Idiopathic pulmonary fibrosis (IPF) is the most frequent and most lethal progressive fibrotic interstitial lung disease.1 The prevalence of obstructive sleep apnea (OSA) in patients with IPF in previous studies was around 76%,2 ranging from 45% to 90% depending on the OSA criteria and the methods used.3–5 The clinical presentation of OSA in IPF differs from that of regular OSA patients, being lower in terms of both symptomatology3,4 and body mass index (BMI).6,7 Many series found a predominance of hypopneas with substantial desaturation.5 Sleep-disordered breathing (SDB) encompassing OSA and nocturnal hypoxemia seem to be predictive of poor outcomes in IPF.3,8 Neither the mechanisms through which IPF might favor this marked prevalence of SDB nor the mechanisms through which SDB might cause a worse prognosis in these specific patients have as yet been elucidated.9
Therefore, the aims of this study were: (i) to better characterize the different SDB patterns observed in patients with IPF and (ii) to study the relationship between SDB and IPF through their clinical characteristics, lung function and blood biological mediators.
This is an observational prospective study of newly diagnosed IPF patients who had started antifibrotic treatment and who had a forced vital capacity (FVC) and diffusing lung capacity for carbon monoxide (DLCO) higher than 50% and 20% of predicted, respectively. Patients with other life-threatening or unstable diseases were excluded. The studies carried out were: video-polysomnography (PSG), capnography, laboratory analysis, pulmonary function test in both upright and supine position, 6-minute walking tests (6MWT), echocardiography and quality of life and sleep questionnaires. All inclusion patients received antifibrotic drugs (Pirfenidone or Nintedanib) after the diagnosis.
Apneas were codified as obstructive, mixed, or central and hypopneas as obstructive or central, according to the American Academy of Sleep Medicine criteria.10–12 The SDB were classified as: OSA when apnea hypopnea index (AHI)≥15h−1 and obstructive events were predominant (>50%); central sleep apnea (CSA) when central events were predominant (>50%) and sleep sustained hypoxemia (SSH) when percentage of total sleep time under SpO2 88% (TST88)>5min and AHI<15h−1.10–12
Laboratory analyses included: haemogram, C-reactive protein (CRP), lactate dehydrogenase (LDH), N-terminal pro-B-type natriuretic peptide, hemoglobin A1c (HbA1c), interleukin 6 (IL-6), matrix metalloproteinase-1, matrix metalloproteinase-7, matrix metalloproteinase-9 (MMP-9), surfactant protein D, tenascin-c large (FNIII-C), Krebs von den Lungen 6 (KL-6), advanced glycation end-products (AGEs), and receptor of advanced glycation end-products (RAGEs).
The quality of life (QoL) evaluation included sleep quality, the Functional Outcomes of Sleep Questionnaire short version, EuroQol 5D-5L and the King's Brief Interstitial Lung Disease questionnaire. The comorbidity questionnaires used were the Beck Depression Inventory-II, the 7-item Generalized Anxiety Disorder Scale and the Gastroesophageal Reflux Disease Questionnaire.
Categorical variables were presented as the number of cases and percentages, while continuous variables were presented as the median and the interquartile range (IQR). The Chi-square test or Fisher test (when appropriate) was used to compare categorical variables between groups and the Kruskal–Wallis test was used to compare continuous variables.
This study was approved by the hospital's Ethics Committee (PR413/18). Further information is provided in Supplementary Material.
