Evaluation of biologic therapy response is vital to monitor its effectiveness. Authors have proposed various response criteria including good responder, super-responder, non-responder, and clinical remission.
ObjectivesTo ascertain the prevalence of response and clinical remission after long-term treatment (>6 months) of anti-IgE and anti-IL-5/IL-5Rα biologics, compare these results with existing criteria, and identify predictors for non-responders and clinical remission.
MethodsA multicenter, real-life study involving severe asthma patients in Spain. Various outcomes were assessed to gauge response and clinical remission against established criteria.
ResultsThe study included 429 patients, 209 (48.7%) omalizumab, 112 (26.1%) mepolizumab, 19 (4.4%) reslizumab and 89 (20.7%) benralizumab, with a mean treatment duration of 55.3±38.8 months. In the final year of treatment, 218 (50.8%) were super-responders, 173 (40.3%) responders, 38 (8.9%) non-responders, and clinical remission in 116 (27%), without differences among biologics. The short-term non-responders (<6 months) were 25/545 (4.6%). Substantial variations in response and clinical remission were observed when applying different published criteria. Predictors of non-response included higher BMI (OR:1.14; 95% CI:1.06–1.23; p<0.001), admissions at ICU (2.69; 1.30–5.56; p=0.01), high count of SAE (1.21; 1.03–1.42; p=0.02) before biologic treatment. High FEV1% (0.96; 0.95–0.98; p<0.001), a high ACT score (0.93; 0.88–0.99; p=0.01) before biologic treatment or NSAID-ERD (0.52; 0.29–0.91; p=0.02) showed strong associations with achieving clinical remission.
ConclusionA substantial proportion of severe asthma patients treated long-term with omalizumab or anti-IL5/IL-5Rα achieved a good response. Differences in response criteria highlight the need for harmonization in defining response and clinical remission in biologic therapy to enable meaningful cross-study comparisons.
Assessing biologic therapy response is crucial for ongoing evaluation due to the potential influence of various factors on suboptimal response.1 These factors encompass patient-related elements such as medication adherence, inhalation technique, and comorbidity control2–4 along with potential differences in targeted mechanisms.5
To gauge biological treatment effectiveness,4 parameters like respiratory symptoms, lung function, asthma control, exacerbations, and systemic corticosteroids (SCS) usage and dosage are generally considered.
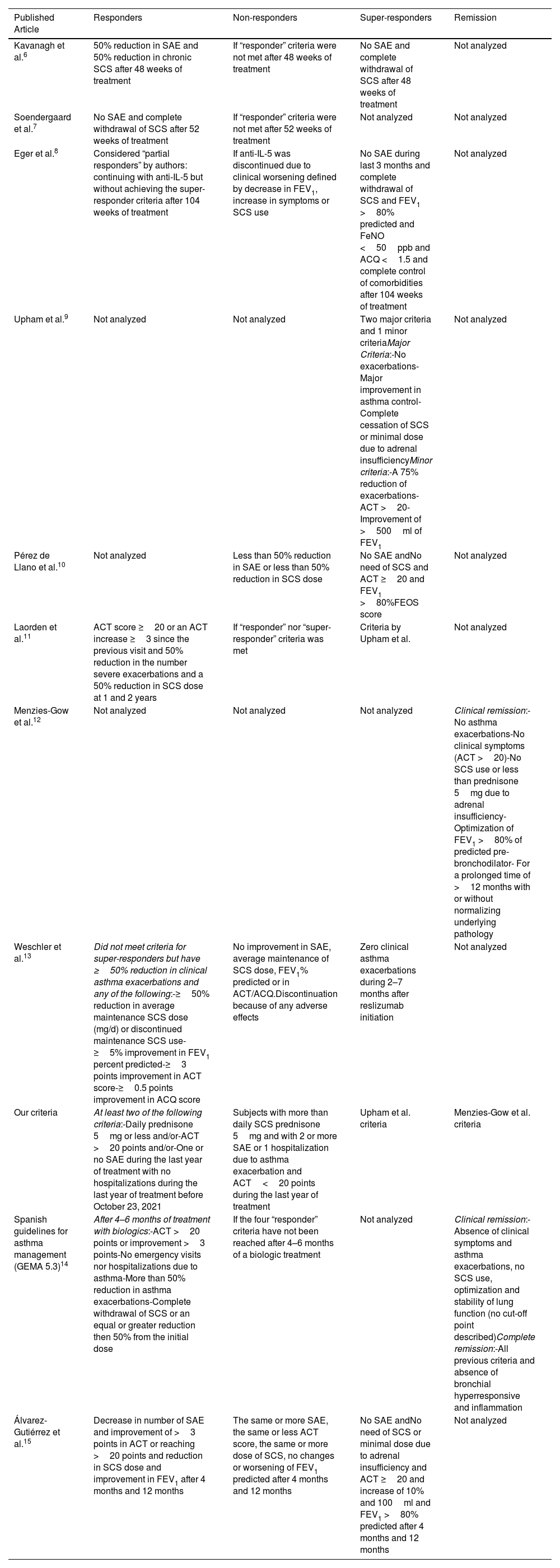
Recent publications have introduced distinct criteria for categorizing biologic-treated patients as “good responder”, “super-responder”, or “non-responder” (Table 1). For instance, Pepper et al. define “responders” as individuals achieving good asthma control (asthma control test, ACT>20 or asthma control questionnaire, ACQ<1.5) and/or <1 severe asthma exacerbation (SAE) and no hospitalization during a year of treatment and/or the tapering of SCS without losing asthma control and/or forced exhaled volume in first second (FEV1) greater or equal to 80% without bronchodilators.1 A “non-responder” is a subject that, under the biological treatment, has started SCS, has >1 SAE or hospitalization due to asthma. The “super-responders” meet even stricter criteria. Recently, a Delphi consensus was reached for a definition of super-responders9 based on major “super-responders” criteria including no exacerbations, improvement in asthma control and cessation of maintenance SCS; the definition requires improvement in two major criteria and one minor criterion. Kavanagh et al.6 proposed a different classification labeling subjects as super-responders, responders, and non-responders based on SAE reduction and SCS usage after 48 weeks of treatment with biologics. A similar classification was used in a Danish cohort6 followed for 52 weeks with anti-IL-5 treatment. Other authors tend to be stricter in the definition of “super-responders”, as Eger et al.8 who besides previous goals add FEV1 >80% and FeNO <50ppb with good comorbidity control with anti-IL-5 treatment for 104 weeks. In the Netherlands, a very different classification was used for a cohort of 215 subjects receiving reslizumab for 30 weeks.13
Summary of Criteria Published for Definition of Response in Severe Eosinophilic Asthma Treated With Biologics.
