Comorbidities are common in patients with chronic obstructive pulmonary disease (COPD), and have a significant impact on health status and prognosis. The PLATINO study provides data on self-reported comorbidities and perceived health status in COPD subjects.
MethodsPLATINO is a population-based study on COPD prevalence in five Latin American cities. COPD diagnosis was defined by GOLD criteria (FEV1/FVC<.70 post-bronchodilator). Information was collected on the following comorbidities: heart disease, hypertension, diabetes, cerebrovascular disease, peptic ulcer and asthma. Health status was evaluated using the SF-12 questionnaire, derived from the question: “In general, would you say your health is excellent, very good, good, fair or poor?” A simple comorbidity score was calculated by adding the total number of comorbid conditions.
ResultsOf a total population of 5314 individuals, 759 had COPD. Reported comorbidities by decreasing frequency were: any cardiovascular disease, hypertension, peptic ulcer, heart disease, diabetes, cerebrovascular disease, asthma and lung cancer. COPD patients had a higher comorbidity score and prevalence of lung cancer (P<.0001) and asthma (P<.0001), as well as a higher tendency to have hypertension (P=.0652) and cerebrovascular disease (P=.0750). Factors associated with comorbidities were age, body mass index (BMI) and female gender. The number of comorbidities increased as the health status deteriorated.
ConclusionsIn the PLATINO population-based study, COPD individuals had an increased number of comorbidities. Age, female gender and higher BMI were the factors associated with comorbidity in these patients. Comorbid conditions were associated with impaired health status, independently of the COPD status.
La enfermedad pulmonar obstructiva crónica (EPOC) se asocia a comorbilidades que influyen en el estado de salud y en el pronóstico de los pacientes. El estudio PLATINO aporta datos sobre comorbilidades autorreportadas y percepción del estado general de salud (EGS) en la EPOC.
MétodosPLATINO es un estudio poblacional, sobre prevalencia de EPOC en 5 ciudades de Latinoamérica. El diagnóstico de EPOC se realizó según el criterio de GOLD (FEV1/FVC<0,70 post-broncodilatador). Se recogió información sobre las siguientes comorbilidades: cardíaca, hipertensión, diabetes, accidente cerebrovascular (ACV), úlcera y asma. El EGS se evaluó mediante el cuestionario SF-12, con la pregunta: «En general ¿diría usted que su salud es: excelente, muy buena, buena, regular o pobre?». Sumando las comorbilidades, se elaboró un índice de comorbilidad.
ResultadosSobre una población total de 5.314individuos se realizó diagnóstico de EPOC en 759. Las comorbilidades reportadas en orden decreciente fueron: cualquier tipo de enfermedad cardiovascular, hipertensión, úlcera péptica, enfermedad cardíaca, diabetes, ACV, asma y cáncer de pulmón. Los sujetos con EPOC tuvieron mayor índice de comorbilidad, prevalencia de cáncer de pulmón (p<0,0001) y asma (p<0,0001), así como mayor tendencia a hipertensión (p=0,0652) y ACV (p=0,0750). Los factores asociados a comorbilidad en EPOC fueron la edad, el índice de masa corporal (IMC) y el género femenino. Con el deterioro del EGS aumenta el número de comorbilidades.
ConclusionesEn población no seleccionada los individuos con EPOC presentan más comorbilidades. La edad, el sexo femenino y mayor IMC son los principales factores asociados a comorbilidad en estos pacientes. Independientemente de la condición de EPOC, un mayor número de comorbilidades se asocia a peor EGS.
Chronic obstructive pulmonary disease (COPD) is a major public health problem1 that frequently presents with other chronic diseases or comorbidities and can have a significant impact on health status and prognosis. Although the prevalence of comorbidities varies depending on the series under study,2 the most common are cardiovascular diseases, malignant neoplasms (particularly lung cancer), diabetes and psychiatric disorders.3
Barr et al.4 evaluated the prevalence and distribution of comorbidities in COPD patients in a telephone survey. Over half of the patients interviewed had hypertension or hypercholesterolemia and one-third or more had depression, cataracts or osteoporosis.
