2 years 0.60 (0.78), p<0.001Adherence100%, 2 months 100%, 2 years 95%Adverse eventsMinor epistaxis (n=1)Interface discomfort (n=1),Dysphagia (n=2) |
| Weinreich et al., 202328 | Denmark | Retrospective study | Inclusion criteria- Patients with COPD- Treated with LTOT plus either long-term HFNC or NIV, in whom a 2nd add-on (NIV or HFNC) was initiated | HFNC+LTOT baseline17 patients with COPD66.8 (7.3) years35% malesFEV1 27.1 (8.2)% predNIV+LTOT baseline16 patients with COPD68.3 (7.5) years43.8% malesFEV1 30.5 (8.8)% pred | HFNC+LTOT baselinePrescribed according to the Danish Respiratory Society guidelines (or physician’ discretion before 2019)12 patient started also NIV (night) | NIV+LTOT baselinePrescribed according to the Danish Respiratory Society guidelines13 patients started also HFNC(day) | ∼29 months (908 (586) days) | Hospitalizations | HospitalizationsHFNC+LTOT 2.5 (0.4) vs 12 months 1.5 (0.4), p=0.022NIV+LTOT 2.9 (0.5) vs 12 months 1.6 (0.4), p=0.014 |
Abbreviations: 6MWT, 6min walking test; FEV1, forced expiratory volume in the first second; FiO2, fraction of inspired oxygen; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HFNC, high-flow nasal cannula; IQR, interquartile range; LTOT, long-term oxygen treatment; MD, mean difference; M, median; mMRC, modified Medical Research Council scale; NIV, non-invasive ventilation; PaCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; pred predicted; PtcCO2, transcutaneous carbon dioxide pressure; SGRQ, St George Respiratory Questionnaire; SRI, Severe Respiratory Insufficiency.
Most studies were conducted in patients with COPD (n=6), 2 studies in patients with bronchiectasis, one in both COPD and bronchiectasis, and one in patients with ILD. Inclusion criteria varied among studies, but prescription of long-term oxygen therapy (LTOT) (n=5),23,27–29,31 chronic respiratory failure/hypercapnia (n=4),22,24,29,31 history of exacerbations/hospitalizations (n=4),25,26,29,30 stable hypercapnia (n=422,24,29,31), sputum production (n=2)25,30 and body mass index<30kg/m2 (n=2)22,24 were some of the most commonly used. The studies included a total of 701 stable patients (sample sizes from 9 to 200). The average age of the patients was 65–76 years old. The average FEV1% predicted at baseline was 25–69%, with the lowest values found in studies with patients with COPD.
HFNC was mainly compared with standard care (n=3)25,27,30; LTOT (n=3),23,29,31 and VNI+LTOT (n=2) and NIV (n=3).22,24,28 HFNC was prescribed at flow rates between 20-60L/min, with or without O2 supplementation. Patients were recommended to use HFNC between 2 and 8hours/day, preferably during sleep. The effects of the intervention were evaluated both in the short-term (6 weeks, n=422,24,27,31) and in the long-term (123,25,28,30 and 2 years26).
Summary of findingsExacerbationsMost articles used exacerbations (n=9) as an outcome measure, with 6 showing the ability of HFNC to reduce these events when compared with usual care25,26,30,33 or LTOT.23,29 The other three articles (2 post hoc analyses of23) also showed significant improvements in exacerbations, particularly in hypercapnic patients and those with 2 or more exacerbations per year.31,32,34
HospitalizationsHFNC was also able to reduce the rate of hospitalizations in patients with COPD and bronchiectasis (n=723,25,26,28,30,32,34), although not superior to LTOT,23 or even usual care.30 Based on non-randomized studies and considering only patients with bronchiectasis, HFNC seemed to be more beneficial than usual care.25,26
PaCO2PaCO2 was a selected outcome in 6 articles. HFNC improved PaCO2 in patients with COPD and was shown to be superior to LTOT23,31,34 or non-invasive ventilation (NIV).24 This was not observed in patients with ILD.27
Health-related quality of lifeQuality of life was used as an outcome measure in 8 articles, 6 of which showed results in favor of HFNC in patients with COPD,23,24,31 bronchiectasis,25,33 or both.30 Different patient-reported outcome measures were used to assess this health domain: SGRQ general or the COPD specific version,23–25,30,31,33 EQ-5D-5L,31 health state visual analog scale,24 Severe Respiratory Insufficiency Questionnaire.2
Lung functionLung function, specifically FEV1 and FVC, were one of the most common used outcome measures (n=9 articles). However, only two studies with long-term follow up of patients with COPD and bronchiectasis were able to demonstrate the superiority of HFNC over usual care for lung function.25,30
Other outcomesThe 6MWT (n=7) and mMRC (n=6) were other commonly used outcomes, with the 6MWT demonstrating the superior effect of HFNC compared to LTOT and usual care in patients with COPD and ILD23,27; and the mMRC compared to usual care patients with bronchiectasis.26
AdherenceAdherence to HFNC has been reported in 8 studies, with most reporting adherences between 5.2 and 8.6h/day,24,25,27,29,31 or a percentage of users above a certain threshold (32%30 and 100%26 ≥2h, 86% 7h/day23). In four studies HFNC was compared to NIV/LTOT, but only Bräunlich et al. showed that adherence of HFNC was superior to NIV.24