| Published Article | Responders | Non-responders | Super-responders | Remission |
|---|---|---|---|---|
| Kavanagh et al.6 | 50% reduction in SAE and 50% reduction in chronic SCS after 48 weeks of treatment | If “responder” criteria were not met after 48 weeks of treatment | No SAE and complete withdrawal of SCS after 48 weeks of treatment | Not analyzed |
| Soendergaard et al.7 | No SAE and complete withdrawal of SCS after 52 weeks of treatment | If “responder” criteria were not met after 52 weeks of treatment | Not analyzed | Not analyzed |
| Eger et al.8 | Considered “partial responders” by authors: continuing with anti-IL-5 but without achieving the super-responder criteria after 104 weeks of treatment | If anti-IL-5 was discontinued due to clinical worsening defined by decrease in FEV1, increase in symptoms or SCS use | No SAE during last 3 months and complete withdrawal of SCS and FEV1 >80% predicted and FeNO <50ppb and ACQ <1.5 and complete control of comorbidities after 104 weeks of treatment | Not analyzed |
| Upham et al.9 | Not analyzed | Not analyzed | Two major criteria and 1 minor criteriaMajor Criteria:-No exacerbations-Major improvement in asthma control-Complete cessation of SCS or minimal dose due to adrenal insufficiencyMinor criteria:-A 75% reduction of exacerbations-ACT >20-Improvement of >500ml of FEV1 | Not analyzed |
| Pérez de Llano et al.10 | Not analyzed | Less than 50% reduction in SAE or less than 50% reduction in SCS dose | No SAE andNo need of SCS and ACT ≥20 and FEV1 >80%FEOS score | Not analyzed |
| Laorden et al.11 | ACT score ≥20 or an ACT increase ≥3 since the previous visit and 50% reduction in the number severe exacerbations and a 50% reduction in SCS dose at 1 and 2 years | If “responder” nor “super-responder” criteria was met | Criteria by Upham et al. | Not analyzed |
| Menzies-Gow et al.12 | Not analyzed | Not analyzed | Not analyzed | Clinical remission:-No asthma exacerbations-No clinical symptoms (ACT >20)-No SCS use or less than prednisone 5mg due to adrenal insufficiency-Optimization of FEV1 >80% of predicted pre-bronchodilator- For a prolonged time of >12 months with or without normalizing underlying pathology |
| Weschler et al.13 | Did not meet criteria for super-responders but have ≥50% reduction in clinical asthma exacerbations and any of the following:-≥50% reduction in average maintenance SCS dose (mg/d) or discontinued maintenance SCS use-≥5% improvement in FEV1 percent predicted-≥3 points improvement in ACT score-≥0.5 points improvement in ACQ score | No improvement in SAE, average maintenance of SCS dose, FEV1% predicted or in ACT/ACQ.Discontinuation because of any adverse effects | Zero clinical asthma exacerbations during 2–7 months after reslizumab initiation | Not analyzed |
| Our criteria | At least two of the following criteria:-Daily prednisone 5mg or less and/or-ACT >20 points and/or-One or no SAE during the last year of treatment with no hospitalizations during the last year of treatment before October 23, 2021 | Subjects with more than daily SCS prednisone 5mg and with 2 or more SAE or 1 hospitalization due to asthma exacerbation and ACT<20 points during the last year of treatment | Upham et al. criteria | Menzies-Gow et al. criteria |
| Spanish guidelines for asthma management (GEMA 5.3)14 | After 4–6 months of treatment with biologics:-ACT >20 points or improvement >3 points-No emergency visits nor hospitalizations due to asthma-More than 50% reduction in asthma exacerbations-Complete withdrawal of SCS or an equal or greater reduction then 50% from the initial dose | If the four “responder” criteria have not been reached after 4–6 months of a biologic treatment | Not analyzed | Clinical remission:-Absence of clinical symptoms and asthma exacerbations, no SCS use, optimization and stability of lung function (no cut-off point described)Complete remission:-All previous criteria and absence of bronchial hyperresponsive and inflammation |
| Álvarez-Gutiérrez et al.15 | Decrease in number of SAE and improvement of >3 points in ACT or reaching >20 points and reduction in SCS dose and improvement in FEV1 after 4 months and 12 months | The same or more SAE, the same or less ACT score, the same or more dose of SCS, no changes or worsening of FEV1 predicted after 4 months and 12 months | No SAE andNo need of SCS or minimal dose due to adrenal insufficiency and ACT ≥20 and increase of 10% and 100ml and FEV1 >80% predicted after 4 months and 12 months | Not analyzed |
ACQ: asthma control questionnaire; ACT: asthma control test; FeNO: forced exhaled fraction of nitric oxide; FEV1: forced exhaled volume in first second; SCS: systemic corticosteroid; SAE: severe asthma exacerbation.
Pérez de Llano et al.10 developed the (FEOS) score, emphasizing SAE, SCS dose, symptoms, and FEV1. This weighted system quantifies response quality with higher scores indicating better response (ranges from 0=worsening to 100=best possible response). A score >75 in the FEOS system, suggests a super-responder.11
In asthma, remission has been explored as spontaneous occurrence reached in the population with asthma without treatment.16 The concept of achieving asthma remission through treatment has been less investigated.17 Menzies-Gow et al. proposed criteria for clinical remission, including symptoms absence, lung function stabilization, of clinical symptoms and asthma exacerbations, stabilization of lung function, no SCS usage, and agreement between patient and physician on disease remission, all must be maintained through the last >12 months (with or without normalizing underlying pathology) or complete remission (current negative bronchial hyperresponsiveness test).12 Recently, in the German severe asthma registry Milger et al. demonstrated that clinical remission has more possibility of being achieved at 12 months with biologic treatment (37.6% of 210 subjects) against no biologic treatment (17.2% of 233 subjects).18 The results with long-term treatment (>12 months) are scarce and especially in large cohorts.
Our study aims to determine the prevalence of response (responders, super-responders, and non-responders) and clinical remission in a large real-world cohort of patients with the long-term treatment (>6 months) of anti-IgE and anti-IL-5/IL-5Rα biologics. We also seek to compare various response criteria and identify predictors for non-responders and clinical remission to biologics.
MethodsStudy DesignA multicenter, retrospective, observational, real-life study was performed across (Allergology or Pneumology) nine hospitals within the Spanish Network of Asthma (A Coruña, Badalona, Barcelona, Madrid, Navarra, Santiago de Compostela). Ethical approval was obtained from all participating hospitals, and patients provided informed consent. A Table E1 with the number of subjects included per hospital is available in the supplementary file.
The study included 545 adult patients diagnosed with severe uncontrolled asthma and treated with biological therapies (dupilumab, omalizumab, reslizumab, benralizumab and/or mepolizumab) based on GEMA 5.0 guidelines criteria,14 analyzed by Rial et al.19 A retrospective review of electronic medical records collected demographic data, comorbidities related to asthma, cardiovascular risk factors, characteristics of asthma and corticosteroids usage. More information in supplementary file. Clinical assessments were performed at baseline and at the last visit during biologic treatment up to October 23, 2021. Subjects who discontinued biologic treatment for different reasons were excluded. More information in supplementary file.
Criteria Used for Classification of Response to TreatmentAuthors defined “responders” to achieve at least two of the following criteria: daily prednisone dose 5mg or less and/or <1 SAE with no hospitalizations in the last 12 months of treatment before October 23, 2021 and/or ACT >20 points. The possible causes of why an absolute reduction in asthma exacerbations is not achieved despite biological treatment have been studied, and it has been suggested that they could be due to causes unrelated to the target mechanism of the drug.6 Therefore, the occurrence of one asthma exacerbation that does not require a hospital admission seems justified to us. Considering the reduction of SCS, the authors consider the complete reduction of SCS as a goal, instead of establishing an arbitrary percentage of reduction of the initial dose of SCS, especially because there is no literature on what a clinically important reduction of SCS will be. “non-responders” were those with >5mg prednisone daily and with >1 SAE or one asthma-related hospitalization and ACT <20 during the last year of treatment. Patients requiring a prednisone dose of ≤5mg for adrenal insufficiency were classified as not requiring SCS therapy for asthma.
Among responders, super-responders were identified according to Upham et al.9 definition. Super-responders met at least two major criteria, such as elimination of exacerbations, major improvement in asthma control (>6 points for ACT), complete cessation of SCS or justified minimal dose by adrenal insufficiency, and one minor criterion such as 75% exacerbation reduction, ACT >20 or a >500ml improvement in FEV1.8
The study compared the proportion of responders, non-responders and super-responders within different biological therapy subgroups.
