One of the pathways involved in pulmonary arterial hypertension (PAH) is the nitric oxide (NO) pathway. A polymorphism in the inducible NO synthase (NOS2) gene has been described, consisting of the CCTTT pentanucleotide repeat, which causes a reduction in NO production. The aim of this study was to determine if this polymorphism increases susceptibility to developing PAH.
MethodsSixty-four patients with a diagnosis of PAH groups i and iv and 50 healthy controls were compared. DNA genotyping of the samples for this polymorphism was performed using PCR. The distribution between both groups was compared and correlated with clinical and hemodynamic parameters and therapeutic response.
ResultsA significantly different distribution was observed in the number of repeats between patients and controls (P<.0001). When the samples were categorized by short forms (both alleles with less than 12 repeats) and long forms (≥12 repeats), it was observed that the former had an almost 4-fold risk of developing PAH (odds ratio: 3.83; 95% CI: 1.19–12.32, P=.024). There were no differences between the most common types of PAH, either in therapeutic response or survival. There was no correlation between hemodynamic parameters and the number of repeats in the patients, and only a weak correlation with systolic PAH.
ConclusionsThere are significant differences in the distribution of the NOS2 promotor CCTTT polymorphism between patients with PAH and the healthy population. A minor CCTTT pentanucleotide repeat in the NOS2 gene may increase the risk of developing PAH.
La hipertensión arterial pulmonar (HAP) es una enfermedad en la que se implican, entre otras, la vía del óxido nítrico (NO). Se ha descrito un polimorfismo en el gen de la sintasa inducible del NO (NOS2) que consiste en la repetición del pentanucleótido CCTTT dando lugar a menor producción de NO. El objetivo del estudio fue conocer si este polimorfismo incrementa la susceptibilidad para desarrollar HAP.
MétodosSe compararon 64 pacientes diagnosticados de HAP grupos i y iv y 50 controles sanos. Mediante PCR se genotiparon las muestras de ADN para este polimorfismo. Se comparó la distribución en ambos grupos y se correlacionó con parámetros clínicos, hemodinámicos y respuesta terapéutica.
ResultadosSe observó una distribución significativamente diferente en el número de repeticiones entre pacientes y controles (p<0,0001). Agrupando las muestras en formas cortas (ambos alelos con menos de 12repeticiones) y largas (≥12repeticiones) se observó que los primeros tenían un riesgo casi 4veces superior de desarrollar HAP (odds ratio: 3,83; IC 95%: 1,19-12,32; p=0,024). No hubo diferencias entre los tipos más frecuentes de HAP ni en la respuesta terapéutica o supervivencia. No existió correlación entre parámetros hemodinámicos y el número de repeticiones en los pacientes, solo débil correlación con la presión arterial pulmonar sistólica.
ConclusionesExisten diferencias significativas en la distribución del polimorfismo CCTTT del gen NOS2 entre pacientes con HAP y población sana. Una menor repetición del pentanucleótido CCTTT en el gen de la NOS2 podría incrementar el riesgo de desarrollar HAP.
Pulmonary arterial hypertension (PAH) is a rare disease that leads to a progressive increase in the resistance of the pulmonary arteries. This is due to endothelial dysfunction, characterized by the increased production of substances with vasoconstrictor and proliferative effects and the reduced production of substances with the opposite effect. One of the latter is nitric oxide (NO), which, in addition to being a potent pulmonary vasodilator, also acts as an inhibitor of platelet activation and smooth muscle proliferation in the blood vessels.1 NO is synthesized from l-arginine by NO synthase (NOS), of which there are 3 isoforms: NOS1 or neuronal, NOS2 or inducible and NOS3 or endothelial. NOS2 is the leading source of NO in inflammatory situations involving exposure to cytokines, such as tumor necrosis factor alpha, IL-1, interferon gamma and endothelin-1 (ET-1).2 The NOS2 gene is located on chromosome 12, as is the gene coding for the serotonin transporter protein, another important player in pulmonary vascular tone regulation. Numerous reports have shown a reduction in NO concentration in both the plasma and lungs of patients with idiopathic PAH or PAH associated with other conditions.3,4 The cause of this reduction appears to be a reduction in NOS activity that leads to a drop in the endogenous synthesis of NO.5 In proinflammatory situations, NOS activity is probably largely NOS2-dependent, since the NOS3 constitutive form appears in much lower concentrations. In patients with PAH, ET-1 also acts as a potent inducer of NOS2 activity. However, in patients with systemic sclerosis and PAH, it has been shown that the NO concentration does not correlate with the increase in ET-1, and NO concentrations remain at normal levels despite a considerable increase in ET-1.6 This could be explained by the presence of some type of polymorphism on the NOS2 gene with a lesser capacity for NO synthesis. A polymorphism located in the promoter region of the NOS2 gene consisting of a CCTTT pentanucleotide repeat7 has been described that appears to confer protection against malaria, in addition to reducing the risk of diabetic nephropathy.8,9 A correlation between this pentanucleotide repeat and the serum NO/ET-1 ratio has been shown in patients with scleroderma, irrespective of whether there is PAH or not. This could suggest that in the presence of high concentrations of ET-1, as occurs in PAH, this polymorphism may be responsible for low NO production6 and thus represents an important factor in the pathogenicity of this disease entity. All these findings indicate that this polymorphism may be involved in the development of PAH.
