Pleuroparenchymal fibroelastosis (PPFE) is a rare interstitial lung disease (ILD) characterized by the presence of intra-alveolar fibrosis, defective expansion of elastic fibers in the interstitial space and dense fibrous thickening of the pleura occurring in the upper pulmonary lobes.1 In this case, we will analyze respiratory mechanics of a critically ill patient with PPFE and discuss the role of pulmonary stress in the pathophysiology of lung injury.
A 59-year-old female was being studied for lung transplantation eligibility due to advanced PPFE. The diagnostic computed tomography (CT) scan of the thorax performed two years before referral to a transplant center is shown in Fig. 1A. While being evaluated for inclusion in the transplant waitlist, she was admitted in the pulmonology ward due to acute respiratory failure. A thoracic CT scan revealed a pattern of ground glass opacities on top of the known PPFE image findings. Antimicrobials and high dose methylprednisolone pulses were started without improvement. Patient presented rapid clinical deterioration with increased work of breathing and life-threatening respiratory failure. Invasive mechanical ventilation followed by veno-venous extracorporeal membrane oxygenation (VV-ECMO) was initiated as a bridge to lung transplantation. During invasive mechanical ventilation and prior to ECMO start, blood gas analyses revealed that, while oxygenation impairment was mild (PaO2/FiO2: 231), patient developed extreme hypercarbia (PaCO2: 83mmHg) and respiratory acidosis (pH: 7.09) due to high dead space ventilation (ventilatory ratio: 3.82).2 Respiratory microbiological analyses, including a bronchoalveolar lavage with conventional bacterial and fungal cultures as well as viral and fungal PCRs, did not yield any infectious etiology.
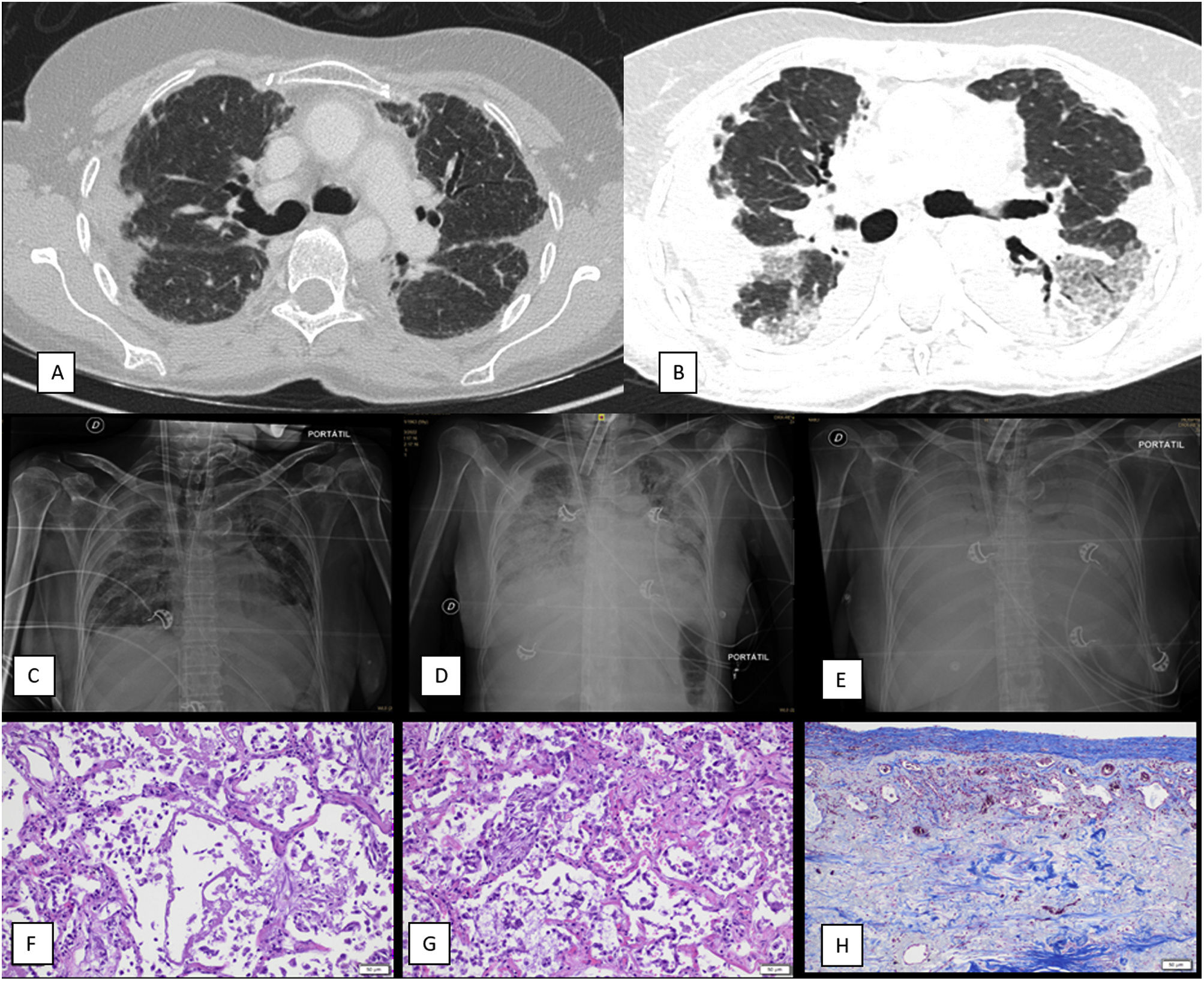
(A) Non-contrast thoracic CT scan that shows pleural thickening and irregular apical opacities as well as traction bronchiectasis suggestive of pleuroparenchymal fibroelastosis (diagnostic CT, two years before referral to a transplant center). (B) Contrast-enhanced thoracic CT scan that reveals progression of the images consistent with pleuroparenchymal fibroelastosis in the upper lobes as well as ground glass opacities and consolidation on the lower lobes, suggestive of diffuse alveolar damage (CT performed during acute exacerbation). (C) Chest X-ray, antero-posterior (AP) projection. First day of ECMO weaning attempt. Patient had been weaned from mechanical ventilation several weeks before. X-ray reveals mild bilateral pulmonary opacities and consolidation of the upper pulmonary lobes consistent with PPFE. Femoral and jugular ECMO cannulas and tracheostomy tube are also identified. (D) Chest X-ray, antero-posterior (AP) projection. Third day of ECMO weaning attempt. Patient had been weaned from mechanical ventilation several weeks before. Thoracic X-ray shows an increase in pulmonary opacities with consolidation in the lower pulmonary lobes and the PPFE pattern. (E) Fifth day after ECMO weaning attempt (weaning attempts ceased on day three). Thoracic X-ray shows a diffuse pattern of severe bilateral consolidation (white lung pattern). (F) Autopsy sample of lung (H/E staining, 20× magnification) reveals presence of hyaline membranes and neutrophilic inflammation in the alveoli with disrupted epithelium, compatible with diffuse alveolar damage in exudative (acute) stage. (G) Autopsy sample of lung (H/E staining, 20× magnification) shows thickening of the alveolar septa, in addition to proliferation of type II pneumocytes and myofibroblasts, compatible with diffuse alveolar damage in proliferative (subacute) stage. (H) Autopsy sample of lung (trichrome staining, 20× magnification) shows marked pleural thickening with fibrosis with abundant collagen deposition.