Criteria Used for Clinical RemissionIn our cohort clinical, defined clinical remission aligns with Menzies-Gow et al.’s criteria considering no asthma exacerbation, absence of clinical symptoms (ACT>20), no SCS and optimization of lung function (FEV1>80%, predicted before bronchodilation).11
Statistical MethodsContinuous variables are expressed as mean±standard deviation (SD) or median and interquartile range (IQR). The Kolgomorov–Smirnov test evaluated the normal distribution when n>50 and the Shapiro–Wilk test when n<50. Frequencies of comorbidities were compared among different biologic subgroups, and their associations with improvement categories (responders, super-responders, non-responders) were assessed using the Chi-square test or Fisher's exact test. Paired data comparisons were conducted using two-tailed Paired t-test or with Wilcoxon matched-pairs tests based on normality.
Kruskal–Wallis tests and Mann–Whitney tests were applied for multiple and two-group comparisons, respectively.
Univariable and multivariable logistic regression models were used to identify variables associated with non-responder status and clinical remission. More information in supplementary file.
Statistical significance was defined as p<0.05. GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA) and R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for data analysis.
ResultsTotal SampleInitially, there were 545 patients with severe uncontrolled asthma receiving biologic treatment.
Nine subjects with dupilumab were excluded due to small sample size, and 62 subjects because of missing data. An additional 45 subjects had discontinued biologic treatment for different reasons, including 25 subjects (4.6%) due to lack of efficacy (ACT<20 and >1 SAE) at short-term (first 6 months of treatment) evaluation (Fig. EI in supplementary file). The final analysis focused on 429 subjects receiving their first biologic treatment for more than six months.
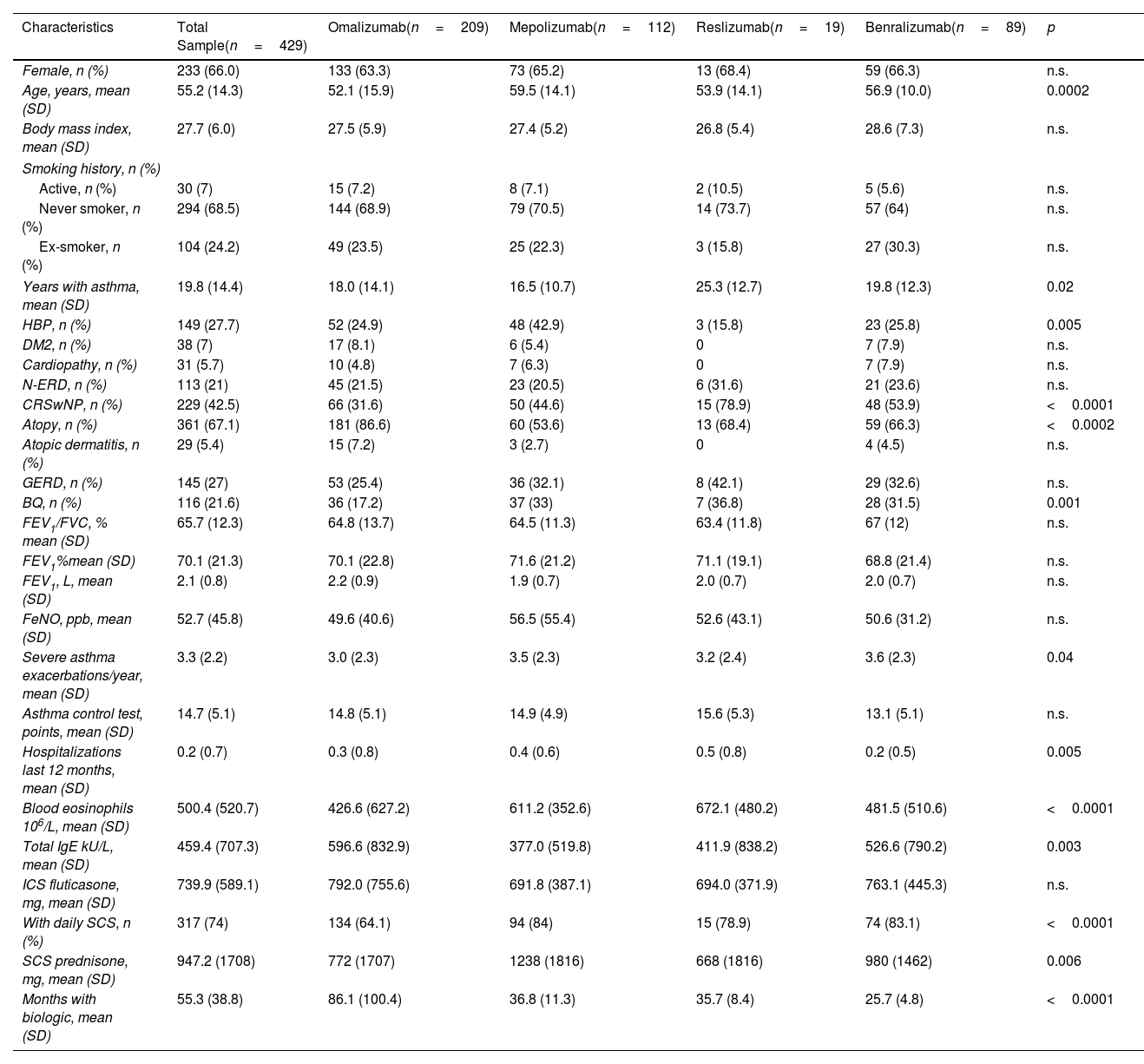
Among these, 209 (48.7%) received omalizumab, 112 (26.1%) mepolizumab, 19 (4.4%) reslizumab and 89 (20.7%) benralizumab with a mean of 55.3±38.8 months of treatment. Females are the prevalent sex (66%); the mean age was 55.2±14.3 years old. Clinical parameters at baseline (Table 2) align with expectations for severe uncontrolled asthma. Subjects had a high dose of ICS/day, and 74% of subjects used daily SCS. They had a mean of 19.8±14.4 years of asthma disease before the biologic treatment.
Baseline Characteristics of Total Sample and Biologic Subgroups.