In a population-based sample of 14828 subjects (≥45 years of age), including 995 subjects with a medical diagnosis of COPD, Schnell et al.5 found that, in these patients, comorbidities were the rule rather than the exception. Over 90% of COPD patients had at least one condition that could impact negatively on treatment, the most common being arthritis, depression, osteoporosis, cancer, coronary disease, heart failure and cerebrovascular disease.
Comorbidities have repercussions on the COPD patient's general state of health, the use of healthcare resources, hospitalizations and mortality.6–11 Indeed, while the most common cause of death in patients with advanced disease is respiratory, in patients with mild to moderate COPD, mortality is associated with cardiovascular comorbidities and lung cancer.9,10
Recently, Divo et al.12 evaluated the risk of mortality due to comorbidities in COPD. In a patient cohort followed up for an average of 51 months, 12 comorbidities associated with greater mortality were identified and used to draw up a comorbidity index: COTE (CO-morbidity TEst). Thus, comorbidities such as hypertension and hypercholesterolemia, which are common in COPD patients, would not be associated with increased mortality, while others such as cancer (particularly of the lung, esophagus, pancreas and breast), anxiety, liver cirrhosis, atrial fibrillation, diabetes, pulmonary fibrosis, heart failure, gastroduodenal ulcer and coronary disease would be.
Very little information is available on the incidence of comorbidities in COPD patients from multicenter epidemiological studies that also include diagnosis by spirometry and evaluation of the repercussions of such comorbidities on general health status.
The objectives of this study were: a) to determine the incidence of self-reported comorbidities in the overall population and in subjects with and without COPD in the PLATINO study; b) to analyze the possible factors associated with the incidence of self-reported comorbidities in subjects with and without COPD; and c) to examine the perceived general health status of the overall population and COPD subjects in relation to their various comorbidities.
Materials and MethodsPLATINO is a multicenter, cross-sectional, population-based study designed to measure the prevalence of COPD in 5 Latin American cities: São Paulo (Brazil), Mexico City (Mexico), Montevideo (Uruguay), Santiago de Chile (Chile) and Caracas (Venezuela). Details of the sampling methods and the size of the population-based sample have been published previously.13
To summarize, a representative sample of subjects aged 40 years and over were selected using a multi-stage cluster sampling strategy. The working protocol was approved by the ethics committee of each center.
Subjects interviewed completed a questionnaire and underwent spirometry testing using portable equipment (Easy-One™; NDD Medical Technologies, Chelmsford MA and Zurich, Switzerland). Data were obtained on symptoms, smoking habit, years of education, employment, previous spirometry, respiratory medication and previous diagnosis of COPD, asthma or tuberculosis.
The general health status data were evaluated using the SF-12 questionnaire and the patient's perceived health status was derived from the question “In general, would you say that your health is excellent, very good, good, fair or poor?”
COPD diagnosis was defined by the GOLD criterion (FEV1/FVC ratio <0.70 post-bronchodilator),1 resulting in 2 subject groups, those with and those without COPD. Both groups were divided into subgroups of smokers or ex-smokers and non-smokers.
Self-reported data on comorbidities were gathered from the following questions:
- •
Has the doctor told you at any time in your life that you have or have had any of the following diseases? (Yes/No)
- a.
Heart disease?
- b.
High blood pressure (hypertension)?
- c.
High blood sugar (diabetes)?
- d.
Lung cancer?
- e.
Brain embolism, ischemia or hemorrhage (cerebrovascular disease)?
- f.
Tuberculosis?
- g.
Gastritis or ulcer?
- a.
- •
Has the doctor ever told you that you have asthma, asthmatic bronchitis, bronchospasm or allergic bronchitis?
A simple comorbidity index (range 0 to 7) was drawn up, adding one point for each of the self-reported comorbidities (heart disease, hypertension, cerebral infarction, diabetes, lung cancer, ulcer, asthma). For example, a patient who replied “yes” to the question on previous diagnosis of diabetes, hypertension and asthma would have a comorbidity score of 3.
