There is conflicting information regarding the severity and outcomes of COVID-19 in people living with human immunodeficiency virus (PLHIV). Data from previously published matched case–control studies showed no differences in severity or outcome between HIV-infected and HIV-negative patients.1–3 Also, a cohort study of 2988 HIV-infected patients evaluating the association between HIV and COVID-19 diagnoses, hospitalisation, and in-hospital death in New York State reported poor outcomes in HIV-infected patients compared with HIV-negative patients.4 There are no specific studies investigating the severity and outcomes of critically ill patients with COVID-19 and HIV-infection. We aim to investigate whether the clinical presentation, severity and outcomes of COVID-19 in critically ill HIV-infected patients were comparable to those seen in non-HIV-infected patients.
The CIBERESUCICOVID is a multicentre, observational, prospective/retrospective cohort study (NCT04457505) of 6512 consecutive patients with SARS-CoV-2 infection who were admitted to 69 ICUs in Spain between February 2020 and July 2022. Approved by the ethical committee – HCB/2020/0370. Data was collected as previously described.5 The primary outcome was all-cause 90-day mortality. Secondary outcomes included all-cause in-hospital, 15-day, 30-day and 1-year mortality, length of ICU and hospital stay, and ventilator and ICU-free days. Propensity score matching (PSM) method6,7 was used to obtain the balance among baseline variables between patients with and without HIV. We used a 1:2 nearest-neighbour matching with age, sex, number of comorbidities and SOFA as covariates and an exact matching constraint on wave of COVID-19 and centre, without replacement and within a calliper width of 0.6. We estimated the 15-, 30- and 90-day, and 1-year mortality with 95% confidence intervals (CI).8 We analysed the association between HIV infection and mortality by means of Cox regression analyses. Survival curves of patients with and without HIV were obtained using the Kaplan–Meier method and compared using the Gehan–Breslow–Wilcoxon test.9
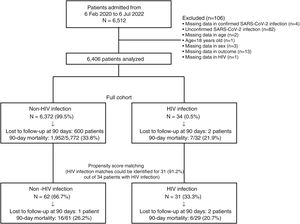
Fig. 1 depicts patients flow-chart and Table 1 describes the full cohort (N=6406: 6372 HIV-negative and 34 PLHIV) and the propensity score matching (N=93, 62 HIV-negative and 31 PLHIV) cohort. In the full cohort, patients with HIV infection were younger, presented higher numbers of comorbidities, were more frequently current smokers, alcohol abusers, had higher APACHE II scores at ICU admission than patients without HIV infection. After PSM, no significant differences were found in age, number of comorbidities, proportion of smokers or alcohol abusers. However, the APACHE II score was higher in PLHIV (Table 1). Sex and SOFA did not differ between groups before and after PSM. In the full cohort, cardiac ischaemia was the only complication that differed between groups, showing a higher rate in the HIV infection group compared to non-HIV infection group. After PSM, no significant differences were found in any complication. Finally, all outcomes did not differ between groups before and after PSM. In the full cohort, the 15-, 30-, 90-day, and 1-year mortality rates were 12% (95% CI, 12–13%), 25% (95% CI, 23–26%), 34% (95% CI, 33–35%) and 39% (95% CI, 38–40%) in the non-HIV infection group, compared with 16% (95% CI, 3–28%), 22% (95% CI, 8–36%), 22% (95% CI, 8–36%) and 27% (95% CI, 11–42%) in the HIV infection group, respectively. After PSM, the 15-, 30-, 90-day, and 1-year mortality rates were 10% (95% CI, 2–17%), 21% (95% CI, 11–31%), 26% (95% CI, 15–37%) and 30% (95% CI, 18–42%) in the non-HIV infection group, compared with 14% (95% CI, 1–26%), 21% (95% CI, 6–35%), 21% (95% CI, 6–35%) and 26% (95% CI, 9–42%) in the HIV infection group, respectively. In the full cohort, Cox regression analyses for 15-, 30-, 90-day, and 1-year mortality did not find significant differences between groups, giving HRs of 1.24 (95% CI, 0.52–2.99), 0.92 (95% CI, 0.44–1.94), 0.66 (95% CI, 0.32–1.40) and 0.73 (95% CI, 0.36–1.46), respectively. After PSM, Cox regression analyses for 15-, 30-, 90-day, and 1-year mortality did not find significant differences between groups, giving HRs of 1.40 (95% CI, 0.39–4.95), 1.01 (95% CI, 0.38–2.65), 0.81 (95% CI, 0.32–2.07) and 0.89 (95% CI, 0.37–2.15), respectively.