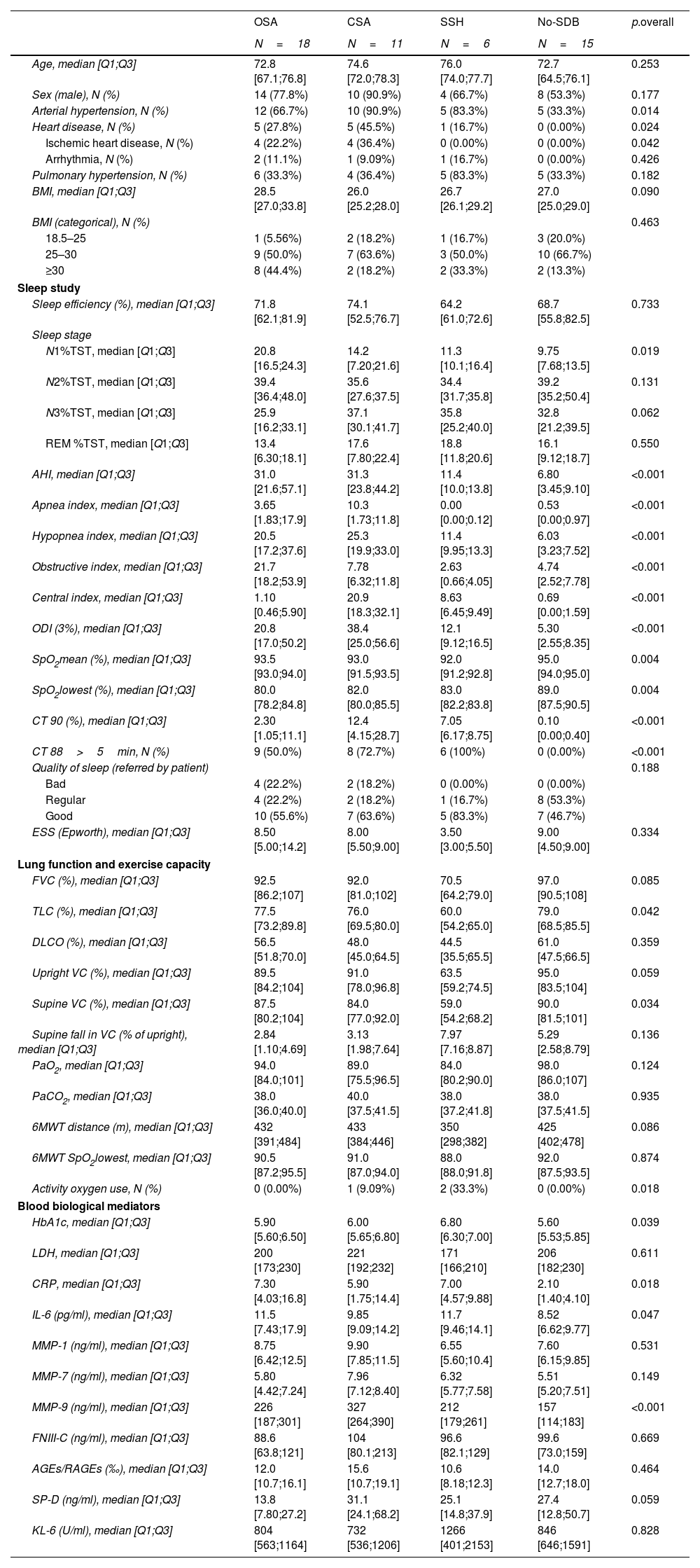
Fifty patients with IPF were enrolled (Table 1). Median age was 74.1 years and 72% of patients were male. Thirty-five (70%) patients had SDBs, 18 (36%) patients were diagnosed as OSA, 11 (22%) as CSA, and six (12%) as SSH. Both OSA and CSA were accompanied by the typical pattern of intermittent hypoxia (IH) with variable degrees of hypoxia, whereas desaturation was sustained in SSH. Median AHI was similar in OSA and CSA patterns, with a predominance of hypopneas vs. apnea in both groups. The SSH group had the lowest nocturnal SpO2 average. The CSA group had three patients with periodic breathing and one with hypoventilation and nocturnal hypercapnia measured by transcutaneous capnography (this patient was under opioid treatment as part of their chronic pain treatment). Sleep efficiency was reduced in all groups. There were no significant differences between groups in perceived sleep quality or excessive daytime sleepiness measured by Epworth sleepiness scale (ESS) and only 12% reported sleeping poorly. There were no significant differences between groups in the quality of life or symptoms questionnaire.