The incidence of comorbidities was compared between individuals with and without COPD and also according to their smoking status.
The PLATINO questionnaire is available on the website http://www.platino-alat.org.
Statistical AnalysisThe descriptive analysis included group comparisons carried out using the Pearson's χ2 test for categorical variables and the Wald test for continuous variables. Multivariate regression models were constructed using key variables considered influential for reasons of clinical rationale, bivariate analysis or previous analyses of the PLATINO database. The Poisson regression was used, since the dependent variable (comorbidity) was a very biased count variable. To facilitate interpretation, the results of the regression analysis were expressed as rate ratio. Standard regression diagnostics was carried out on all the models tested. All analyses were adjusted for survey design and carried out using a STATA statistical software package (STATA version 11.2; STATA Corporation, College Station, TX, USA).
The incidence rate ratio represents the modeled incidence of comorbidity in the group with the characteristics compared to the group without the characteristics. This is interpreted in the same way as other rates, where a value >1 represents a greater risk. For example, Table 2 shows that women have a rate ratio of 1.32. This means that women are 32% more likely to have comorbidities than men (after adjusting for age, BMI and smoking habit).
ResultsOf a total eligible population of 6711 subjects, 5571 were interviewed and spirometry was performed in 5314. Of these, 759 subjects had a FEV1/FVC ratio <.70 post-bronchodilator (COPD) and 4555 had an FEV1/FVC post-bronchodilator ≥.70 (no COPD); 2261 subjects were smokers or ex-smokers and 3050 were non-smokers.
Table 1 shows the general characteristics and self-reported comorbidity of the groups evaluated (subjects with and without COPD) and the overall population. Compared to subjects without COPD, a greater proportion of the COPD subjects were male, older, had a lower BMI and greater consumption of tobacco, and as expected, reported a higher incidence of respiratory symptoms. Comorbidities reported in decreasing order of incidence in the general population were: any type of cardiovascular disease, hypertension, peptic ulcer disease, heart disease, diabetes, cerebrovascular accident (CVA), asthma and, finally, lung cancer. A similar distribution was observed in patients with and without COPD. Compared to non-COPD subjects, COPD subjects more frequently reported lung cancer (P<.0001) and asthma (P<.0001). The group of subjects with COPD also tended to report hypertension (P=.0652) and CVA (P=.0750) more frequently. The incidence of other comorbidities was similar in the two groups (Table 2).
Description of General Characteristics and Self-reported Comorbidity of the General Population and Subjects With and Without COPD.
| Variables | COPD (n=759) | No COPD (n=4555) | Total population (n=5314) | P-value COPD vs no COPD |
| Age, years, mean (SD) | 64.1 (0.44) | 55.0 (0.22) | 56.3 (0.22) | <.0001 |
| Age groups, years, mean (SD) | ||||
| 40–49 | 109 (14.4) | 1840 (40.4) | 1949 (36.7) | <.0001 |
| 50–59 | 179 (23.6) | 1407 (30.9) | 1586 (29.9) | |