| Characteristics | Total Sample(n=429) | Omalizumab(n=209) | Mepolizumab(n=112) | Reslizumab(n=19) | Benralizumab(n=89) | p |
|---|---|---|---|---|---|---|
| Female, n (%) | 233 (66.0) | 133 (63.3) | 73 (65.2) | 13 (68.4) | 59 (66.3) | n.s. |
| Age, years, mean (SD) | 55.2 (14.3) | 52.1 (15.9) | 59.5 (14.1) | 53.9 (14.1) | 56.9 (10.0) | 0.0002 |
| Body mass index, mean (SD) | 27.7 (6.0) | 27.5 (5.9) | 27.4 (5.2) | 26.8 (5.4) | 28.6 (7.3) | n.s. |
| Smoking history, n (%) | ||||||
| Active, n (%) | 30 (7) | 15 (7.2) | 8 (7.1) | 2 (10.5) | 5 (5.6) | n.s. |
| Never smoker, n (%) | 294 (68.5) | 144 (68.9) | 79 (70.5) | 14 (73.7) | 57 (64) | n.s. |
| Ex-smoker, n (%) | 104 (24.2) | 49 (23.5) | 25 (22.3) | 3 (15.8) | 27 (30.3) | n.s. |
| Years with asthma, mean (SD) | 19.8 (14.4) | 18.0 (14.1) | 16.5 (10.7) | 25.3 (12.7) | 19.8 (12.3) | 0.02 |
| HBP, n (%) | 149 (27.7) | 52 (24.9) | 48 (42.9) | 3 (15.8) | 23 (25.8) | 0.005 |
| DM2, n (%) | 38 (7) | 17 (8.1) | 6 (5.4) | 0 | 7 (7.9) | n.s. |
| Cardiopathy, n (%) | 31 (5.7) | 10 (4.8) | 7 (6.3) | 0 | 7 (7.9) | n.s. |
| N-ERD, n (%) | 113 (21) | 45 (21.5) | 23 (20.5) | 6 (31.6) | 21 (23.6) | n.s. |
| CRSwNP, n (%) | 229 (42.5) | 66 (31.6) | 50 (44.6) | 15 (78.9) | 48 (53.9) | <0.0001 |
| Atopy, n (%) | 361 (67.1) | 181 (86.6) | 60 (53.6) | 13 (68.4) | 59 (66.3) | <0.0002 |
| Atopic dermatitis, n (%) | 29 (5.4) | 15 (7.2) | 3 (2.7) | 0 | 4 (4.5) | n.s. |
| GERD, n (%) | 145 (27) | 53 (25.4) | 36 (32.1) | 8 (42.1) | 29 (32.6) | n.s. |
| BQ, n (%) | 116 (21.6) | 36 (17.2) | 37 (33) | 7 (36.8) | 28 (31.5) | 0.001 |
| FEV1/FVC, % mean (SD) | 65.7 (12.3) | 64.8 (13.7) | 64.5 (11.3) | 63.4 (11.8) | 67 (12) | n.s. |
| FEV1%mean (SD) | 70.1 (21.3) | 70.1 (22.8) | 71.6 (21.2) | 71.1 (19.1) | 68.8 (21.4) | n.s. |
| FEV1, L, mean (SD) | 2.1 (0.8) | 2.2 (0.9) | 1.9 (0.7) | 2.0 (0.7) | 2.0 (0.7) | n.s. |
| FeNO, ppb, mean (SD) | 52.7 (45.8) | 49.6 (40.6) | 56.5 (55.4) | 52.6 (43.1) | 50.6 (31.2) | n.s. |
| Severe asthma exacerbations/year, mean (SD) | 3.3 (2.2) | 3.0 (2.3) | 3.5 (2.3) | 3.2 (2.4) | 3.6 (2.3) | 0.04 |
| Asthma control test, points, mean (SD) | 14.7 (5.1) | 14.8 (5.1) | 14.9 (4.9) | 15.6 (5.3) | 13.1 (5.1) | n.s. |
| Hospitalizations last 12 months, mean (SD) | 0.2 (0.7) | 0.3 (0.8) | 0.4 (0.6) | 0.5 (0.8) | 0.2 (0.5) | 0.005 |
| Blood eosinophils 106/L, mean (SD) | 500.4 (520.7) | 426.6 (627.2) | 611.2 (352.6) | 672.1 (480.2) | 481.5 (510.6) | <0.0001 |
| Total IgE kU/L, mean (SD) | 459.4 (707.3) | 596.6 (832.9) | 377.0 (519.8) | 411.9 (838.2) | 526.6 (790.2) | 0.003 |
| ICS fluticasone, mg, mean (SD) | 739.9 (589.1) | 792.0 (755.6) | 691.8 (387.1) | 694.0 (371.9) | 763.1 (445.3) | n.s. |
| With daily SCS, n (%) | 317 (74) | 134 (64.1) | 94 (84) | 15 (78.9) | 74 (83.1) | <0.0001 |
| SCS prednisone, mg, mean (SD) | 947.2 (1708) | 772 (1707) | 1238 (1816) | 668 (1816) | 980 (1462) | 0.006 |
| Months with biologic, mean (SD) | 55.3 (38.8) | 86.1 (100.4) | 36.8 (11.3) | 35.7 (8.4) | 25.7 (4.8) | <0.0001 |
BQ: bronchiectasis; CRSwNP: chronic rhinosinusitis with nasal polyps; DM2: diabetes mellitus type 2; ICS: inhaled corticosteroids; IgE: immunoglobulin E; FeNO: fraction exhaled of nitric oxide; FEV1: forced exhaled volume in first second; FVC: forced volume capacity; GERD: gastroesophageal reflux disease; HBP: high blood pressure; N-ERD: NSAID exacerbated respiratory disease; SCS: systemic corticosteroids; SD: standard deviation.
Notably, significant differences among biologic subgroups can be observed in Table 2. Also, the distribution of comorbidities varies among biologic subgroups.
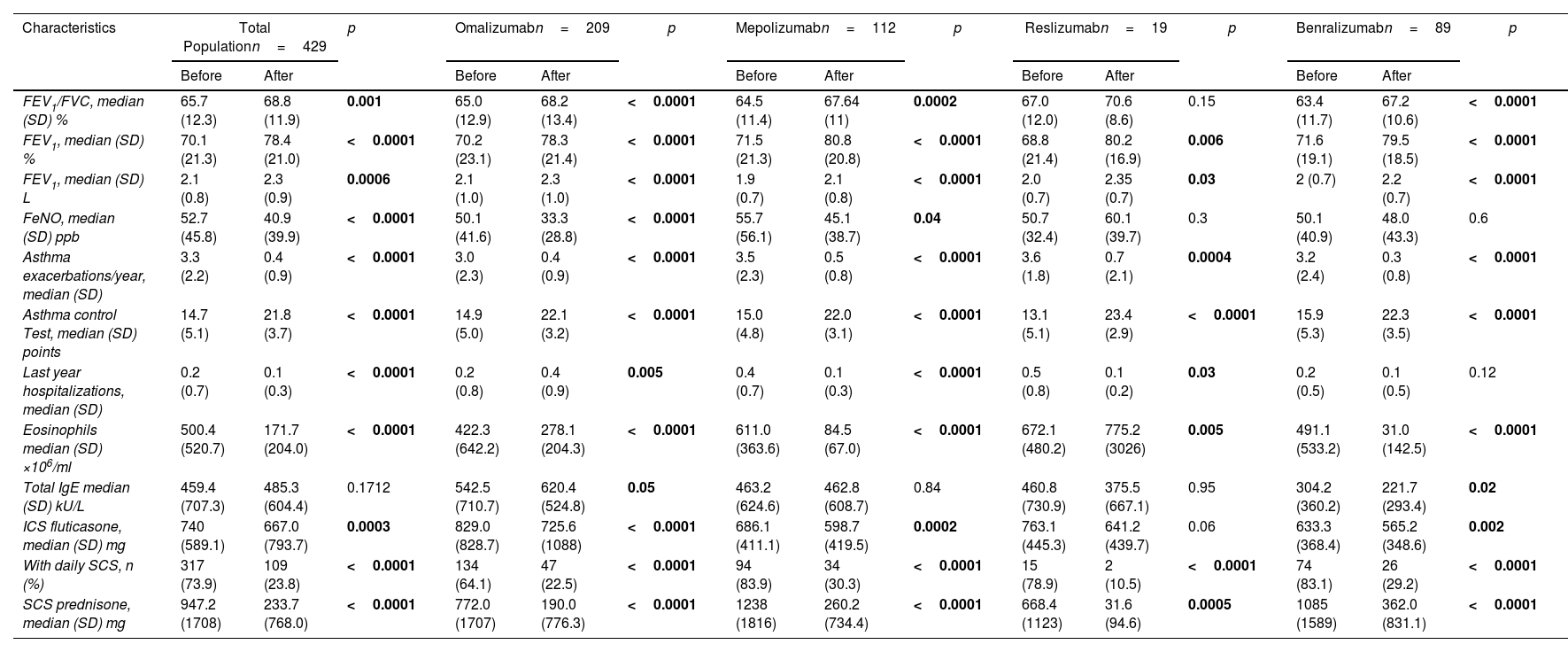
After biologic treatment, there was a significant improvement in lung function tests (FEV1, FEV1/FVC, FeNO); ACT >20 points in 335 (78.1%) subjects, a reduction in SAE and hospitalizations, 298 (69.5%) subjects were free of SAE and hospitalizations, had a significant reduction of subjects on SCS usage and dose in the last 12 months of treatment. This data is shown in Table 3.
