The coronavirus disease-19 (COVID-19) pandemic continues to determine a significant impact on the health system globally, not only related to the acute phase, but also to the long-term manifestations. The persistence of post-acute symptoms is commonly identified and can be defined as post-COVID-19 syndrome if it lasts more than 12 weeks.1 Respiratory symptoms are one of the most common post-COVID-19 manifestations that need to be assessed during follow-up, mainly in patients who had pulmonary involvement in the acute phase of the disease.2
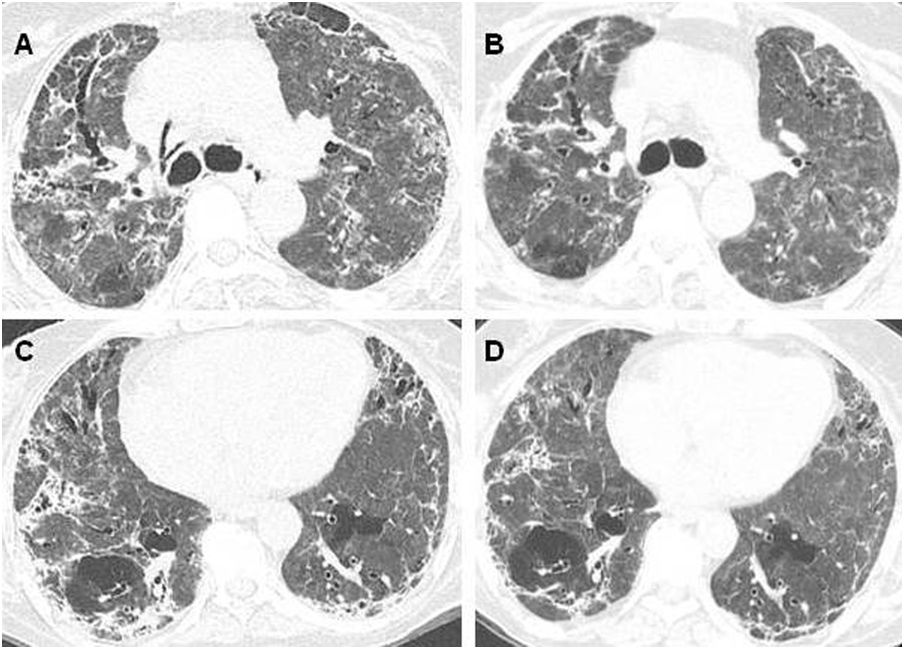
The presence of pulmonary fibrosis secondary to COVID-19 is a new entity, whose exact definition, prevalence, pathophysiology and treatment are not fully understood. A systematic review demonstrated that pulmonary fibrosis after COVID-19 was identified in 7.0% of patients in five included studies.3 The concept of when persistent post-COVID-19 pulmonary fibrosis is defined remains poorly understood once patients may present progressive improvement in the follow-up. Several case reports and series that describe pulmonary fibrosis after COVID-19 and its potential treatment have been published. The resolution of long-term lung lesions may occur more than six months after the acute phase, and seems to be related to the predominant pattern of pulmonary abnormalities, such as ground-glass opacities and consolidations, which may improve over time (Fig. 1).4–7 Additionally, further studies are warranted to determine the role of antifibrotic drugs in preventing or attenuating changes consistent with post-COVID-19 pulmonary fibrosis, and in which scenarios they will be recommended.
Patient with COVID-19, who was admitted to the ICU and needed mechanical ventilation. (A and C) Chest CT four months after the onset of symptoms demonstrates diffuse ground-glass and reticular opacities, consolidations, and bilateral peripheral bronchiectasis and bronchiolectasis. (B and D) At 7-month follow-up, chest CT shows partial improvement of the lesions.