The aim of our study was to determine the distribution of this polymorphism in patients with different types of PAH and to investigate the possible relationship between the number of pentanucleotides and hemodynamic and clinical parameters.
Materials and MethodsConsecutive adult patients with a diagnosis of group I and IV PAH (Dana Point 2008) were enrolled in our unit. They gave their consent for the study after receiving an explanation of the objectives. In all cases, the diagnosis was based initially on a suspicious echocardiogram and later confirmed with resting right heart catheterization (mean pulmonary artery pressure [mPAP] ≥25mmHg, wedge pressure ≤15mmHg). The following data were collected: age at diagnosis, sex, onset of symptoms, PAH type, functional class (FC), hemodynamic values from echocardiogram and catheterization, clinical laboratory results, including autoimmune testing and brain natriuretic peptide (BNP), distance achieved on 6-minute walking test and response to treatment, particularly phosphodiesterase-5 inhibitors. University students with no personal or family history of PAH disease were selected as controls. The study was approved by the local ethics committee.
SamplesDNA was extracted from peripheral leukocytes obtained from venous blood using the FlexiGene DNA kit (Qiagen, Germany) and frozen at −70°C until processing. The corresponding promoter region was amplified by PCR with the following primers: 5′-ACC CCT GGA AGC CTA CAA CTG CAT-3′ (forward); 5′-GCC ACT GCA CCC TAG CCT GTC TCA-3′ (reverse). The various alleles were obtained by capillary electrophoresis using an ABI Prism 3100 genetic analyzer (Applied Biosystems, California, U.S.). Allele size was calculated using GeneScan Analysis software with the GeneScanTM400 ROXTM Size Standard (Applied Biosystems, CA, USA).
Statistical AnalysisThe main aim was to determine differences in the distribution of the number of CCTTT pentanucleotides in PAH patients compared to the healthy population. Given the sample size, non-parametric tests were used. For the comparison between the numbers of repeats, the Mann–Whitney test was used. Qualitative variables were analyzed using contingency tables with Chi-square or Fisher tests. The Spearman test was used for correlations between the number of nucleotides and hemodynamic parameters. A P-value of ≤.05 was considered significant. All calculations were carried out using the SPSS v19 software package.
ResultsThe characteristics of the 64 patients, of whom 37 were women, are shown in Table 1. The most common types of PAH were idiopathic (19 cases, 29.6%), connective tissue disease-related (17 cases, 26.5%) and thromboembolic (14 cases, 21.8%). Mean age at the time of diagnosis was 52±16 years, with a mPAP of 49±15mmHg.
Patient Characteristics.
| Patients | 64 |
| Men/women | 37/27 |
| Age (years) | 52±16 |
| mPAP (mmHg) | 49±15 |
| sPAP (mmHg) | 77±19 |
| Pulmonary vascular pressure (Wood U) | 10.7±6 |
| Cardiac index (l/min/m2) | 2.3±0.7 |
| 6-minute walking test (m) | 338±72 |
| PAH type | |
| Idiopathic | 19 |
| Connective disease | 17 |
| Thromboembolic | 14 |
| Congenital heart disease | 7 |
| Pulmonary portal | 5 |
| HIV | 2 |
PAH: pulmonary arterial hypertension; mPAP: mean pulmonary arterial pressure sPAPs: systolic pulmonary arterial pressure; HIV: human immunodeficiency virus.