SafetyWith regard to safety, 8 articles presented data on adverse events associated with HFNC, of which 3 reported no events,23,25,30 and the remaining presented common non-serious events26,27,31 or showed no differences between events observed under LTOT/NIV.24,29
DiscussionIn this systematic review, we comprehensively evaluated the effectiveness, adherence and safety of HFNC therapy in stable patients with COPD, bronchiectasis and ILD with chronic respiratory failure. We found that HFNC seems more effective as a long-term strategy for reducing exacerbations and improving quality of life than usual care or other home respiratory therapies, although more robust evidence is still needed. HFNC appears to have also beneficial effects on hospitalizations, paCO2, and lung function, while being safe and having good adherence.
This review shows that HFNC is associated with a reduction in exacerbations in patients with COPD and bronchiectasis that is not inferior to NIV and greater than LTOT or usual care.23,25,26,29,30,33 This is an important benefit, demonstrating that HFNC contributes to the key long-term goal of reducing the frequency and severity of exacerbations in patients with chronic respiratory diseases. In addition, the reduction appears to be more significant in both hypoxic and hypercapnic patients and in those with 2 or more exacerbations in the last year.31,32,34 HFNC can therefore be considered as an alternative to consider in a selective group: patients with COPD and frequent acute exacerbations. As exacerbations are a major determinant of health status, this effect is probably related to the observed improvement in quality of life. It is noteworthy that most of the studies showing effects on quality of life used the SGRQ and exceeded its minimal clinical important difference (MICD) of 4 units. The improvement in quality of life highlighted in this narrative synthesis is in line with meta-analytic findings from previous systematic reviews on the effects of HFNC in COPD.12,13 The effectiveness of HFNC in these two health domains has also been assessed using cost-effectiveness analyses, which have shown that HFNC has the potential to provide substantial cost savings.35,36
HFNC improved paCO2 in patients with COPD and has shown to be superior to LTOT or NIV.23,24,31,34 This finding is consistent with a previous review in patients with COPD.14 Nevertheless, this improvement in paCO2 needs to be considered with caution as it may be a result of the selection process and not translate the improvement expected in real-world patients with COPD. Indeed, in 4 of the 6 studies recruiting solely patients with COPD, stable hypercapnia (defined as paCO2>45mmHg or >50mmHg) was one of inclusion criteria. Benefits of HFNC on hospitalizations and lung function were also found,25,26,30 but this evidence comes mainly from non-randomized studies. The potential of HFNC in comparison with usual care or other home respiratory therapies in changing these outcomes needs to be further explored in future studies with larger samples and long-term follow-up. Two trials are underway that will shed light on the effect of HFNC on these outcomes.37,38
The effects of HFNC on exercise tolerance and dyspnea are fragile,23,26,27 which may be related to the short follow-up of most studies, but also to the responsiveness of the selected outcome measures to HFNC. The MICD of the 6MWT has been estimated to be 30m in chronic respiratory diseases,39 and unless HFNC is combined with specific interventions to improve exercise tolerance, it is unlike that its benefit will be demonstrated with such a specific measure of fitness. It has already been shown that the mMRC scale is a good tool to discriminate patients in terms of their dyspnea, but is not sensitive enough to change to be useful as an outcome in clinical trials.40 Future studies should therefore consider including other measures that replicate activities of daily living and associated dyspnea, such as 1-min sit to stand41 and London Chest Activity of Daily Living scale.42
The effectiveness of HFNC will be more clearly demonstrated if the patients’ perspective is considered in the design and evaluation of interventions. Unfortunately, none of the studies in this review mentioned that the design of the interventions included input from patients or carers, or assessed patient comfort or experience. Twelve participants in one of the trials included in this review and 8 relatives participated in a qualitative study addressing the experience with HFNC.23 Patients reported improved sleep and more energy for daily activities and found the ease of use of the device to be a strong motivator for adherence.43 Future trials evaluating the effectiveness of home-based care should consider including patient-reported outcome and experience measures.44 A combination of both is essential to fully understand the performance of home respiratory therapies and to allow patient-centered comparisons. Currently, there is no specific patient-reported experience measure for this health context, and this should be a research priority.45 In the meantime, a COPD-specific46 and other generic47,48 measures can be used. This, together with the design of pragmatic trials that take into account patient preference and experience, will provide robust real-world evidence on the role of HFNC.