Demographics and clinical characteristics at admission, and complications and outcomes during ICU admission.
| Variables | Full cohort (N=6406) | Propensity score matching (N=93) | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Non-HIV infection (N=6372) | HIV infection (N=34) | P-value | No | Non-HIV infection (N=62) | HIV infection (N=31) | P-value | |
| Waves of COVID-19 | 6406 | 0.729 | 93 | >0.999 | ||||
| First: 8/1/2020 to 9/5/2020 | 3048 (48) | 18 (53) | – | 32 (52) | 16 (52) | – | ||
| Second: 10/5/2020 to 7/12/2020 | 1839 (29) | 11 (32) | – | 22 (35) | 11 (35) | – | ||
| Third: 8/12/2020 to 9/3/2021 | 873 (14) | 5 (15) | – | 8 (13) | 4 (13) | – | ||
| Fourth: 10/3/2021 to 15/6/2021 | 187 (3) | 0 (0) | – | 0 (0) | 0 (0) | – | ||
| Fifth: 16/6/2021 to 13/10/2021 | 245 (4) | 0 (0) | – | 0 (0) | 0 (0) | – | ||
| Sixth: 14/10/2021 to 27/3/2022 | 163 (3) | 0 (0) | – | 0 (0) | 0 (0) | – | ||
| Seventh or higher: 28/3/2022 to 6/7/2022 | 16 (0.3) | 0 (0) | – | 0 (0) | 0 (0) | – | ||
| Age, median (Q1;Q3), years | 6406 | 63 (54; 71) | 51 (47; 65) | <0.001 | 93 | 57.5 (46; 64) | 51 (48; 65) | 0.619 |
| Male sex,n(%) | 6406 | 4484 (70) | 26 (76) | 0.437 | 93 | 46 (74) | 23 (74) | >0.999 |
| BMI, median (Q1;Q3), kg/m2 | 5631 | 28.9 (26; 32.3) | 26.9 (23.6; 33.1) | 0.174 | 77 | 29.4 (26.5; 31.9) | 26.9 (23.9; 34.6) | 0.277 |
| Number of comorbidities, median (Q1;Q3) | 5745 | 2 (1; 3) | 2.5 (2; 4) | <0.001 | 84 | 3 (1; 4) | 2 (2; 4) | 0.931 |
| Comorbidities,n(%) | ||||||||
| Diabetes mellitus | 6406 | 1577 (25) | 11 (32) | 0.306 | 93 | 24 (39) | 8 (26) | 0.217 |
| Hypertension | 6405 | 3208 (50) | 18 (53) | 0.763 | 93 | 41 (66) | 15 (48) | 0.099 |
| Metabolic disease | 6396 | 1955 (31) | 8 (24) | 0.364 | 93 | 35 (56) | 7 (23) | 0.002 |
| Chronic liver disease | 6405 | 225 (4) | 4 (12) | 0.032 | 93 | 3 (5) | 3 (10) | 0.397 |
| Chronic heart disease | 6405 | 819 (13) | 3 (9) | 0.614 | 93 | 21 (34) | 3 (10) | 0.012 |
| Chronic lung disease | 6406 | 985 (15) | 4 (12) | 0.811 | 93 | 16 (26) | 3 (10) | 0.069 |
| Chronic renal failure | 6405 | 455 (7) | 3 (9) | 0.732 | 93 | 8 (13) | 2 (6) | 0.487 |
| Immunosuppressiona | 6403 | 237 (4) | 34 (100) | <0.001 | 93 | 5 (8) | 31 (100) | <0.001 |
| Solid transplantation | 109 (2) | 2 (6) | 0.117 | 3 (5) | 2 (6) | >0.999 | ||
| Bone transplantation | 6 (0.1) | 0 (0) | >0.999 | 0 (0) | 0 (0) | – | ||
| AIDS or HIV infection | 0 (0) | 34 (100) | – | 0 (0) | 31 (100) | – | ||
| Other | 147 (2) | 1 (3) | 0.549 | 2 (3) | 1 (3) | >0.999 | ||
| Smoking habit | 5915 | 0.007 | 88 | 0.396 | ||||
| No smoke | 3706 (63) | 17 (55) | 0.349 | 31 (53) | 16 (55) | – | ||
| Current smoke | 347 (6) | 6 (19) | 0.009 | 7 (12) | 6 (21) | – | ||
| Former smoke | 1831 (31) | 8 (26) | 0.524 | 21 (36) | 7 (24) | – | ||
| Alcohol abuse (current or former) | 5847 | 317 (5) | 7 (23) | <0.001 | 85 | 4 (7) | 5 (18) | 0.148 |
| Nursing-home,n(%) | 6252 | 103 (2) | 1 (3) | 0.416 | 91 | 0 (0) | 1 (3) | 0.319 |
| Previous 30 days admission,n(%) | 6404 | 229 (4) | 3 (9) | 0.124 | 93 | 5 (8) | 2 (6) | >0.999 |
| Days since initial symptoms to ICU admission, median (Q1;Q3) | 6365 | 9 (7; 12) | 7 (5; 10) | 0.013 | 93 | 8.5 (6; 12) | 9 (5; 10) | 0.508 |
| Treatment before admission,n(%) | ||||||||
| Angiotensin-converting enzyme inhibitor | 3175 | 1225 (39) | 5 (29) | 0.429 | 55 | 21 (51) | 2 (14) | 0.016 |