Pulmonary and chest wall mechanics were monitored with an esophageal catheter and a dedicated software (Fluxmed®, Argentina). At the time of this analysis, the patient was under neuromuscular blockade and ECMO. Initially, tidal volume and positive end-expiratory pressure were set at 250mL (5mL/kg predicted body weight) and 5cmH2O. Plateau pressure was 21cmH2O and end-inspiratory and end-expiratory esophageal pressures were 6 and 3cmH2O, respectively. Transpulmonary expiratory pressure was 2cmH2O and total transpulmonary pressure (elastance-derived method)3 was 17cmH2O.
We performed a tracheostomy early after the start of ECMO to ease sedation withdrawal and to start physical rehabilitation to ameliorate lung transplantation suitability. Importantly, this strategy included maintaining a high CO2 clearance by ECMO aiming to diminish inspiratory drive and reduce the harm of negative intrathoracic pressures and subsequent pulmonary stress during rehabilitation.
After several weeks of rehabilitation, the patient was weaned from mechanical ventilation, tracheostomy was closed, and oral nutrition was started. Unfortunately, the lung transplant committee considered the patient not suitable for lung transplant.
We therefore attempted to switch off the ECMO gas (ECMO weaning), but this was poorly tolerated. Hypercarbia and vigorous work of breathing was identified when ECMO sweep flow was reduced. As esophageal catheter had been removed due to epistaxis, we could not evaluate pleural and transpulmonary pressures during the ECMO weaning attempts. However, transpulmonary pressures (airway pressure minus pleural pressure) in patients with an acute exacerbation of ILD breathing spontaneously are even higher than those from patients with ARDS due to high inspiratory efforts.4 After three days of such attempts, we identified prominent new-onset bilateral pulmonary opacities (Fig. 1C–E) and patient died shortly.
A necropsy was performed and revealed different stages of diffuse alveolar damage and histological features consistent with PPFE in the upper lobes (Fig. 1F–H). Of note, a pattern suggestive of infectious pneumonia was not observed and microbiology of the pulmonary tissue was negative.
In this case we could examine pathophysiological characteristics of PPFE and elaborate hypothesis for the onset of the exacerbation and the clinical evolution during the weaning attempts. Respiratory mechanics analyses revealed that low respiratory system compliance (15.6mL/cmH2O, normal ∼64mL/cmH2O)3 was caused by both an extremely diminished lung compliance (19.2mL/cmH2O, normal 90–140mL/cmH2O)3 and a decreased chest wall compliance (83.3mL/cmH2O, normal 100–200mL/cmH2O).3 However, as revealed by the lung elastance to respiratory system elastance ratio (0.81, normal 0.5–0.7),3 less than 20% of the applied pressure was transmitted to the chest wall during positive pressure ventilation. Overall, these findings reveal that such lungs were at extreme risk of injury when ventilation was not fully controlled as the parenchymal fibrosis and elastosis were the driving mechanisms of altered respiratory mechanics.
We believe that the progression of lung injury during the ECMO weaning trials (Fig. 1C, D), the findings of DAD in different stages (Fig. 1F–H), and the absence of other causes for DAD such as pneumonia, suggest that high transpulmonary pressures might have contributed to DAD development in this patient, especially when breathing spontaneously.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.