Patient data.
| OSA | CSA | SSH | No-SDB | p.overall | |
|---|---|---|---|---|---|
| N=18 | N=11 | N=6 | N=15 | ||
| Age, median [Q1;Q3] | 72.8 [67.1;76.8] | 74.6 [72.0;78.3] | 76.0 [74.0;77.7] | 72.7 [64.5;76.1] | 0.253 |
| Sex (male), N (%) | 14 (77.8%) | 10 (90.9%) | 4 (66.7%) | 8 (53.3%) | 0.177 |
| Arterial hypertension, N (%) | 12 (66.7%) | 10 (90.9%) | 5 (83.3%) | 5 (33.3%) | 0.014 |
| Heart disease, N (%) | 5 (27.8%) | 5 (45.5%) | 1 (16.7%) | 0 (0.00%) | 0.024 |
| Ischemic heart disease, N (%) | 4 (22.2%) | 4 (36.4%) | 0 (0.00%) | 0 (0.00%) | 0.042 |
| Arrhythmia, N (%) | 2 (11.1%) | 1 (9.09%) | 1 (16.7%) | 0 (0.00%) | 0.426 |
| Pulmonary hypertension, N (%) | 6 (33.3%) | 4 (36.4%) | 5 (83.3%) | 5 (33.3%) | 0.182 |
| BMI, median [Q1;Q3] | 28.5 [27.0;33.8] | 26.0 [25.2;28.0] | 26.7 [26.1;29.2] | 27.0 [25.0;29.0] | 0.090 |
| BMI (categorical), N (%) | 0.463 | ||||
| 18.5–25 | 1 (5.56%) | 2 (18.2%) | 1 (16.7%) | 3 (20.0%) | |
| 25–30 | 9 (50.0%) | 7 (63.6%) | 3 (50.0%) | 10 (66.7%) | |
| ≥30 | 8 (44.4%) | 2 (18.2%) | 2 (33.3%) | 2 (13.3%) | |
| Sleep study | |||||
| Sleep efficiency (%), median [Q1;Q3] | 71.8 [62.1;81.9] | 74.1 [52.5;76.7] | 64.2 [61.0;72.6] | 68.7 [55.8;82.5] | 0.733 |
| Sleep stage | |||||
| N1%TST, median [Q1;Q3] | 20.8 [16.5;24.3] | 14.2 [7.20;21.6] | 11.3 [10.1;16.4] | 9.75 [7.68;13.5] | 0.019 |
| N2%TST, median [Q1;Q3] | 39.4 [36.4;48.0] | 35.6 [27.6;37.5] | 34.4 [31.7;35.8] | 39.2 [35.2;50.4] | 0.131 |
| N3%TST, median [Q1;Q3] | 25.9 [16.2;33.1] | 37.1 [30.1;41.7] | 35.8 [25.2;40.0] | 32.8 [21.2;39.5] | 0.062 |
| REM %TST, median [Q1;Q3] | 13.4 [6.30;18.1] | 17.6 [7.80;22.4] | 18.8 [11.8;20.6] | 16.1 [9.12;18.7] | 0.550 |
| AHI, median [Q1;Q3] | 31.0 [21.6;57.1] | 31.3 [23.8;44.2] | 11.4 [10.0;13.8] | 6.80 [3.45;9.10] | <0.001 |
| Apnea index, median [Q1;Q3] | 3.65 [1.83;17.9] | 10.3 [1.73;11.8] | 0.00 [0.00;0.12] | 0.53 [0.00;0.97] | <0.001 |
| Hypopnea index, median [Q1;Q3] | 20.5 [17.2;37.6] | 25.3 [19.9;33.0] | 11.4 [9.95;13.3] | 6.03 [3.23;7.52] | <0.001 |
| Obstructive index, median [Q1;Q3] | 21.7 [18.2;53.9] | 7.78 [6.32;11.8] | 2.63 [0.66;4.05] | 4.74 [2.52;7.78] | <0.001 |
| Central index, median [Q1;Q3] | 1.10 [0.46;5.90] | 20.9 [18.3;32.1] | 8.63 [6.45;9.49] | 0.69 [0.00;1.59] | <0.001 |
| ODI (3%), median [Q1;Q3] | 20.8 [17.0;50.2] | 38.4 [25.0;56.6] | 12.1 [9.12;16.5] | 5.30 [2.55;8.35] | <0.001 |