| Statin | 6366 | 1983 (31) | 12 (35) | 0.618 | 91 | 26 (42) | 10 (32) | 0.366 |
| Non-steroidal anti-inflammatory drug | 6280 | 755 (12) | 2 (6) | 0.422 | 90 | 13 (21) | 2 (7) | 0.131 |
| Corticosteroids | 6363 | 521 (8) | 3 (9) | 0.750 | 90 | 9 (15) | 3 (10) | 0.744 |
| Antibiotics | 6358 | 860 (14) | 5 (15) | 0.802 | 93 | 11 (18) | 4 (13) | 0.550 |
| SARS-CoV-2 vaccine | 3561 | 269 (8) | 0 (0) | 0.622 | 43 | 0 (0) | 0 (0) | – |
| Two doses of SARS-CoV-2 vaccine | 216 | 151 (70) | 0 (0) | <0.001 | 0 | 0 (0) | 0 (0) | – |
| Characteristics at ICU admission | ||||||||
| Glasgow Coma Scale, median (Q1; Q3) | 5196 | 15 (15; 15) | 15 (15; 15) | 0.664 | 74 | 15 (15; 15) | 15 (15; 15) | 0.652 |
| APACHE-II score, median (Q1; Q3) | 3708 | 12 (9; 15) | 16.5 (14; 20) | <0.001 | 51 | 10 (6; 13) | 16.5 (14; 18) | <0.001 |
| SOFA score, median (Q1; Q3) | 4416 | 5 (3; 7) | 5.5 (4; 8) | 0.288 | 61 | 4 (3; 7) | 5.5 (4; 8) | 0.297 |
| SOFA haemodynamic component, median (Q1; Q3) | 6165 | 0 (0; 4) | 0 (0; 4) | 0.890 | 87 | 0 (0; 4) | 0 (0; 4) | 0.779 |
| SOFA renal component, median (Q1; Q3) | 6305 | 0 (0; 0) | 0 (0; 1) | 0.052 | 93 | 0 (0; 1) | 0 (0; 1) | 0.614 |
| Temperature, median (Q1; Q3), °C | 5982 | 36.7 (36; 37.5) | 36.8 (35.8; 37.8) | 0.693 | 88 | 36.8 (36; 37.7) | 37 (35.9; 38) | 0.572 |
| Respiratory rate, median (Q1; Q3), bpm | 5719 | 25 (21; 30) | 26 (23; 35) | 0.153 | 81 | 24.5 (22; 30) | 25 (23; 35) | 0.379 |
| Arterial blood gases at ICU admission | ||||||||
| PaO2/FiO2ratio, median (Q1; Q3) | 5351 | 112 (80; 164) | 94 (79; 151) | 0.343 | 77 | 112 (79; 176) | 92.5 (79; 151) | 0.176 |
| PaO2/FiO2ratio in ventilated patients, median (Q1; Q3) | 5063 | 111 (80; 162) | 94 (79; 148) | 0.367 | 70 | 107 (79; 174) | 89 (76; 130) | 0.145 |
| PaO2/FiO2ratio categories in ventilated patients, n (%) | 5063 | 0.055 | 70 | 0.222 | ||||
| Severe (<100) | 2126 (42) | 15 (60) | – | 22 (47) | 15 (65) | – | ||
| Moderate (≥100 to <200) | 2160 (43) | 4 (16) | – | 15 (32) | 3 (13) | – | ||
| Mild (≥200 to <300) | 546 (11) | 4 (16) | – | 3 (6) | 3 (13) | – | ||
| No ARDS (≥300) | 206 (4) | 2 (8) | – | 7 (15) | 2 (9) | – | ||
| pH, median (Q1; Q3) | 5787 | 7.41 (7.34; 7.46) | 7.38 (7.3; 7.42) | 0.094 | 86 | 7.42 (7.36; 7.46) | 7.38 (7.31; 7.43) | 0.142 |
| PaCO2, median (Q1; Q3), mmHg | 5628 | 39 (34; 46.8) | 38.5 (31; 59.2) | 0.869 | 81 | 38 (34.1; 43) | 39.8 (31; 60) | 0.504 |
| PaCO2in ventilated patients, median (Q1; Q3), mmHg | 5265 | 39.3 (34; 47) | 38.5 (31; 59.2) | 0.811 | 71 | 38 (34.2; 45.5) | 41 (31; 60) | 0.535 |
| Laboratory findings at ICU admission | ||||||||
| Haemoglobin, median (Q1; Q3), g/dL | 6144 | 13.2 (12; 14.4) | 12.4 (11; 14) | 0.037 | 92 | 12.3 (11.5; 14.8) | 12.5 (11; 14.1) | 0.661 |
| Leucocyte count, median (Q1; Q3), 109/L | 6293 | 8.9 (6.4; 12.5) | 8.9 (7.1; 14) | 0.516 | 93 | 8.1 (6.1; 12.1) | 8.7 (7.1; 14) | 0.401 |
| Lymphocyte count, median (Q1; Q3), 109/L | 6202 | 0.69 (0.47; 0.98) | 0.79 (0.6; 1.4) | 0.032 | 93 | 0.73 (0.4; 1.2) | 0.73 (0.53; 1.4) | 0.498 |
| Neutrophil count, median (Q1; Q3), 109/L | 6171 | 7.7 (5.2; 11.1) | 7.4 (5.7; 12.5) | 0.696 | 93 | 7.1 (4.7; 11.3) | 7.3 (5.7; 12.9) | 0.436 |
| Monocyte count, median (Q1; Q3), 109/L | 5988 | 0.36 (0.2; 0.53) | 0.35 (0.28; 0.5) | 0.569 | 92 | 0.4 (0.2; 0.54) | 0.33 (0.25; 0.5) | 0.822 |