Changes Between Before and After the Long-term With Biologics in the Total Population and among Biologics Subgroups.
| Characteristics | Total Populationn=429 | p | Omalizumabn=209 | p | Mepolizumabn=112 | p | Reslizumabn=19 | p | Benralizumabn=89 | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | ||||||
| FEV1/FVC, median (SD) % | 65.7 (12.3) | 68.8 (11.9) | 0.001 | 65.0 (12.9) | 68.2 (13.4) | <0.0001 | 64.5 (11.4) | 67.64 (11) | 0.0002 | 67.0 (12.0) | 70.6 (8.6) | 0.15 | 63.4 (11.7) | 67.2 (10.6) | <0.0001 |
| FEV1, median (SD) % | 70.1 (21.3) | 78.4 (21.0) | <0.0001 | 70.2 (23.1) | 78.3 (21.4) | <0.0001 | 71.5 (21.3) | 80.8 (20.8) | <0.0001 | 68.8 (21.4) | 80.2 (16.9) | 0.006 | 71.6 (19.1) | 79.5 (18.5) | <0.0001 |
| FEV1, median (SD) L | 2.1 (0.8) | 2.3 (0.9) | 0.0006 | 2.1 (1.0) | 2.3 (1.0) | <0.0001 | 1.9 (0.7) | 2.1 (0.8) | <0.0001 | 2.0 (0.7) | 2.35 (0.7) | 0.03 | 2 (0.7) | 2.2 (0.7) | <0.0001 |
| FeNO, median (SD) ppb | 52.7 (45.8) | 40.9 (39.9) | <0.0001 | 50.1 (41.6) | 33.3 (28.8) | <0.0001 | 55.7 (56.1) | 45.1 (38.7) | 0.04 | 50.7 (32.4) | 60.1 (39.7) | 0.3 | 50.1 (40.9) | 48.0 (43.3) | 0.6 |
| Asthma exacerbations/year, median (SD) | 3.3 (2.2) | 0.4 (0.9) | <0.0001 | 3.0 (2.3) | 0.4 (0.9) | <0.0001 | 3.5 (2.3) | 0.5 (0.8) | <0.0001 | 3.6 (1.8) | 0.7 (2.1) | 0.0004 | 3.2 (2.4) | 0.3 (0.8) | <0.0001 |
| Asthma control Test, median (SD) points | 14.7 (5.1) | 21.8 (3.7) | <0.0001 | 14.9 (5.0) | 22.1 (3.2) | <0.0001 | 15.0 (4.8) | 22.0 (3.1) | <0.0001 | 13.1 (5.1) | 23.4 (2.9) | <0.0001 | 15.9 (5.3) | 22.3 (3.5) | <0.0001 |
| Last year hospitalizations, median (SD) | 0.2 (0.7) | 0.1 (0.3) | <0.0001 | 0.2 (0.8) | 0.4 (0.9) | 0.005 | 0.4 (0.7) | 0.1 (0.3) | <0.0001 | 0.5 (0.8) | 0.1 (0.2) | 0.03 | 0.2 (0.5) | 0.1 (0.5) | 0.12 |
| Eosinophils median (SD) ×106/ml | 500.4 (520.7) | 171.7 (204.0) | <0.0001 | 422.3 (642.2) | 278.1 (204.3) | <0.0001 | 611.0 (363.6) | 84.5 (67.0) | <0.0001 | 672.1 (480.2) | 775.2 (3026) | 0.005 | 491.1 (533.2) | 31.0 (142.5) | <0.0001 |
| Total IgE median (SD) kU/L | 459.4 (707.3) | 485.3 (604.4) | 0.1712 | 542.5 (710.7) | 620.4 (524.8) | 0.05 | 463.2 (624.6) | 462.8 (608.7) | 0.84 | 460.8 (730.9) | 375.5 (667.1) | 0.95 | 304.2 (360.2) | 221.7 (293.4) | 0.02 |
| ICS fluticasone, median (SD) mg | 740 (589.1) | 667.0 (793.7) | 0.0003 | 829.0 (828.7) | 725.6 (1088) | <0.0001 | 686.1 (411.1) | 598.7 (419.5) | 0.0002 | 763.1 (445.3) | 641.2 (439.7) | 0.06 | 633.3 (368.4) | 565.2 (348.6) | 0.002 |
| With daily SCS, n (%) | 317 (73.9) | 109 (23.8) | <0.0001 | 134 (64.1) | 47 (22.5) | <0.0001 | 94 (83.9) | 34 (30.3) | <0.0001 | 15 (78.9) | 2 (10.5) | <0.0001 | 74 (83.1) | 26 (29.2) | <0.0001 |
| SCS prednisone, median (SD) mg | 947.2 (1708) | 233.7 (768.0) | <0.0001 | 772.0 (1707) | 190.0 (776.3) | <0.0001 | 1238 (1816) | 260.2 (734.4) | <0.0001 | 668.4 (1123) | 31.6 (94.6) | 0.0005 | 1085 (1589) | 362.0 (831.1) | <0.0001 |
ICS: inhaled corticosteroid; IgE: immunoglobulin E; FeNO: fraction exhaled of nitric oxide; FEV1: forced exhaled volume in first second; FVC: forced volume capacity; SCS: systemic corticosteroids; ppb: parts per billion; SD: standard deviation.
A p<0.05 was considered significant.
When the population is divided by the treatment they received, the FEV1 increases with all biologics. The improvement in FEV1/FVC can be observed with all biologics except reslizumab. A statistically significant decrease in FeNO occurs with omalizumab and mepolizumab. The improvement in SAE and hospitalizations after biologics were similar between treatments.
The ACT also has a statistically significant change with all biologics. As expected, the reduction in eosinophils was greater after anti-IL-5/anti-IL-5Rα than omalizumab (p<0.0001).
There were 317 (74%) subjects who used daily SCS at baseline, and with biologics, 215 (67.8% of 317) reduced the SCS to prednisone <5mg. One hundred subjects were not able to reduce the SCS dose to prednisone <5mg, two had missing data and nine additional subjects needed to start SCS. Based on the biologic received, omalizumab had greater reduction than mepolizumab (p<0.0001) and benralizumab (p=0.002). The nine (2.1%) subjects who needed to start SCS were receiving omalizumab.
Prevalence of Response in Our CohortOf the cohort, 391 (91.1%) subjects were classified as “responders”, with 218 (55.8% of 391) subjects being super-responders according to Upham et al.9 criteria. A total of 363 (84.6%) subjects had <1 SAE and no hospitalizations due to asthma, 335 (78.1%) subjects improved the ACT >20 points and 318 (74%) subjects received prednisone <5mg of SCS. The majority of the 173/391 subjects who did not meet the super-responder criteria failed to reduce the prednisone dose to <5mg.
Only 38 (8.9%) subjects of our cohort were “non-responders”. Most subjects (20/38) failed to achieve zero asthma hospitalizations and had prednisone >5mg. Interestingly, 20 subjects (20/38 and 20/429) failed the three goals. Among these non-responders, none were receiving reslizumab.