| SpO2mean (%), median [Q1;Q3] | 93.5 [93.0;94.0] | 93.0 [91.5;93.5] | 92.0 [91.2;92.8] | 95.0 [94.0;95.0] | 0.004 |
| SpO2lowest (%), median [Q1;Q3] | 80.0 [78.2;84.8] | 82.0 [80.0;85.5] | 83.0 [82.2;83.8] | 89.0 [87.5;90.5] | 0.004 |
| CT 90 (%), median [Q1;Q3] | 2.30 [1.05;11.1] | 12.4 [4.15;28.7] | 7.05 [6.17;8.75] | 0.10 [0.00;0.40] | <0.001 |
| CT 88>5min, N (%) | 9 (50.0%) | 8 (72.7%) | 6 (100%) | 0 (0.00%) | <0.001 |
| Quality of sleep (referred by patient) | 0.188 | ||||
| Bad | 4 (22.2%) | 2 (18.2%) | 0 (0.00%) | 0 (0.00%) | |
| Regular | 4 (22.2%) | 2 (18.2%) | 1 (16.7%) | 8 (53.3%) | |
| Good | 10 (55.6%) | 7 (63.6%) | 5 (83.3%) | 7 (46.7%) | |
| ESS (Epworth), median [Q1;Q3] | 8.50 [5.00;14.2] | 8.00 [5.50;9.00] | 3.50 [3.00;5.50] | 9.00 [4.50;9.00] | 0.334 |
| Lung function and exercise capacity | |||||
| FVC (%), median [Q1;Q3] | 92.5 [86.2;107] | 92.0 [81.0;102] | 70.5 [64.2;79.0] | 97.0 [90.5;108] | 0.085 |
| TLC (%), median [Q1;Q3] | 77.5 [73.2;89.8] | 76.0 [69.5;80.0] | 60.0 [54.2;65.0] | 79.0 [68.5;85.5] | 0.042 |
| DLCO (%), median [Q1;Q3] | 56.5 [51.8;70.0] | 48.0 [45.0;64.5] | 44.5 [35.5;65.5] | 61.0 [47.5;66.5] | 0.359 |
| Upright VC (%), median [Q1;Q3] | 89.5 [84.2;104] | 91.0 [78.0;96.8] | 63.5 [59.2;74.5] | 95.0 [83.5;104] | 0.059 |
| Supine VC (%), median [Q1;Q3] | 87.5 [80.2;104] | 84.0 [77.0;92.0] | 59.0 [54.2;68.2] | 90.0 [81.5;101] | 0.034 |
| Supine fall in VC (% of upright), median [Q1;Q3] | 2.84 [1.10;4.69] | 3.13 [1.98;7.64] | 7.97 [7.16;8.87] | 5.29 [2.58;8.79] | 0.136 |
| PaO2, median [Q1;Q3] | 94.0 [84.0;101] | 89.0 [75.5;96.5] | 84.0 [80.2;90.0] | 98.0 [86.0;107] | 0.124 |
| PaCO2, median [Q1;Q3] | 38.0 [36.0;40.0] | 40.0 [37.5;41.5] | 38.0 [37.2;41.8] | 38.0 [37.5;41.5] | 0.935 |
| 6MWT distance (m), median [Q1;Q3] | 432 [391;484] | 433 [384;446] | 350 [298;382] | 425 [402;478] | 0.086 |
| 6MWT SpO2lowest, median [Q1;Q3] | 90.5 [87.2;95.5] | 91.0 [87.0;94.0] | 88.0 [88.0;91.8] | 92.0 [87.5;93.5] | 0.874 |
| Activity oxygen use, N (%) | 0 (0.00%) | 1 (9.09%) | 2 (33.3%) | 0 (0.00%) | 0.018 |
| Blood biological mediators | |||||
| HbA1c, median [Q1;Q3] | 5.90 [5.60;6.50] | 6.00 [5.65;6.80] | 6.80 [6.30;7.00] | 5.60 [5.53;5.85] | 0.039 |
| LDH, median [Q1;Q3] | 200 [173;230] | 221 [192;232] | 171 [166;210] | 206 [182;230] | 0.611 |