| 60+ | 471 (33.5) | 1308 (28.7) | 1779 (33.5) | |
| Gender, n (%) | ||||
| Male | 397 (52.3) | 1705 (37.4) | 2102 (39.6) | <.0001 |
| Female | 362 (47.7) | 2850 (62.6) | 3212 (60.4) | |
| BMI, kg/m2, mean (SD) | 26.8 (0.18) | 28.3 (0.10) | 28.1 (0.90) | <.0001 |
| Smoking habit, pack-years, mean (SD) | 19.4 (0.98) | 9.1 (0.27) | 10.5 (0.29) | <.0001 |
| Smoking status, n (%) | ||||
| Current smoker | 273 (36.0) | 1310 (28.8) | 1583 (29.8) | <.0001 |
| Ex-smoker | 247 (32.5) | 1220 (26.8) | 1467 (27.6) | |
| Non-smoker | 239 (31.5) | 2022 (44.4) | 2261 (42.6) | |
| Respiratory symptoms, n (%) | ||||
| Cough (Yes) | 238 (31.4) | 870 (19.1) | 1108 (20.9) | <.0001 |
| Phlegm (Yes) | 215 (28.3) | 779 (17.1) | 994 (18.7) | <.0001 |
| Wheezing (Yes) | 295 (38.9) | 973 (21.4) | 1268 (23.9) | <.0001 |
| Dyspnea (Yes) | 379 (50.7) | 2030 (45.1) | 2409 (45.9) | <.0046 |
| Any symptom (Yes) | 562 (74.0) | 2704 (59.4) | 3266 (61.5) | <.0001 |
| Heart disease (Yes), n (%) | 104 (13.7) | 579 (12.7) | 683 (12.9) | .3996 |
| Hypertension (Yes), n (%) | 282 (37.2) | 1533 (33.7) | 1815 (34.2) | .0652 |
| CVA (Yes), n (%) | 24 (3.2) | 96 (2.1) | 120 (2.3) | .0750 |
| Any cardiovascular disease (Yes), n (%) | 315 (41.5) | 1.767 (38.8) | 2.082 (39.2) | .1458 |
| Diabetes (Yes), n (%) | 64 (8.4) | 450 (9.9) | 514 (9.7) | .2200 |
| Peptic ulcer disease (Yes), n (%) | 241 (31.8) | 1.361 (29.9) | 1.602 (30.2) | .3144 |
| Lung cancer (Yes), n (%) | 8 (1.1) | 5 (0.1) | 13 (0.0) | <.0001 |
| Asthma (Yes), n (%) | 173 (22.8) | 478 (10.5) | 651 (12.3) | <.0001 |
| Comorbidity score, mean (SD) | 1.17 (0.04) | 0.99 (0.02) | 1.01 (0.02) | <.0001 |
BMI, body mass index; CVA, cerebrovascular accident.
Statistical analysis: Pearson's χ2 for categorical variables; Wald test for continuous variables. Statistical significance: P<.05.
Multivariate Analysis of Factors Associated With Comorbidity in Subjects With and Without COPD.
| Variables | Incidence rate ratio | Standard error | P | 95% confidence interval | ||||
| COPD (n=759) | No-COPD (n=4555) | COPD (n=759) | No-COPD (n=4555) | COPD (n=759) | No-COPD (n=4555) | COPD (n=759) | No-COPD (n=4555) | |
| Age | 1.01 | 1.02 | 0.003 | 0.001 | .001 | <.001 | 1.00–1.01 | 1.01–1.02 |
| BMI | 1.03 | 1.02 | 0.007 | 0.003 | <.001 | <.001 | 1.01–1.04 | 1.01–1.03 |
| Female | 1.32 | 1.35 | 0.096 | 0.049 | <.001 | <.001 | 1.14–1.52 | 1.26–1.45 |
| Ex-smoker | 1.14 | 1.16 | 0.085 | 0.040 | .086 | <.001 | 0.98–1.32 | 1.08–1.24 |
| Current smoker | 0.94 | 1.01 | 0.079 | 0.037 | .479 | .785 | 0.80–1.11 | 0.94–1.09 |
BMI, Body Mass Index.
Multivariate analysis: Poisson logistic regression model.
Results are expressed as incidence rate ratios.
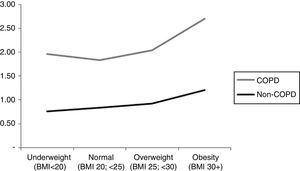
Subjects with COPD had a significantly higher comorbidity score compared to non-COPD subjects (P<.0001). Similar behavior was observed in the comorbidity score by gender, BMI, smoking habit and the presence or absence of dyspnea. Fig. 1 shows the comorbidity score in subjects with and without COPD by BMI category. It is important to highlight that for COPD subjects, the relationship between the comorbidity score and BMI is curvilinear (U-shaped), while for non-COPD subjects the score is observed to increase progressively in line with the BMI.