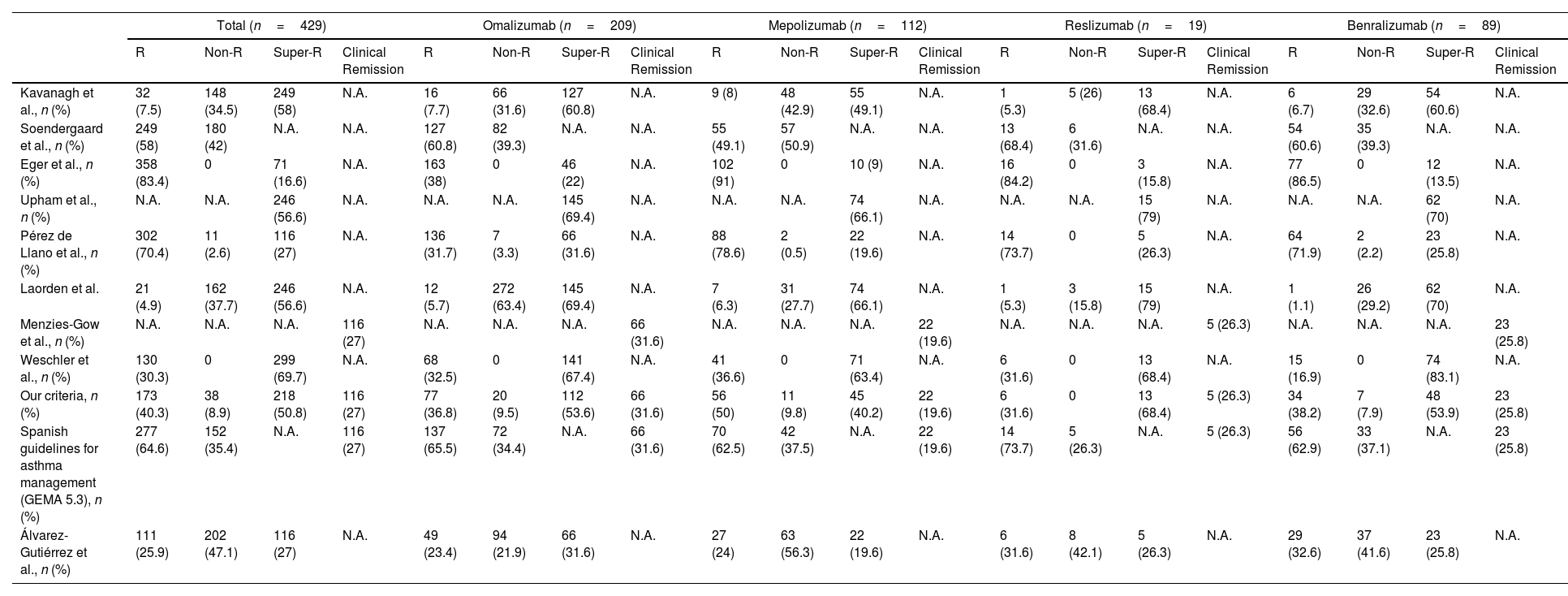
Comparison of the Response to Biologics According to Different Criteria in our CohortVarious response criteria from the literature were applied (Table 1) to our cohort to indirectly compare the results of response and clinical remission (Table 4). The criteria influenced the classification of response to biologics.
Comparison of the Response to Long-term (>6 Months) Treatment With Biologics in Our Sample According to Different Criteria.
| Total (n=429) | Omalizumab (n=209) | Mepolizumab (n=112) | Reslizumab (n=19) | Benralizumab (n=89) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | Non-R | Super-R | Clinical Remission | R | Non-R | Super-R | Clinical Remission | R | Non-R | Super-R | Clinical Remission | R | Non-R | Super-R | Clinical Remission | R | Non-R | Super-R | Clinical Remission | |
| Kavanagh et al., n (%) | 32 (7.5) | 148 (34.5) | 249 (58) | N.A. | 16 (7.7) | 66 (31.6) | 127 (60.8) | N.A. | 9 (8) | 48 (42.9) | 55 (49.1) | N.A. | 1 (5.3) | 5 (26) | 13 (68.4) | N.A. | 6 (6.7) | 29 (32.6) | 54 (60.6) | N.A. |
| Soendergaard et al., n (%) | 249 (58) | 180 (42) | N.A. | N.A. | 127 (60.8) | 82 (39.3) | N.A. | N.A. | 55 (49.1) | 57 (50.9) | N.A. | N.A. | 13 (68.4) | 6 (31.6) | N.A. | N.A. | 54 (60.6) | 35 (39.3) | N.A. | N.A. |
| Eger et al., n (%) | 358 (83.4) | 0 | 71 (16.6) | N.A. | 163 (38) | 0 | 46 (22) | N.A. | 102 (91) | 0 | 10 (9) | N.A. | 16 (84.2) | 0 | 3 (15.8) | N.A. | 77 (86.5) | 0 | 12 (13.5) | N.A. |
| Upham et al., n (%) | N.A. | N.A. | 246 (56.6) | N.A. | N.A. | N.A. | 145 (69.4) | N.A. | N.A. | N.A. | 74 (66.1) | N.A. | N.A. | N.A. | 15 (79) | N.A. | N.A. | N.A. | 62 (70) | N.A. |
| Pérez de Llano et al., n (%) | 302 (70.4) | 11 (2.6) | 116 (27) | N.A. | 136 (31.7) | 7 (3.3) | 66 (31.6) | N.A. | 88 (78.6) | 2 (0.5) | 22 (19.6) | N.A. | 14 (73.7) | 0 | 5 (26.3) | N.A. | 64 (71.9) | 2 (2.2) | 23 (25.8) | N.A. |
| Laorden et al. | 21 (4.9) | 162 (37.7) | 246 (56.6) | N.A. | 12 (5.7) | 272 (63.4) | 145 (69.4) | N.A. | 7 (6.3) | 31 (27.7) | 74 (66.1) | N.A. | 1 (5.3) | 3 (15.8) | 15 (79) | N.A. | 1 (1.1) | 26 (29.2) | 62 (70) | N.A. |
| Menzies-Gow et al., n (%) | N.A. | N.A. | N.A. | 116 (27) | N.A. | N.A. | N.A. | 66 (31.6) | N.A. | N.A. | N.A. | 22 (19.6) | N.A. | N.A. | N.A. | 5 (26.3) | N.A. | N.A. | N.A. | 23 (25.8) |
| Weschler et al., n (%) | 130 (30.3) | 0 | 299 (69.7) | N.A. | 68 (32.5) | 0 | 141 (67.4) | N.A. | 41 (36.6) | 0 | 71 (63.4) | N.A. | 6 (31.6) | 0 | 13 (68.4) | N.A. | 15 (16.9) | 0 | 74 (83.1) | N.A. |
| Our criteria, n (%) | 173 (40.3) | 38 (8.9) | 218 (50.8) | 116 (27) | 77 (36.8) | 20 (9.5) | 112 (53.6) | 66 (31.6) | 56 (50) | 11 (9.8) | 45 (40.2) | 22 (19.6) | 6 (31.6) | 0 | 13 (68.4) | 5 (26.3) | 34 (38.2) | 7 (7.9) | 48 (53.9) | 23 (25.8) |
| Spanish guidelines for asthma management (GEMA 5.3), n (%) | 277 (64.6) | 152 (35.4) | N.A. | 116 (27) | 137 (65.5) | 72 (34.4) | N.A. | 66 (31.6) | 70 (62.5) | 42 (37.5) | N.A. | 22 (19.6) | 14 (73.7) | 5 (26.3) | N.A. | 5 (26.3) | 56 (62.9) | 33 (37.1) | N.A. | 23 (25.8) |
| Álvarez-Gutiérrez et al., n (%) | 111 (25.9) | 202 (47.1) | 116 (27) | N.A. | 49 (23.4) | 94 (21.9) | 66 (31.6) | N.A. | 27 (24) | 63 (56.3) | 22 (19.6) | N.A. | 6 (31.6) | 8 (42.1) | 5 (26.3) | N.A. | 29 (32.6) | 37 (41.6) | 23 (25.8) | N.A. |
N.A.: not available; Non-R: non-responders; R: responders; Super-R: super-responders.