Patients adhered well to HFNC, with most studies showing adherence between 5 and 8h/day.24,25,27,29,31 Unfortunately at this stage we cannot know if adherence to HFNC is better than other home respiratory therapies (LTOT/NIV) as only one study made this comparison and showed results favoring HFNC.24 Nevertheless the adherence reported for HFNC seems in line with the real-world adherence to NIV49 and the common cut-offs of 4–5h/day to define good adherence.50,51 The study with lower adherence (mean 1.6h/day) was also the one in which patients were advised to use the therapy for a shorter period (2h), which is understandable as it was one of the pioneers in testing the feasibility and safety of implementing long-term HFNC.30 In addition, HFNC has been shown to be an overall safe therapy that can be deliverable at home, with adverse events similar to those known for LTOT or NIV.52 The concern in reporting adverse events is a stronger point of the included studies (8 out of 10 original studies). However, the method of collecting adverse events was poorly reported, with some studies appearing to use standard collection methods, while others may have relied on spontaneous patient reporting. Future trials should improve the consistency of reporting important adverse events.53
Different HFNC protocols were used, differing mainly in the flow provided, the use of O2 and the prescribed hours per day. Most studies used flows of 20–40L/min and recommended sessions of 6–8h per day, preferably at night. In patients on LTOT, the supplemental oxygen flow was maintained unchanged during HFNC unless a SpO2<88% was detected. Differences may be related to the characteristics of the devices, but mainly to the lack of specific guidelines at the time the studies were conducted. The Danish guidelines published this year are pioneering,5 although based mostly on narrative review of findings and expert opinion. Other clinical practice guidelines are likely to follow, ideally using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach.54 This is crucial because HFNC is already being used in routine practice, as evidenced by the two retrospective studies included in this review,25,28 the number of editorials published,11,55–58 and the European Respiratory Society survey on HFNC practice (although with results not yet available). Recently, three strategies or ventilatory modalities have been described related to HFNC settings.55 Future studies are necessary to enhance our understanding of this technique and the impact of different HFNC settings on clinical outcomes.
This systematic review has some limitations that need to be considered. Our search strategy did not include the effectiveness of HFNC in stable patients during exercise or pulmonary rehabilitation programs, for which there is also a growing body of evidence.59–61 This can be considered as a limitation of our work and should be addressed in future reviews. This review is the first attempt to gather evidence on the long-term use of HFNC in patients with different chronic respiratory diseases. As it was expected, the number of studies is still limited and most of the evidence comes from patients with COPD and bronchiectasis, with only one study including patients with ILD. This limits the ability to generalize the results to patients with chronic respiratory diseases. Different study designs were included that used different outcome measures. This prevented us from doing a meta-analysis. In addition, the included reports were generally of moderate to low quality. Future studies can use these previous works to better select the most responsive outcome measures, to substantiate their sample size estimates and to design feasible HFNC protocols. This will improve the overall quality of the evidence being produced, which will allow stronger research synthesis of the evidence with the addition of meta-analysis.
ConclusionsHFNC seems more effective than usual care or other home respiratory therapies as a long-term strategy for reducing exacerbations and improving quality of life in patients with COPD and bronchiectasis. This review also showed that HFNC has good adherence levels and is safe in the home setting. Real-world pragmatic trials are nevertheless needed to better clarify the effectiveness of HFNC in patients with stable chronic respiratory diseases with chronic respiratory failure.
FundingNo funding was received for this work.
Conflict of interestsCristina Jácome, Marta Jácome, Mónica Duarte, João Carlos Winck, Savador Díaz Lobato, Manel Luján and Cátia Caneiras have no competing interests to declare. Sara Correia, Inês Flores and Patrícia Farinha are employees of ResMed. Javier Sayas Catalan received lecture honoraria from ResMed and Philips.