| CRP, median [Q1;Q3] | 7.30 [4.03;16.8] | 5.90 [1.75;14.4] | 7.00 [4.57;9.88] | 2.10 [1.40;4.10] | 0.018 |
| IL-6 (pg/ml), median [Q1;Q3] | 11.5 [7.43;17.9] | 9.85 [9.09;14.2] | 11.7 [9.46;14.1] | 8.52 [6.62;9.77] | 0.047 |
| MMP-1 (ng/ml), median [Q1;Q3] | 8.75 [6.42;12.5] | 9.90 [7.85;11.5] | 6.55 [5.60;10.4] | 7.60 [6.15;9.85] | 0.531 |
| MMP-7 (ng/ml), median [Q1;Q3] | 5.80 [4.42;7.24] | 7.96 [7.12;8.40] | 6.32 [5.77;7.58] | 5.51 [5.20;7.51] | 0.149 |
| MMP-9 (ng/ml), median [Q1;Q3] | 226 [187;301] | 327 [264;390] | 212 [179;261] | 157 [114;183] | <0.001 |
| FNIII-C (ng/ml), median [Q1;Q3] | 88.6 [63.8;121] | 104 [80.1;213] | 96.6 [82.1;129] | 99.6 [73.0;159] | 0.669 |
| AGEs/RAGEs (‰), median [Q1;Q3] | 12.0 [10.7;16.1] | 15.6 [10.7;19.1] | 10.6 [8.18;12.3] | 14.0 [12.7;18.0] | 0.464 |
| SP-D (ng/ml), median [Q1;Q3] | 13.8 [7.80;27.2] | 31.1 [24.1;68.2] | 25.1 [14.8;37.9] | 27.4 [12.8;50.7] | 0.059 |
| KL-6 (U/ml), median [Q1;Q3] | 804 [563;1164] | 732 [536;1206] | 1266 [401;2153] | 846 [646;1591] | 0.828 |
OSA: obstructive sleep apnea; CSA: central sleep apnea; SSH: sleep sustained hypoxemia; No-SDB: sleep-disordered breathing; BMI: body mass index; TST: total sleep time; AHI: apnea–hypopnea index; REM: rapid eye movement; SpO2: arterial oxygen saturation measured by pulse oximetry; ODI: oxygen desaturation index; TST90: percentage of TST with SpO2<90%; TC-CO2: partial pressure of carbon dioxide measured by tracutaneous capnograpy; PaCO2: partial pressure of carbon dioxide measured by arterial gasometry at 8am after PSG; ESS: Epworth sleepiness scale; FVC: forced vital capacity; TLC: total lung capacity; FEV1: forced expiratory volume in 1s; VC: vital capacity; DLCO: diffusing capacity of the lungs for carbon monoxide; KCO: carbon monoxide transfer coefficient; PaO2: partial pressure of oxygen measured by arterial gasometry at 7.00am after PSG; 6MWT: 6-min walking test; HbA1c: hemoglobin A1c; LDH: lactate dehydrogenase; CRP: C-reactive protein; IL-6: human interleukin 6; MMP-1: matrix metalloproteinase 1; MMP-7: matrix metalloproteinase 7; MMP-9: matrix metalloproteinase 9; FNIII-C: tenascin-c large; AGEs: advanced glycation end-products; RAGEs: receptor for advanced glycation end products; SP-D: surfactant protein D; KL-6: Krebs von den Lungen-6.