The criteria applied the criteria used by Kavanagh et al.6 to our cohort, we obtained 7.5% (32) of responders, 58% (249) of super-responders and 34.5% (148) of non-responders.
Using the criteria used in Soendergaard et al.7 58% (249) from our cohort achieved a complete response.
Compared with the criteria of response by Eger et al.,8 we would find 16.6% (71).
When criteria of super-responders by FEOS10 was applied, 67.6% (290) of subjects from our cohort surpassed this cut-off.
Under Weschler et al.13 criteria, we obtained 69.7% (299) of super-responders.
The GEMA guidelines are much stricter, we had 64.6% of responders and 35.4% of non-responders.14 Against the criteria of Álvarez-Gutiérrez et al.,15 our cohort reached 25.9% of responders, 27% of super-responders and 47.1% of non-responders.
Predictors for “Non-responders”Our model had an area under curve (AUC) ROC of 0.88 (0.93–0.93). Factors associated with being a non-responder included a higher BMI (OR:1.14; 95% CI:1.06–1.23; p<0.001), ICU admission (2.69; 1.30–5.56; p=0.01), a higher number of SAE (1.21; 1.03–1.42; p=0.02) before biologic treatment. Conversely, a lower ACT score (0.89; 0.80–0.99; p=0.03), FEV1 (0.92; 0.86–0.99; p=0.02) or fewer years with asthma (0.96; 0.93–0.99; p=0.01) before treatment, reduced the risk of being non-responder. More information in supplementary file.
Clinical RemissionClinical remission, as defined by Menzies-Gow et al.12 was experienced by 116 (27%) subjects. No significant differences were found between different biologic treatments [66 (31.6%) omalizumab, 22 (19.6%) mepolizumab, 5 (26.3%) reslizumab, 23 (25.8%) benralizumab].
Predictors for Clinical RemissionOur model had an AUC ROC of 0.78 (0.93–0.93). Factors associated with achieving clinical remission included higher FEV1% (0.96; 0.95–0.98; p<0.001), higher ACT score (0.93; 0.88–0.99; p=0.01) or N-ERD (0.52; 0.29–0.91; p=0.02) before the biologic treatment. Conversely, higher ICU hospitalizations (5.42; 0.76–38.65; p=0.008) or having heart disease (5.54; 1.12–27.77; p=0.01) were associated with not reaching clinical remission. More information in supplementary file.
DiscussionThis paper analyzed patients with severe uncontrolled asthma treated with omalizumab and anti-IL-5/IL-5R with a mean treatment duration of 55.3±38.8 months. Patient baseline characteristics varied due to the real-life nature of the study. Notably, subjects receiving reslizumab were limited due to its intravenous administration, making it less favorable. In this real-life cohort, there was an excellent long-term response to biologic treatment with 91.1% of responders from which 55.7% were “super-responders”, and only 8.9% as “non-responders”, with similar results among biologic used.
When analyzing the clinical response and applying the criteria from other authors to our cohort, there were differences between the cohorts’ results. The study by Kavanagh et al.6 obtained 86% of “responders”, 39% of “super-responders”, and 13.8% of “non-responders” after 48 weeks of benralizumab treatment. The cohort from Kavanagh's study had more SAE per year and worse FEV1. Our cohort had a longer duration with biologic treatment with a better chance to achieve the established goals. If we apply their criteria to our cohort, we obtained 7.5% (32) of responders, 58% (249) of super-responders and 34.5% (148) of non-responders. This comparison highlights the difference between subjective (ACT) and objective (SCS and SAE) response criteria.
Results from the Soendergaard et al.7 of subjects treated with anti-IL-5 treatments for 52 weeks reached a complete response by 58%. Applying their criteria, we had the same results, 58% (249). The comparison between both cohorts before biologic treatment reveals slight differences.
Compared with the criteria of response by Eger et al.,8 we would find 16.6% (71) being super-responders which is very similar to 14% in their study. The super-responder criteria are more stringent than Upham et al.9 because of the FEV1 improvement, which is harder to achieve and because anti-IL-5/IL-5Rα biologic treatments do not usually have a decrease in FeNO. By their criteria, there are no non-responders in our analyzed cohort with long-term biologic therapy. Nevertheless, we should mention that from the original 545 subjects in the cohort of Rial et al.,19 25 subjects (4.6%) are non-responders at short-term evaluation using Eger et al. criteria.8 On the other hand, 83.4% (358) would be considered “partial responders” by the authors’ criteria.
With the FEOS10 system, 67.6% (290) of subjects are super-responders. In their cohort, with 3 years of treatment with anti-IL-5/anti-IL-5R, they had 72% super-responders, 22% responders and 5% non-responders.
Under Weschler et al.13 criteria, we obtained 69.7% (299) of super-responders, an excellent result because it is only based on the improvement of one goal (no SAE). All our subjects improved to the minimum criteria. In short-term we had four subjects with adverse effects with omalizumab.
The GEMA14 guidelines are much stricter, we had 64.6% of responders and 35.4% of non-responders.
Using the criteria of Álvarez-Gutiérrez et al.,15 our cohort reached 25.9% of responders, 27% of super-responders and 47.1% of non-responders. The higher percentage of non-responders is because we have many subjects with less than 10% or 100ml of improvement in FEV1.
Our criteria for responders are somewhat flexible, allowing subjects to be considered “responder” by achieving a complete clinically satisfactory goal in two of the three main aspects of severe uncontrolled asthma. We applied the definition of “super-responders” suggested by Upham et al.,9 similar to Kavanagh et al.6 and Soendergaard et al.7 criteria for responders. When the different criteria are applied to our cohort, we show that we are all using the same tools to measure the results, and the combination of them is the differentiating point. We want to highlight that Eger et al.8 and Alvarez-Gutiérrez et al.15 were the only two references considering worsening or the absence of improvement as criteria for non-responders.
Factors such as higher total number of SAE (OR:1.21), ICU admissions (OR:2.69) were associated with being non-responders.
The Soendergaard et al.7 authors determined the need for SCS, a younger onset of asthma and concomitant DM2 as predictors of failure at achieving a complete response to biologics. They also obtained that >0.3×109L eosinophils, allergic rhinitis and lower ACQ score were predictors of complete response. In a regression analysis, Eger et al.8 obtained that <10 years with asthma have a greater OR:3.5 of achieving a super-response.