| Platelet count, median (Q1; Q3), 109/L | 6291 | 232 (177; 303) | 222 (144; 299) | 0.210 | 93 | 212 (156; 272) | 230 (160; 316) | 0.483 |
| D-dimer, median (Q1; Q3), ng/mL | 5376 | 980 (500; 2300) | 949 (522; 1855) | 0.737 | 82 | 647 (356; 2250) | 1100 (544; 1970) | 0.200 |
| C-reactive protein, median (Q1; Q3), mg/L | 5977 | 127 (61; 219) | 167 (82; 245) | 0.240 | 91 | 111 (69; 194) | 172 (91; 221) | 0.065 |
| Serum creatinine, median (Q1; Q3), mg/dL | 6303 | 0.83 (0.67; 1.08) | 0.99 (0.78; 1.51) | 0.037 | 93 | 0.89 (0.74; 1.5) | 0.99 (0.72; 1.51) | 0.741 |
| LDH, median (Q1; Q3), U/L | 5203 | 477 (362; 654) | 465 (351; 529) | 0.194 | 81 | 439 (303; 591) | 472 (357; 589) | 0.347 |
| Ferritin, median (Q1; Q3), ng/mL | 3341 | 1151 (609; 1915) | 921 (339; 1756) | 0.520 | 48 | 800 (473; 1365) | 921 (339; 1756) | 0.556 |
| Respiratory support at ICU admissionb | 6391 | 0.376 | 93 | 0.531 | ||||
| Conventional oxygen therapy | 442 (7) | 4 (12) | – | 8 (13) | 3 (10) | – | ||
| High-flow nasal cannula | 1760 (28) | 12 (35) | – | 27 (44) | 11 (35) | – | ||
| Non-invasive mechanical ventilation | 750 (12) | 2 (6) | – | 1 (2) | 2 (6) | – | ||
| Invasive mechanical ventilation | 3405 (54) | 16 (47) | – | 26 (42) | 15 (48) | – | ||
| Septic shock at ICU admissionc | 5864 | 428 (7) | 3 (10) | 0.494 | 87 | 4 (7) | 1 (4) | >0.999 |
| Complications during ICU admission,n(%) | ||||||||
| Bacterial pneumoniad | 6366 | 1748 (28) | 11 (33) | 0.463 | 92 | 21 (34) | 10 (33) | 0.959 |
| Pneumothorax | 6394 | 511 (8) | 2 (6) | >0.999 | 93 | 2 (3) | 2 (6) | 0.598 |
| Pleural effusion | 6389 | 658 (10) | 6 (18) | 0.159 | 93 | 5 (8) | 5 (16) | 0.292 |
| Organising pneumonia | 6307 | 291 (5) | 3 (9) | 0.186 | 91 | 3 (5) | 3 (10) | 0.379 |
| Tracheobronchitis | 6358 | 55 (1) | 0 (0) | >0.999 | 91 | 2 (3) | 0 (0) | >0.999 |
| Pulmonary embolism | 6245 | 594 (10) | 2 (6) | 0.765 | 91 | 8 (13) | 2 (7) | 0.488 |
| Endocarditis | 6391 | 20 (0.3) | 0 (0) | >0.999 | 92 | 0 (0) | 0 (0) | – |
| Myocarditis/pericarditis | 6396 | 107 (2) | 0 (0) | >0.999 | 92 | 0 (0) | 0 (0) | – |
| Cardiomyopathy | 6394 | 118 (2) | 0 (0) | >0.999 | 92 | 0 (0) | 0 (0) | – |
| Heart failure | 6395 | 146 (2) | 0 (0) | >0.999 | 93 | 0 (0) | 0 (0) | – |
| Cardiac ischaemia | 6393 | 114 (2) | 3 (9) | 0.024 | 93 | 1 (2) | 3 (10) | 0.106 |
| Bacteraemia | 6386 | 1645 (26) | 5 (15) | 0.160 | 92 | 16 (26) | 5 (17) | 0.328 |
| Stroke | 6392 | 111 (2) | 0 (0) | >0.999 | 93 | 0 (0) | 0 (0) | – |
| Delirium | 6380 | 1198 (19) | 8 (24) | 0.490 | 93 | 11 (18) | 8 (26) | 0.363 |
| Coagulation disordere | 6384 | 1975 (31) | 8 (24) | 0.396 | 92 | 19 (31) | 7 (23) | 0.465 |
| Disseminated intravascular coagulationf | 1944 | 806 (42) | 3 (38) | >0.999 | 26 | 9 (47) | 2 (29) | 0.658 |
| Anaemiag | 6398 | 3939 (62) | 22 (65) | 0.736 | 93 | 40 (65) | 20 (65) | >0.999 |
| Rhabdomyolysis | 6374 | 225 (4) | 1 (3) | >0.999 | 93 | 1 (2) | 1 (3) | >0.999 |
| Acute renal failureh | 6397 | 2081 (33) | 11 (32) | 0.965 | 93 | 25 (40) | 10 (32) | 0.449 |
| Pancreatitis | 6384 | 57 (1) | 0 (0) | >0.999 | 93 | 1 (2) | 0 (0) | >0.999 |
| Liver dysfunction | 6396 | 2036 (32) | 7 (21) | 0.155 | 93 | 17 (27) | 7 (23) | 0.615 |
| Hyperglycaemia | 6394 | 4687 (74) | 24 (71) | 0.682 | 93 | 46 (74) | 23 (74) | >0.999 |