The acute phase of COVID-19 is crucial for the development and long-term persistence of pulmonary lesions after hospital discharge. The most important risk factors are mainly related to the patient and the severity of the infection. Elderly, obese, smokers and diabetics, and patients that required mechanical ventilation and had severe acute respiratory distress syndrome, with high levels of C-reactive protein, D-dimer and interleukin-6 seem to have a greater risk of long-term pulmonary involvement.7–9 Additionally, the hyperstimulation of the immune system associated with systemic inflammation secondary to COVID-19 can trigger autoimmune responses, with production of cytokines and autoantibodies, which may contribute to the development and progression of pulmonary parenchymal lesions.7,8 Genetic predisposition, such as the identification of shortening of leucocyte telomeres, is also speculated as a potential risk factor for the occurrence of definitive pulmonary fibrosis after COVID-19.10,11 Patients with chronic interstitial lung diseases (ILDs) are at increased risk of progression of lung parenchymal lesions after the acute phase of COVID-19.9
There are scarce studies that evaluated patients beyond one year from the acute phase of COVID-19. Although most patients improve over time, a systematic review and meta-analysis of studies within one year following hospitalization demonstrated the presence of opacities suggestive of lung fibrotic lesions in almost one third of the exams (0.29; 95% CI 0.22–0.37).12 Techniques that employ automatic quantification of tomographic abnormalities can be used to assess the progression of the extent of pulmonary impairment over time in COVID-19. A Brazilian study found pulmonary abnormalities in 80% of patients evaluated 90 days after hospital discharge, with ground-glass opacities and parenchymal bands as the predominant lesions. There was a good correlation between the analysis made by an experienced radiologist with artificial intelligence techniques in the quantification of the pulmonary involvement.6
Autopsies and explanted lungs from patients with COVID-19 demonstrated the presence of diffuse alveolar damage in different stages, micro-thrombosis, organizing pneumonia, and pulmonary fibrosis.7 Few studies described the histopathological findings in patients with COVID-19 after a long follow-up period. A series of patients that underwent transbronchial biopsy 4–15 months after the acute phase demonstrated mostly the presence of bronchiolocentric interstitial pneumonia, with architectural distortion and extracellular matrix deposition.13 Another study showed three different histopathological clusters in 10 patients who underwent transbronchial lung cryobiopsy on average 3.5 months after recovery from COVID-19. The clusters included a chronic fibrosing pattern, with usual interstitial pneumonia and smoking-related fibrosis, an acute/subacute injury, with organizing pneumonia, nonspecific interstitial pneumonia and diffuse alveolar damage, and a pattern with vascular modifications.14 Further studies with a greater number of patients are necessary to determine the most common histopathological patterns in post-COVID-19 pulmonary fibrosis.
Acute exacerbation of post-COVID fibrosis may occur and can be idiopathic or associated with reinfection, air travel, other viral and bacterial infections, and environmental exposures.15 Additionally, viral infections, including COVID-19, may determine progression and acute exacerbation in patients with baseline interstitial lung abnormalities and ILDs, increasing the risk of hospitalization and death.8,10,15
Despite significant advances in the management of the acute phase of COVID-19, there are still several uncertainties about the treatment of post-COVID-19 pulmonary fibrosis that need to be clarified. The use of antifibrotics nintedanib and pirfenidone is established in fibrotic and progressive ILDs, such as idiopathic pulmonary fibrosis.16 However, the role of antifibrotic drugs in post-COVID-19 fibrosis is unclear and may be considered in rare scenarios, such as those with the progressive phenotype over time, although this needs to be confirmed in randomized controlled trials.5,7,9 Although corticosteroids may be used in the acute phase of COVID-19, their role in preventing or attenuating chronic ILD is controversial, but may be considered in those with areas suggestive of organizing pneumonia on CT scan.7 Lung transplantation may be an alternative for patients with irreversible disease undergoing long periods of extracorporeal membrane oxygenation, although the main indications and contraindications remain under discussion.
Therefore, most patients with chronic ILD secondary to COVID-19 present clinical, functional and tomographic improvement over time, especially those with a mild impairment. There are still several uncertainties regarding post-COVID-19 pulmonary fibrosis that need to be better understood in the near future. First, the definition, prevalence and impact on healthy systems in the long-term of this entity are still unclear. Moreover, further studies are warranted to determine the subgroup of patients that will benefit from the use of antifibrotic drugs. The COVID-19 pandemic was responsible for several hospitalizations and deaths around the world, the number of survivors is even greater and the prevalence of patients with sequelae of the disease is still increasing. It is essential to follow these patients regularly with clinical, functional and tomographic evaluation, to organize and support the health services, preferably with a multidisciplinary approach, in order to clarify all uncertainties regarding post-COVID-19 pulmonary fibrosis.