A recent prospective study from Japan20 prospectively analyzed 54 subjects with biologic treatment for 12 months, looking for clinical remission (corresponding to Upham et al.9 super-responder criteria) and functional remission (clinical remission and FEV1 predicted >80%, eosinophils <300×106L, FeNO <35ppb), corresponding to Menzies-Gow et al.12 definition for clinical remission. They observed 68% of clinical remission (vs. 56.6% of super-responders in our cohort) and 70% of functional remission (vs. 27% of clinical remission in our cohort). The differences observed between the Japanese series and our cohort could be explained by a worse baseline FEV1% predicted (77% vs. 70%, respectively) in our cohort. In this Japanese study, the cut-off value for clinical remission for FEV1% is a higher predicted FEV1 >75% with a sensitivity of 94% and a specificity of 64%.20
Clinical remission by Menzies-Gow et al.12 was experienced by 116 (27%). Our results agreed with those of Milger et al.18 No statistically significant differences were found between biologic treatments. In retrospective phase III studies, only 14.5% (85/586) on benralizumab and 7.7% (48/620) on placebo achieved clinical remission at 12 months.21 In an observational retrospective study (ORBE II) of 204 subjects performed in Spain with benralizumab for 12 months, 43% achieved clinical remission under the Menzies-Gow et al. definition. The greater result in ORBE II could be related to less duration of asthma disease (mean 15 years vs. 19 years, in our cohort) and a higher percentage of subjects with SCS before biologic treatment (29% vs. 74%, in our cohort).22 A recently published cohort of 486 subjects with severe uncontrolled asthma with biologic treatment from Italy, obtained a percentage of clinical remission (Menzies-Gow et al.12 criteria) like ours.23
With mepolizumab, they obtained clinical remission was obtained in 29% at 52 weeks and increased to 33% at 104 weeks of treatment in a real-life cohort (REALITI-A study),24 like our result. A multicenter observational study performed in Spain (REDES study) followed 318 subjects with mepolizumab with the reduction in exacerbation rate as the primary endpoint achieving a reduction of 77.5% compared with the exacerbation rate from the 12 months before mepolizumab.25 A post hoc analysis looking for clinical remission under Menzies-Gow et al.12 obtained that 37% achieved no SCS plus no SAE and ACT >20 (consistent with our definition of super-responder by Upham et al.) and 30% of clinical remission adding the improvement in FEV1.26 We did obtain better results with the super-responder criteria of 56%, some clear differences are observed in the mean SAE rate (3.3 vs. 4.5 in REDES) and subjects with BMI >25 in our cohort were 65% compared to 72.8% in REDES.
A high FEV1% (OR: 0.96), high ACT score (OR: 0.93) before the biologic treatment or N-ERD (OR: 0.52) were associated with reaching clinical remission. A history of ICU admissions (OR: 5.42) and heart disease (OR: 5.54) have a higher risk of not achieving clinical remission.
This study's limitations are its retrospective nature and missing data. However, this weakness is part of real-life studies. Nevertheless, comparative real-life studies are recommended.1,6
These results demonstrated that most patients achieved a satisfactory response to biologics by combining multiple goals. Reducing SAE and hospitalizations, lowering SCS and decreasing the frequency of asthma symptoms were all important outcomes. The most challenging goal is improving FEV1, likely due to asthma's severity and remodeling.27
The study suggests that personalizing the evaluation time based on asthma severity before the biologic treatment could yield better results.
ConclusionIn a large, multicenter, Spanish cohort of severe uncontrolled asthma treated with biologics (anti-IgE, anti-IL-5, anti-IL-5Rα) over an extended period, most of the patients were able to achieve a satisfactory response to biologics and only 8.9% were considered as “non-responders”. Clinical remission was obtained in 27% of subjects. Comparing these results with other studies proves challenging due to variations in the criteria used for assessment. Harmonizing definitions of response and clinical remission to biologics is needed to compare results from different studies.
During the preparation of this work, the authors used ChatGPT in order to reduce the original text. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
FundingNo funding was received for the research or preparation of this manuscript.
Conflict of InterestMVM declares to have received consulting fee from Leti Pharma, fees for lectures from GSK and AstraZeneca and support for attending international meetings from AstraZeneca. EA declares to have received honoraria for lectures from GSK, MSD, AstraZeneca, Sanofi, Gebro Pharma and to have participated in data safety monitoring board for GSK, MSD and AstraZeneca. LSR declares to have received honoraria from AstraZeneca, Sanofi, Takeda and has received support for attending international meetings from Hall-Allergy and an institutional grant for research. MJR declares to have received honoraria from AstraZeneca, GSK, Leti Pharma, Chiesi Pharmaceutics. JM declares to have a contract with GSK, AstraZeneca, Viatris (Mylan-Meda Pharmaceuticals), Sanofi-Genzyme, Regeneron Pharmaceuticals, NOUCOR/Uriach group; has received consulting fee from Sanofi-Genzyme and NOUCOR/Uriach group; has received honoraria from AstraZeneca, GSK, Menarini, MSD, Mitsubishi-Tanabe Pharma, Novartis, Procter&Gamble, Viatris (Mylan-Meda Pharmaceuticals), Sanofi-Genzyme, Regeneron Pharmaceuticals, UCB Pharma; support for attending meetings from GSK, AstraZeneca and has participated in data safety monitoring board for GSK, MSD, Novartis, AstraZeneca, Lilly, Sanofi-Genzyme, Regeneron Pharmaceuticals, Procter&Gamble, NOUCOR/Uriach group. JDO declares to have received consulting fees from GSK, Sanofi, AstraZeneca; has received honoraria from AstraZeneca, GSK, Sanofi, TEVA, Novartis, Chiesi Pharmaceutics. JMRM declares to have received a grant for research from SEAIC, payment for lectures from GSK and AstraZeneca. SQ declares to have received consulting fees from GSK and Sanofi; payment for lectures from GSK, AstraZeneca, Sanofi, TEVA, Novartis and Chiesi Pharmaceutics. JALP declares to have participated in data safety monitoring board for AstraZeneca. FJGB declares to have received grants from GSK; consulting fees from AstraZeneca, ALK-Abelló, Bial, Gebro Pharma, GSK, Sanofi, TEVA, Novartis, Chiesi Pharmaceutics, Rovi, Stallergenes-Greer, Menarini; payment for lectures from AstraZeneca, ALK-Abelló, Bial, Gebro Pharma, GSK, Sanofi, TEVA, Novartis, Chiesi Pharmaceutics, Rovi, Stallergenes-Greer, Menarini; support for attending meetings from ALK-Abelló, Menarini, Sanofi; to have participated in data safety monitoring board for AstraZeneca, GSK, ALK-Abelló, Menarini, Novartis, Sanofi, TEVA. CMR declares to have received payment for lectures from AstraZeneca, Chiesi Pharmaceutics, GSK, Sanofi, TEVA, Novartis; support for attending meetings from Chiesi Pharmaceutics; to have participated in data safety monitoring board for MundiPharma, GSK, AstraZeneca and Sanofi. JMO declares to have received consulting fees from ALK-Abelló; payment for lectures from AstraZeneca, MundiPharma, GSK. AV declares to have received payment for lecture from GSK, AstraZeneca, Novartis and Sanofi. BB declares to have received payment for lecture from Bial; support for attending meetings from Allergy Therapeutics, Sanofi, Stallergenes-Greer, GSK, Bial, Leti Pharma. DB declares to have received a Río Hortega Research Grant from Instituto Carlos III. XMG declares to have received payment for lectures from AstraZeneca, GSK, Gebro Pharma, Sanofi, Chiesi Pharmaceutics; consulting fees from AstraZeneca, GSK, Gebro Pharma, Sanofi, Chiesi Pharmaceutics; support for attending meetings from Sanofi, AstraZeneca, Menarini, FAES Pharma, GSK; to have participated in data safety monitoring board for Sanofi, GSK, AstraZeneca. VdP declares to have received payment for lectures from AstraZeneca, GSK and an institutional grant for research from AstraZeneca. PSC, BC, CP, IM, MJC, LPP, IB declare no conflict of interest.