| Haemorrhage | 6395 | 481 (8) | 1 (3) | 0.514 | 93 | 6 (10) | 1 (3) | 0.418 |
| Outcomes | ||||||||
| In-hospital mortality, n (%) | 6406 | 1950 (31) | 8 (24) | 0.372 | 93 | 16 (26) | 7 (23) | 0.734 |
| 15-Day mortality, n (%)i | 6191 | 769 (12) | 5 (16) | 0.589 | 91 | 6 (10) | 4 (14) | 0.720 |
| 30-Day mortality, n (%)j | 5998 | 1463 (25) | 7 (22) | 0.728 | 91 | 13 (21) | 6 (21) | 0.976 |
| 90-Day mortality, n (%)k | 5804 | 1952 (34) | 7 (22) | 0.154 | 90 | 16 (26) | 6 (21) | 0.568 |
| 1-Year mortality, n (%)l | 5191 | 2015 (39) | 8 (27) | 0.166 | 83 | 17 (30) | 7 (26) | 0.677 |
| Length of ICU stay, median (Q1; Q3), days | ||||||||
| All patients | 6402 | 14 (7; 27) | 11.5 (7; 20) | 0.404 | 94 | 17 (8; 29) | 12 (7; 23) | 0.392 |
| Surviving patients | 4445 | 13 (7; 27) | 11.5 (7; 23) | 0.659 | 70 | 17 (7; 29) | 12 (7.5; 25.5) | 0.642 |
| Length of hospital stay, median (Q1; Q3), days | ||||||||
| All patients | 6400 | 23 (14; 39) | 18 (12; 40) | 0.477 | 93 | 25 (15; 43) | 22 (12; 42) | 0.729 |
| Surviving patients | 4446 | 26 (16; 44) | 25.5 (15; 42) | 0.699 | 70 | 26.5 (15; 45) | 27.5 (15; 42.5) | 0.995 |
| Ventilator-free days, median (Q1; Q3) | 4900 | 0 (0; 16) | 9 (0; 18) | 0.306 | 66 | 0 (0; 14) | 9 (0; 18) | 0.399 |
| Invasive mechanical ventilation length, median (Q1; Q3), daysm | ||||||||
| All patients | 4714 | 15 (8; 27) | 13 (8; 19) | 0.348 | 62 | 17.5 (9; 25.5) | 13 (9; 19) | 0.205 |
| Surviving patients | 2939 | 14 (8; 27) | 13 (9; 19) | 0.582 | 43 | 20 (9; 26) | 13.5 (9; 23) | 0.399 |
| ICU-free days, median (Q1; Q3) | 6403 | 3 (0; 19) | 12 (0; 20) | 0.339 | 93 | 2.5 (0; 19) | 12 (0; 19) | 0.567 |
| Readmission, n (%)n | 3254 | 417 (13) | 5 (21) | 0.227 | 62 | 4 (10) | 4 (18) | 0.438 |
| Chest X-ray, n (%)o | ||||||||
| Abnormal | 3171 | 2845 (90) | 23 (96) | 0.723 | 62 | 35 (88) | 21 (95) | 0.409 |
| Persistent infiltrates | 3171 | 71 (2) | 0 (0) | >0.999 | 62 | 2 (5) | 0 (0) | 0.535 |
| Diffuse interstitial lung disease | 3171 | 12 (0.4) | 0 (0) | >0.999 | 62 | 0 (0) | 0 (0) | – |
| Fibrotic tract | 3171 | 69 (2) | 0 (9) | >0.999 | 62 | 2 (5) | 0 (0) | 0.535 |
| Emphysema | 3171 | 5 (0.2) | 0 (0) | >0.999 | 62 | 1 (3) | 0 (0) | >0.999 |
| Others | 3171 | 50 (2) | 1 (4) | 0.323 | 62 | 1 (3) | 1 (5) | >0.999 |
| CT scan, n (%)o | ||||||||
| Abnormal | 3172 | 3020 (96) | 24 (100) | 0.623 | 62 | 39 (98) | 22 (100) | >0.999 |
| Persistent infiltrates | 3172 | 202 (6) | 1 (4) | >0.999 | 62 | 5 (13) | 1 (5) | 0.409 |
| Diffuse interstitial lung disease | 3172 | 35 (1) | 0 (0) | >0.999 | 62 | 1 (3) | 0 (0) | >0.999 |
| Fibrotic tract | 3172 | 183 (6) | 0 (0) | 0.397 | 62 | 2 (5) | 0 (0) | 0.535 |
| Emphysema | 3172 | 58 (2) | 0 (0) | >0.999 | 62 | 0 (0) | 0 (0) | – |
| Pulmonary embolism | 3172 | 7 (0.2) | 0 (0) | >0.999 | 62 | 0 (0) | 0 (0) | – |
| Others | 3172 | 258 (8) | 2 (8) | >0.999 | 62 | 4 (10) | 2 (9) | >0.999 |
Abbreviations: HIV indicates human immunodeficiency virus; Q1, first quartile; Q3, third quartile; BMI, body mass index; AIDS, acquired immunodeficiency syndrome; ICU, intensive care unit; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; LDH, lactate dehydrogenase. Percentages calculated on non-missing data. P-values marked in bold indicate numbers that are statistically significant at the 95% confidence limit.