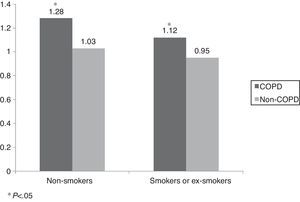
The comorbidity score in patients with and without COPD according to their exposure to tobacco smoke (non-smoker vs current smoker or ex-smoker) is shown in Fig. 2. This score was significantly higher in COPD subjects, independently of their smoking status (COPD 1.28±1.28 vs 1.12±0.05; P=.0388 and non-COPD 1.03±0.02 vs 0.95±0.02; P=.0081).
In COPD subjects, factors associated with comorbidity were age, BMI and female gender, while in the population without COPD, the above factors plus the status of ex-smoker were associated with comorbidity. Since a large number of individuals do not present comorbidities, the analysis of factors associated with comorbidity was carried out using the “incidence rate ratio” calculation, which allows the calculation of how much the risk of comorbidities increases for each variable (after adjustment for other factors) as that variable is increased by one unit. In the non-COPD population, for example, when age is increased by one year, the risk of comorbidities increases by 2% after adjusting for gender, BMI and smoking habit.
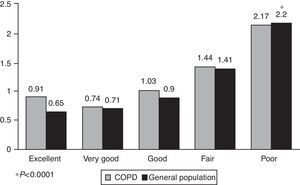
Fig. 3 shows the relationship between the comorbidity score and the general health status of subjects with COPD and the general population. In both populations, a significant increase was observed in the value of the score as the general health status deteriorated (P<.0001).
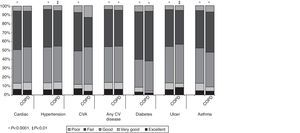
The distribution of self-reported comorbidity in COPD subjects and the general population according to general health status is shown in Fig. 4. In COPD subjects, it can be seen that the proportion of subjects self-reporting any cardiovascular disease, hypertension, heart disease, CVA, diabetes, peptic ulcer disease and asthma increases significantly as the general health status deteriorates. Similar behavior is observed in the general population. In COPD subjects, more than half of those who self-reported any cardiovascular disease, hypertension, heart disease, CVA and peptic ulcer disease considered their general health status to be between good and excellent. However, 62% of subjects who self-reported diabetes and 53.2% of those with asthma considered their general health status to be fair or poor. Similar findings were observed in the overall population.
DiscussionThe PLATINO population-based multicenter study provides information on the prevalence and impact on health status of some self-reported comorbidities in the general population and in subjects with and without COPD. The main findings of this study are the following: firstly, the number of comorbidities was significantly higher in subjects with COPD, regardless of their smoking status; secondly, the primary factors associated with comorbidity in subjects with and without COPD are age, female gender and BMI. Being an ex-smoker was also associated with a greater risk of comorbidity in the population without COPD; thirdly, the number of self-reported comorbidities is associated with a deterioration in general health status in COPD subjects and in the general population. Self-reported diabetes was the comorbidity that most affected the general health status of both populations.
Several studies have evaluated the prevalence of comorbidities in patients with COPD. Van Manen et al.14 reported a higher incidence of comorbidities in COPD patients compared to controls (73 and 64%, respectively). More than 50% of the COPD patients had 1 or 2 comorbidities, 15.8% had 3 or 4 comorbidities and 6.8% had 5 or more comorbid conditions. Other authors have described an average of 3.7 comorbidities in COPD patients vs one comorbidity in control subjects.15 In a population of 2962 subjects with COPD, Crisafulli et al.16 reported that 51% had at least one comorbidity: 61% had metabolic disease (hypertension, diabetes or dyslipidemia) and 24% had heart disease (heart failure or coronary disease).