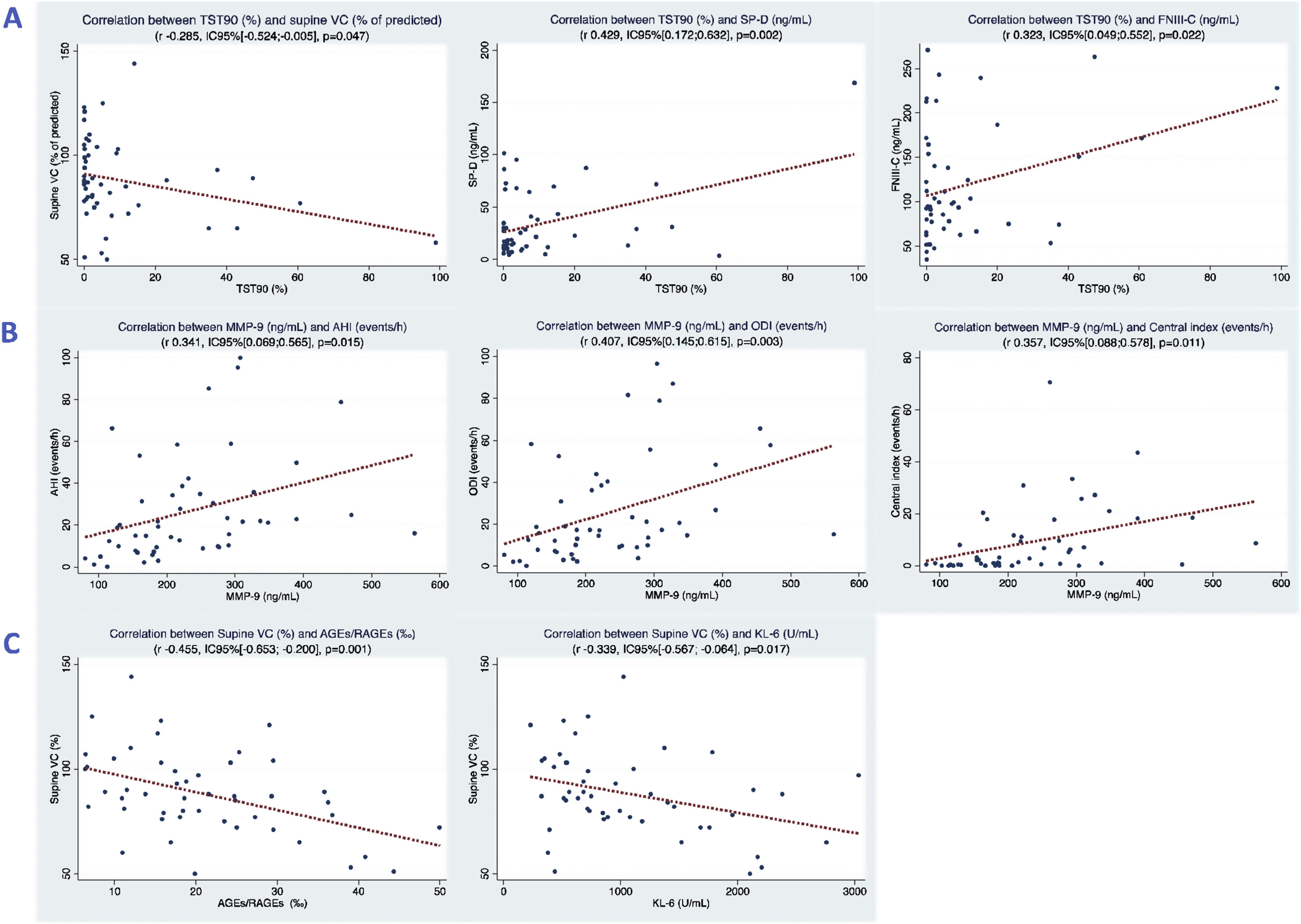
Patients with SDBs showed a worse lung function than patients without SDBs. The SSH group had the worst lung function, with significantly lower total lung capacity (TLC) and supine vital capacity (VC). TST90 (%) showed a correlation with supine VC% (Fig. 1).
(A) Graph showing the relation between TST90 (%) and supine VC (% of predicted), SP-D and FNIII-C. (B) Graph showing the relation between MMP-9 and AHI (events/h), ODI and central index (events/h). (C) Graph showing the relation between supine VC (% of predicted) and AGEs/RAGEs and KL-6. TST90 (%): percentage of TST with SpO2<90%; supine VC: vital capacity in supine position; SP-D: surfactant protein D; FNIII-C: tenascin-c large; MMP-9: matrix metalloproteinase 9; AHI: apnea–hypopnea index; ODI: oxygen desaturation index; AGEs: advanced glycation end-products; RAGEs: receptor for advanced glycation end products; Krebs von den Lungen-6.