Patients who received high-flow nasal cannula but needed non-invasive mechanical ventilation were included in the non-invasive mechanical ventilation group. Patients who received high-flow nasal cannula and/or non-invasive ventilation but needed intubation were included in the invasive mechanical ventilation group.
Criteria for the Sepsis-3 definition of septic shock include vasopressor treatment and a lactate concentration>2mmol/L at ICU admission.
Clinically or radiologically diagnosed bacterial pneumonia managed with antimicrobials. Bacteriologic confirmation was not required.
Abnormal coagulation was identified by abnormal prothrombin time or activated partial thromboplastin time.
Disseminated intravascular coagulation was defined by thrombocytopenia, prolonged prothrombin time, low fibrinogen, elevated D-dimer and thrombotic microangiopathy.
Acute renal injury was defined as either an increase in serum creatinine by ≥0.3mg/dL within 48h or an increase in serum creatinine to ≥1.5 times that at baseline.
Calculated only for patients with 15-day follow-up in the full cohort (6159 in the non-HIV infection group and 32 in the HIV infection group) and in the propensity score matching (62 in the non-HIV infection group and 29 in the HIV infection group).
Calculated only for patients with 30-day follow-up in the full cohort (5966 in the non-HIV infection group and 32 in the HIV infection group) and in the propensity score matching (62 in the non-HIV infection group and 29 in the HIV infection group).
Calculated only for patients with 90-day follow-up in the full cohort (5772 in the non-HIV infection group and 32 in the HIV infection group) and in the propensity score matching (61 in the non-HIV infection group and 29 in the HIV infection group).
Calculated only for patients with 1-year follow-up in the full cohort (5161 in the non-HIV infection group and 30 in the HIV infection group) and in the propensity score matching (56 in the non-HIV infection group and 27 in the HIV infection group).
Of the 34 PLHIV included in the study, 26 (76%) were males, with a median (IQR) age of 51 (47; 65) years. Data on the probable route of exposure were available in 47% of cases. HIV infection was acquired by men who have sex with men and intravenous drug use in 7 (44%) and 5 (31%) cases, respectively. Data on available ART therapy was available in 29 cases (85%). Twenty-five out of 29 cases (86%) were on antiretroviral therapy (ART) at the time of hospital admission. ART regimens were based on integrase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, and other regimens in 11 (44%), 9 (36%), 3 (12%), and 2 (8%) cases, respectively. Data on HIV-RNA viral load in plasma was available in 27 patients (79%). Twenty-one out of 27 patients (84%) had an undetectable HIV-RNA viral load in plasma (<50copies/mL). Data on CD4+ T cell count was available in 21 patients (62%). The median (IQR) last CD4+ T cell count was 518 (421; 823)/mm3. The percentage of PLHIV with less than 200 CD4cells/mm3 was 4.7%. Three patients (10%) presented co-infection with HCV, and three patients (10%) had HBV co-infection. There were also no differences regarding outcomes between HIV-infected patients with undetectable and detectable HIV viral load.
This is the first national multicentre study that describes the clinical characteristics and outcomes of critical COVID-19 in PLHIV admitted to the ICU and compares them with the HIV-negative general population, concluding that the short- and medium-term prognosis is similar in PLHIV who are virological suppressed on ART and are not immunosuppressed (CD4≥200cells/mm3). In our study PLHIV accounted for approximately 0.5% of total hospitalised, critically ill COVID-19 cases in the ICU. This data is in line with previous studies.2–4 Despite PLHIV presenting higher APACHE-II scores at ICU admission, outcomes did not differ between groups before and after PSM (in-hospital, 30-, 90-day and 1-year mortality). Similar results were reported by previous studies in hospitalised patients.10–12 Data from a recent systemic review and meta-analysis that included data from 28 studies found no difference in the risk of death between HIV-infected patients and HIV-negative (OR, 1.09; 95% CI, 0.93–1.26; P>0.001).12
The major strengths of this study include its multicentre nature, the consecutive inclusion of all patients from each unit, thorough checking of data quality, and the high number of patients analysed and long-term follow-up. On the other hand, despite exhaustive propensity score analysis for underlying conditions, a possible limitation of the propensity score methods is their inability to control for unmeasured confounding. A main limitation is the small number of cases, precluding any robust conclusions. In particular, the analysis of outcomes using this size sample may have led to a large type-II error that prevents us from generalising our results. However, there are no similar studies investigating the severity and outcomes of critically ill patients with COVID-19 and HIV-infection, so this information is of value. Also, these results do not apply to PLHIV who are off ART and/or are immunosuppressed, because they are underrepresented in our study. Finally, as we examined real-world data, limitations associated to their observational nature and missing data should be considered.
Although limited by the small sample size, our main conclusion is that critically ill COVID-19 in non-immunosuppressed and virologically suppressed HIV-infected individuals seems to present neither a more severe disease nor a worse clinical outcome than HIV-negative patients. Further research in large cohorts should be encouraged to improve our knowledge on the impact of SARS-CoV-2 in critically ill HIV-infected patients.