The comorbidities most frequently reported among patients with COPD are coronary disease, hypertension, diabetes, heart failure, CVA, depression and cancer.2–5,9 The results of a population-based study selecting patients with COPD4 (1003 subjects) suggested that hypertension (55%) and hypercholesterolemia (52%) were the most common comorbidities, followed by depression (37%), cataracts (31%) and osteoporosis (28%). Angina pectoris was reported in 22% of subjects, myocardial infarction in 19%, history of any cerebrovascular event in 14% and cancer in 6% (0.5% lung cancer).4 Moreover, in subjects defined as having COPD using the criterion of long-term administration of respiratory medication, 98% were found to be receiving at least one “non-respiratory” drug and 68.4% had at least one of the following problems: cardiovascular disease, diabetes or depression.17 Data from the National Health and Nutrition Examination Survey (NHANES)10 showed that subjects with a medical diagnosis of COPD had a higher probability of reporting arthritis (54.6% vs 36.9%), depression (20.6% vs 12.5%), osteoporosis (16.9% vs 8.5%), cancer (16.5% vs 9.9%), coronary disease (12.7% vs 6.1%), heart failure (12.1% vs 3.9%) and cerebrovascular accident (8.9% vs 4.6%).
In our study, we found that the number of self-reported comorbidities expressed by a simple index was significantly higher in COPD subjects compared to subjects without COPD (comorbidity score 1.17 vs 0.99). This finding is in line with those reported previously which described a higher prevalence of comorbidities in COPD subjects. The comorbidities reported in our COPD population in decreasing order of frequency were: any cardiovascular disease (41.5%), hypertension (37.2%), peptic ulcer disease (31.8%), asthma (22.8%), heart disease (13.7%), diabetes (8.4%), cerebrovascular accident (3.2%) and lung cancer (1.1%). These results are in line with those from studies showing that cardiovascular problems are among the most common comorbid conditions in COPD. However, it is important to point out that comparison between these studies is difficult due to the differences in the population samples, the criteria used for the diagnosis of COPD and the severity of the patients. Barr et al.4 evaluated subjects with COPD and moderate to severe dyspnea, while the subjects diagnosed in the PLATINO study were mostly in the early stages of the disease. In other studies, the COPD diagnosis was made according to long-term prescription of respiratory medication5 or previous medical diagnosis,17 while in PLATINO, the diagnosis was made according to the GOLD criterion, thus providing greater diagnostic accuracy in view of the high frequency of erroneous prior diagnoses (69%) previously reported.18
The increase in comorbidities with age has been reported both in general population-based studies and in subjects with COPD.17 Some comorbidities, such as osteoporosis, psychiatric disorders, heart failure and diabetes, are more common in women, while others, such as ischemic heart disease, occur more frequently in men.19 A meta-analysis published by Guh et al.20 concluded that obesity is associated with a higher number of comorbidities. Moreover, in an analysis of the studies Atherosclerosis Risk in Communities Study (ARIC) and Cardiovascular Health Study (CHS), Mannino et al.10 found that older age and higher BMI, a lower level of education and male gender were associated with a greater risk of diabetes, hypertension and cardiovascular disease. They also reported that impaired lung function was associated with greater comorbidity. In logistic regression models adjusted for age, gender, race, smoking habit, BMI and education, subjects with COPD GOLD grade 3–4 had a higher incidence of diabetes (OR: 1.5; 95% CI: 1.1–1.9), hypertension (OR: 1.6; 95% CI: 1.3–1.9) and cardiovascular disease (OR: 2.4; 95% CI: 1.9–3.0).