The SDB groups had significantly higher metabolic glucoside (HbA1c) and inflammatory levels (CRP and IL-6) than the non-SDB group. A profibrotic mediator (MMP-9) was correlated with apnea events (AHI, ODI and central index). Nocturnal hypoxemia (TST90%) was also correlated with increased profibrotic and oxidative molecules (SP-D, FNIII-C and LDH). Concerning pulmonary function, supine VC% was inversely correlated with profibrotic mediators (AGE/RAGEs and KL-6) (Fig. 1).
This study confirms a high prevalence of SDBs in IPF patients when they are systematically evaluated at diagnosis, even without symptoms, and identifies for the first time a high prevalence of CSA, and not only OSA and IH as previously reported.3–5 This was possible through the rigorous coding of the hypopneas, used very exceptionally in IPF.13
Patients with OSA had a higher BMI and higher waist circumference than patients in the other groups, suggesting that overweight plays a role, at least in part, in cases of genuine obstructive events. Physiological changes related to decreased lung capacity in the supine position may be one of the factors contributing to the high prevalence of SDBs in IPF, as was suggested in previous works.14,15 In line with that, apnea groups in our study had lower lung volumes, while profibrotic index AGE/RAGES correlated with central index and had the strongest correlation with supine VC (%).
SDB has been proposed as an aggravating factor in pulmonary fibrosis through recurrent mechanical stretching causing tractional injury, repetitive microaspiration from gastroesophageal reflux, and oxidative stress related to IH.9 In an animal model of bleomycin-induced lung fibrosis, IH increased collagen deposition in the lung through oxidative and inflammatory pathways.16 Nocturnal hypoxemia and OSA were related with subclinical interstitial lung disease and increased blood markers of alveolar epithelial injury and remodeling.17,18 Our data showed a consistent correlation of nocturnal hypoxemia with inflammatory, oxidative and pro-fibrotic mediators. On the other hand, recurrent traction and inspiratory resistive breathing in animal models provoked alveolar damage and fibrosis by inducing a profibrotic mediator, MMP9.19,20 In line with this result, MMP-9 was also correlated with AHI, ODI and central index in our patients.
The main limitation of this study is the limited number of patients by sub-groups, especially for the SSH group. Furthermore, since only IPF patients were included, the conclusions of the results cannot be directly extrapolated to all fibrotic ILDs. However, the homogeneity of IPF allowed the optimal study of sleep patterns and their clinical and biological repercussions, which may be considered for study in other non-IPF progressive fibrotic ILDs.
This study confirms the high prevalence of SDB in IPF patients and highlights the existence of different patterns: obstructive events, but also central events and isolated sustained hypoxemia. These patterns may have different pathophysiological mechanisms and, importantly, entail different therapeutic approaches. Decreased lung capacity in the supine position may play a role in the SDBs of IPF, while SDBs were related to increased oxidative, inflammatory and profibrotic pathways. Hence, systematic assessment of SDBs may be useful for the comprehensive management of IPF patients.
Conference presentationThe results of this study have been partially presented in 55° Sociedad Española de Neumología y Cirugía Torácica (SEPAR) Congress (2022) and European Respiratory Society (ERS) International Congress 2022.
FundingThis study has been funded by: Instituto de Salud Carlos III through the grants CM20/00093 (co-funded by European Social Fund. ESF investing in your future) and PI18/00367 (co-funded by European Regional Development Fund, ERDF, a way to build Europe); Spanish Society of Pneumology and Thoracic Surgery (SEPAR) grants 631/2018 and 685/2018; Emerging ILD Group of SEPAR grant 005 (Boehringer-Roche); Pneumology Foundation of Catalonia (FUCAP) grant 2019; Spanish Sleep Society (SES) grant 2019. Investigation support BRN-Fundació Ramon Pla Armengol. We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Conflict of interestsThe authors state that they have no conflict of interests.
We would like to thank Fujirebio Europe N.V. for providing KL-6 reactives free of charge and David Bridgewater for the English language revision of the manuscript.