Authors’ contributionsAll the authors contributed to the conception and design, acquisition of data, drafting of the article, critical revision, and final approval of the manuscript.
FundingFinancial support was provided by Instituto de Salud Carlos III de Madrid (COV20/00110, ISCIII); Fondo Europeo de Desarrollo Regional (FEDER); “Una manera de hacer Europa”; Centro de Investigación Biomedica En Red–Enfermedades Respiratorias (CIBERES); and Donation program “estar preparados” UNESPA, Madrid, Spain. JMM received a personal 80:20 research grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–23. CC received a grant from the Fondo de Investigación Sanitaria (PI19/00207), Instituto de Salud Carlos III, co-funded by the European Union.
Conflict of interestsJMM has received consulting honoraria and/or research grants from AbbVie, Angelini, Contrafect, Cubist, Genentech, Gilead Sciences, Jansen, Lysovant, Medtronic, MSD, Novartis, Pfizer, and ViiV Healthcare, outside the submitted work. All other authors have no conflicts.
We want to express our appreciation to all CIBERESUCICOVID members for their contribution to this work and their precious task and effort in clinical care and daily practice with all our patients.
Víctor D. Gumucio-Sanguino, Rafael Mañez: Hospital Universitario de Bellvitge, Barcelona. Jordi Solé-Violan, Felipe Rodríguez de Castro: Hospital Dr. Negrín, Las Palmas. Fernando Suarez-Sipmann: Hospital Universitario La Princesa, Madrid. Ruth Noemí Jorge García, María Mora Aznar: Hospital Nuestra Señora de Gracia, Zaragoza. Mateu Torres, María Martínez, Cynthia Alegre, Sofía Contreras: Hospital Universitari Vall d’Hebron, Barcelona, Javier Trujillano, Montse Vallverdú, Miguel León, Mariona Badía, Begoña Balsera, Lluís Servià, Judit Vilanova, Silvia Rodríguez, Neus Montserrat, Silvia Iglesias, Javier Prados, Sula Carvalho, Mar Miralbés, Josman Monclou, Gabriel Jiménez, Jordi Codina, Estela Val, Pablo Pagliarani, Jorge Rubio, Dulce Morales, Andrés Pujol, Àngels Furro, Beatriz García, Gerard Torres, José Javier Vengoechea, David de Gonzalo Calvo, Jessica González, Silvia Gómez: Hospital Universitari Arnau de Vilanova, Lleida. José M. Gómez: Hospital General Universitario Gregorio Marañón, Madrid. José Barberán: Hospital Universitario HM Montepríncipe. Guillermo M. Albaiceta, Lorena Forcelledo Espina, Emilio García Prieto, Paula Martín Vicente, Cecilia del Busto Martínez: Hospital Universitario Central de Asturias, Oviedo. Pablo Vidal: Complexo Hospitalario Universitario de Ourense, Ourense. José Luis García Garmendia, María Aguilar Cabello, Carmen Eulalia Martínez Fernández: Hospital San Juan de Dios del Aljarafe, Sevilla. Nieves Carbonell, María Luisa Blasco Cortés, Ainhoa Serrano Lázaro, Mar Juan Díaz: Hospital Clínic Universitari de València, Valencia. Luis Jorge Valdivia: Hospital Universitario de León, León. María Victoria Boado, David Iglesias: Hospital Universitario de Cruces, Barakaldo. Maria del Carmen de la Torre: Hospital de Mataró. Ignacio Martínez Varela, María Teresa Bouza Vieiro, Inés Esmorís Arijón: Hospital Universitario Lucus Augusti, Lugo. David Campi Hermoso, Rafaela Nogueras Salinas, Teresa Farre Monjo, Ramon Nogue Bou, Gregorio Marco Naya, Núria Ramon Coll: Hospital Universitari de Santa Maria, Lleida. Mercedes Catalán-González, Juan Carlos Montejo-González: Hospital Universitario 12 de Octubre, Madrid. Gloria Renedo Sanchez-Giron, Juan Bustamante-Munguira, Ramon Cicuendez Avila, Nuria Mamolar Herrera: Hospital Clínico Universitario, Valladolid. Raquel Almansa: Instituto de Investigación Biomédica de Salamanca (IBSAL). Víctor Sagredo: Hospital Universitario de Salamanca, Salamanca. Jose M. Añón, Alexander Agrifoglio, Lucia Cachafeiro, Emilio Maseda: Hospital Universitario La Paz-Carlos III, Madrid. Mariana Andrea Novo, Albert Figueras, Maria Teresa Janer, Laura Soliva, Marta Ocón, Luisa Clar, J Ignacio Ayestarán: Hospital Universitario Son Espases, Palma de Mallorca. Sandra Campos Fernández: Hospital Universitario Marqués de Valdecilla, Santander. Mireia Serra-Fortuny, Eva Forcadell-Ferreres, Inmaculada