Our study results suggest that factors associated with the presence of comorbidities in subjects with COPD are age, female gender and being overweight. These data are in line with reports of comorbidities increasing with age17 and obesity.10,20 The curvilinear relationship between the comorbidity score and BMI seen in the COPD group allows us to speculate on the adverse effect of low weight in these patients (unhealthy low weight). The relationship between comorbidity and gender in COPD varies depending on the series.10,21 COPD data from a national sample from the United States indicated that the prevalence of comorbidities was similar between men and women, except for depression, osteoporosis and cardiovascular diseases.4 Another study reported a higher incidence of comorbidity in men with COPD.21 In addition to the Charlson index, Almagro et al.19 used a self-reported comorbidity questionnaire to evaluate the frequency and type of comorbidities in COPD patients according to gender. The mean Charlson index was 2.7 (2.0), with no differences for gender; in contrast, the mean number of all comorbid conditions analyzed was 3.7 (1.7) in men and 1.8 (1.8) in women (P<.05). In a previous publication on gender and COPD using the PLATINO data, we reported a greater number of comorbidities in women, even those without COPD. As we already discussed in that study,22 a possible explanation for the greater comorbidity in women could be the limited number of comorbid conditions offered in PLATINO and the higher BMI of the sample. In subjects without COPD, ex-smoker status is associated with comorbidity, but not in those with the disease. Other authors have also found no associations between the smoking status and the frequency of comorbidities in COPD subjects.5 The lack of association between smoking status and the frequency of comorbidities in COPD is difficult to interpret; however, it may be explained in part by the presence of systemic inflammation in COPD23 and, as some authors maintain, the association between COPD and comorbidities may be the result of the “spill-over” of pulmonary inflammatory mediators into the circulation (with the disease remaining the center of the process).24 Another possible explanation may be the presence of common genetic factors predisposing to COPD and comorbid diseases. In non-COPD subjects, smoking itself is a factor in systemic inflammation. The analysis of these factors is beyond the scope of the design of this study, but there is no doubt that it is an important area for future research.
Some studies have suggested that comorbidities affect the quality of life of the general population.25 This effect has also been studied in COPD populations, including gender-related differences.21 In a recent study, Naberan et al.26 reported the impact of anxiety and depression on the quality of life of women with COPD, but no other comorbidities were included in the analysis. Sundh et al.27 found that the presence of cardiac comorbidity, depression and low body weight were independent factors associated with a poor quality of life in COPD patients. Other authors have reported that depression is the comorbidity which most affects quality of life in these patients and that the impact of cardiovascular comorbidities and diabetes is lower.28
No data are available on the impact of comorbidities on the health status in unselected samples of COPD subjects. Previously published data from the PLATINO study show that the perceived general health status of these patients is poor29 and that the perception differs between men and women.22 In the PLATINO population which presented comorbidities, a poor perceived health status was found both in COPD subjects and in the general population. Over half of the subjects with hypertension or cardiovascular, cardiac, peptic ulcer or cerebrovascular disease said that their health status was good to excellent. However, subjects with diabetes classified their health status as fair to poor. This is one more aspect which speaks of the difference between the comorbidities and their impact, not only on mortality.12
This study has some limitations which must be pointed out. In the original PLATINO questionnaire, only certain comorbidities were recorded: other disorders frequently associated with COPD,12 such as osteoporosis, malignant neoplasms other than lung cancer, depression and anxiety, were not included. However, some of the comorbid conditions that have been associated with higher mortality in COPD12 are included: cardiovascular, cardiac or peptic ulcer disease, diabetes and lung cancer. Another limitation is that the diagnosis of comorbidity is self-reported and underdiagnosis in chronic diseases is not unique to COPD.
Recently, Almagro et al.19 used, in addition to the Charlson index, a self-reported comorbidity questionnaire as a predictor of mortality in COPD, showing that they are related with short-term prognosis. The PLATINO follow-up study included questionnaires on anxiety and depression. These data will probably allow new analyses to be performed in the future and some of these questions may be clarified.30
In conclusion, the results of this study confirm the presence of greater comorbidity in subjects with COPD from a non-selected population (independently of their smoking status), and justifies the active search for these concomitant diseases. Age, female gender and a higher BMI are the main factors associated with comorbidity in these patients. Comorbidities, and in particular diabetes, are associated with a deterioration in general health status, independently of COPD status.
Conflict of InterestsThe authors declare that they do not have any conflict of interests.
María Márquez, María Blanco, Fernanda Rosa, Aquiles Camelier.
PLATINO team members are listed in Annex 1.
Please cite this article as: López Varela MV, Montes de Oca M, Halbert R, Muiño A, Tálamo C, Pérez-Padilla R, et al. Comorbilidades y estado de salud en individuos con y sin EPOC en 5 ciudades de América Latina: Estudio PLATINO. Arch Bronconeumol. 2013;49:468–474.