Salvador-Adell, Neus Bofill, Berta Adell-Serrano, María José Centelles-Serrano, Núria Casacuberta-Barberà, Luis Urrelo-Cerrón, Diego Franch-Llasat, Ferran Roche-Campo: Hospital Verge de la Cinta de Tortosa, Tarragona. Pablo Ryan Murúa, Covadonga Rodríguez Ruíz, Laura Carrión García, Juan I. Lazo Álvarez: Hospital Universitario Infanta Leonor, Madrid. Ana Loza-Vázquez, Desire Macias Guerrero: Hospital Universitario Virgen de Valme, Sevilla. Arturo Huerta, Daniel Tognetti: Clínica Sagrada Familia, Barcelona. Carlos García Redruello, David Mosquera Rodríguez, Eva María Menor Fernández, Sabela Vara Adrio, Vanesa Gómez Casal, Marta Segura Pensado, María Digna Rivas Vilas, Amaia García Sagastume: Hospital de Vigo, Vigo. Raul de Pablo Sánchez, David Pestaña Laguna, Tommaso Bardi: Hospital Universitario Ramón y Cajal, Madrid. Rosario Amaya Villar, Carmen Gómez González, Maria Luisa Gascón Castillo: Hospital Universitario Virgen del Rocío, Sevilla. José Garnacho-Montero, María Luisa Cantón-Bulnes: Hospital Universitario Virgen Macarena, Sevilla. Judith Marin-Corral, Cristina Carbajales Pérez: Hospital Álvaro Cunqueiro, Vigo. Ana Salazar Degracia, Judit Bigas, Rosana Muñoz-Bermúdez, Clara Vilà-Vilardel, Francisco Parrilla, Irene Dot, Ana Zapatero, Yolanda Díaz, María Pilar Gracia, Purificación Pérez, Andrea Castellví, Cristina Climent: Hospital del Mar, Barcelona. Lidia Serra, Laura Barbena, Iosune Cano: Consorci Sanitari del Maresme, Barcelona. Alba Herraiz, Pilar Marcos, Laura Rodríguez, Maria Teresa Sariñena, Ana Sánchez: Hospital Universitari Germans Trias i Pujol, Badalona. Alejandro Úbeda: Hospital Punta de Europa, Algeciras. María Cruz Martin Delgado: Hospital Universitario Torrejón-Universidad Francisco de Vitoria, Madrid. Elena Gallego, Juan Fernando Masa Jiménez: Hospital Universitario San Pedro de Alcántara, Cáceres. Gemma Gomà, Emili Díaz: Hospital Parc Taulí, Sabadell. Mercedes Ibarz, Diego De Mendoza: Hospital Universitari Sagrat Cor, Bacelona. Enric Barbeta, Victoria Alcaraz-Serrano, Joan Ramon Badia, Manuel Castella, Leticia Bueno, Andrea Palomeque, Pamela Conde, Javier Fernández, Karsa Kiarostami, Alexandre López-Gavín, Cecilia L. Mantellini, Carla Speziale, Nil Vázquez, Hua Yang, Minlan Yang, Carlos Ferrando, Pedro Castro, Marta Arrieta, Jose Maria Nicolas, Rut Andrea: Hospital Clinic, Barcelona. Marta Barroso, Raquel Pérez, Sergio Álvarez, Adrián Tormos: Barcelona Supercomputing Center, Barcelona. Luis Tamayo Lomas, Cesar Aldecoa, Rubén Herrán-Monge, José Ángel Berezo García, Pedro Enríquez Giraudo: Hospital Rio Hortega, Valladolid. Pablo Cardinal Fernández, Alberto Rubio López, Orville Báez Pravia: Hospitales HM, Madrid. Juan López Messa, Leire Pérez Bastida, Antonjo Alvarez Ruiz: Complejo Asistencial Universitario de Palencia, Palencia. José Trenado, Anna Parera Pous: Hospital Universitari MutuaTerrassa, Terrassa. Cristóbal Galbán, Ana López Lago, Eva Saborido Paz, Patricia Barral Segade: Hospital de Santiago de Compostela, Santiago. Ana Balan Mariño, Manuel Valledor Mendez: Hospital San Agustin, Avilés. Raúl de Frutos, Luciano Aguilera: Hospital Basurto, Basurto. Felipe Pérez-García, Esther López-Ramos, Ángela Leonor Ruiz-García, Belén Beteré: Hospital Universitario Principe Asturias, Alcala de Henares. Rafael Blancas: Hospital Universitario del Tajo, Aranjuez. Cristina Dólera, Gloria Perez Planelles, Enrique Marmol Peis, Maria Dolores Martínez Juan, Miriam Ruiz Miralles, Eva Perez Rubio, Maria Van der Hofstadt Martin-Montalvo, Tatiana Villada Warrington: Hospital Universitario Sant Joan d’Alacant, Alicante. Juan Carlos Pozo-Laderas: Hospital Universitario Reina Sofia. Ángel Estella, Sara Guadalupe Moreno Cano: Hospital de Jerez, Jerez. Federico Gordo: Hospital Universitario del Henares, Coslada. Basilisa Martínez Palacios: Hospital Universitario Infanta Cristina, Parla. Maite Nieto, Maria Teresa Nieto: Hospital de Segovia, Segovia. Sergio Ossa: Hospital de Burgos, Burgos. Ana Ortega: Hospital Montecelo, Pontevedra. Miguel Sánchez: Hospital Clínico, Madrid. Bitor Santacoloma: Hospital Galdakao, Galdakao.