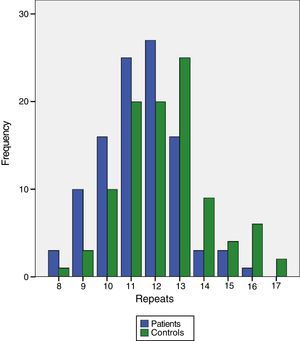
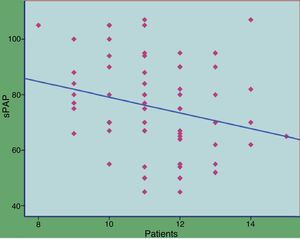
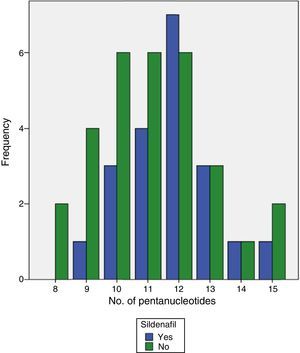
CCTTT polymorphism could be analyzed in all samples from patients and controls. Ten (10) alleles were found between 8 and 17 pentanucleotide repeats. The distribution was significantly different between patients and controls (Table 2) with a median of 12 repeats in patients and 13 in controls (P<.0001). When the 50 patients with group I PAH were analyzed separately, the differences also reached the same degree of statistical significance (P<.0001). To calculate the risk that may be involved in having fewer pentanucleotides, both patients and controls were divided into two groups: short (both alleles with fewer than 12 repeats) or long (12 or more repeats). The differences were clearly significant (odds ratio [OR]: 3.83; 95% confidence interval [CI]: 1.19–12.32; P=.024). When the hemodynamic parameters were analyzed, no significant correlation with the number of pentanucleotides was observed, but statistical significance was reached with systolic PAP measured during the diagnostic catherization, although the r-value was very low (Figs. 1 and 2). No significant differences were found when the number of pentanucleotides was compared among the 3 most common types of PAH. To evaluate the possible relationship between the number of pentanucleotides and the therapeutic response at the end of the first year, the patients were divided into three groups: improvement, stabilization and worsening, using functional class as a measurement. No significant differences were observed (P=.81). Nor were different therapeutic responses observed when the patients were grouped according to the presence of less than 12 repeats or more than 12 repeats (P=.44). We also analyzed the therapeutic responses in the first three months of phosphodiesterase-5 administration by the number of pentanucleotides. In total, 25 patients received sildenafil. Response was considered good in 10 cases (improvement by at least one FC and/or more than 50 meters in the 6-minute walking test). The other 15 did not achieve these endpoints. The distribution of pentanucleotides among these groups was not significantly different (P=.15) (Fig. 3).
Pentanucleotide Repeat Distribution Frequencies.
| Patients | Controls | |
| 8 | 3 (2.4%) | 1 (0.8%) |
| 9 | 14 (11%) | 3 (2.4%) |
| 10 | 20 (15.7%) | 10 (7.9%) |
| 11 | 27 (21.3%) | 20 (15.7%) |
| 12 | 31 (24.4%) | 20 (15.7%) |
| 13 | 19 (15%) | 25 (19.7%) |
| 14 | 6 (4.7%) | 9 (7.1%) |
| 15 | 4 (3.1%) | 4 (3.1%) |
| 16 | 1 (0.8%) | 6 (4.7%) |
| 17 | 1 (0.8%) | 2 (1.6%) |
A clear difference was observed in the frequencies of pentanucleotide repeats (P<.0001): the number was predominantly lower in patients compared to controls.
All patients were followed up for minimum of 2 years. To determine if there was a difference in the distribution of alleles between patients who died and patients who survived, patients with thromboembolic PAH, PAH associated with congenital heart disease and lung transplantation recipients were excluded, as having a different prognosis. Of the 43 patients included in this analysis, 7 died. No significant differences were observed (P=.67) (Table 3).
DiscussionThis study revealed a significantly different distribution in the number of CCTTT pentanucleotides in the NOS2 gene between patients with various types of PAH and the general population. The PAH patients had shorter enzyme forms associated with reduced transcriptional capacity. This is the first study to evaluate this polymorphism in different types of PAH, including the idiopathic forms. One of the most interesting findings was a 3-fold increase in the risk of developing this disease if the number of pentanucleotides in the two alleles was less than 12. It was observed previously that patients with scleroderma-related PAH also had a lower number of repeats compared to scleroderma patients without PAH. This finding seems to indicate that scleroderma patients with this polymorphism are more susceptible to the development of PAH.6 This same study, using a vector that inserted sequences containing between 6 and 14 pentanucleotide repeats in human fibroblasts, showed a gradual increase in the transcriptional activity of NOS2 as the number of pentanucleotides increased.
The NO pathway plays a critical role in the control of pulmonary vascular tone. It is produced mainly in the lung endothelium and epithelium, i.e., regulation is local.10 Initially the effect of NO in the pulmonary vascular tree was studied using non-selective inhibitors of the various NOS isoforms, but later, when genetically modified animals that did not express any isoform became available, the role of each could be studied in depth. For example, NOS3 depletion causes significant systemic hypertension and only mild pulmonary hypertension with no apparent remodeling.11 NOS2 is regulated by inflammatory mediators and plays little part in the absence of disease. A marked increase in NOS2-mediated NO has been observed in respiratory distress syndrome, Paradoxically, this increased NOS2-mediated NO reacts with peroxynitrite, a very abundant oxygen reactive species in this situation, and leads to increased endothelial damage and the development of pulmonary hypertension.12 The same occurs in experimental animals exposed to tobacco smoke, in which NOS2 and peroxynitrite are key players in the genesis of emphysema and PAH. NOS2 inhibition prevents the development of both and even repairs some of the lesions,13 which only serves to underline the importance of NOS2 in situations of stress or inflammatory activity.
The CCTTT pentanucleotide is highly polymorphic, as was also observed in this study, in which the number of alleles ranged from 8 to 17. This variation appears to be of importance in the regulation of NOS2 transcription and protection against some diseases. For example, in an Irish study, carriers of 14 repeats had a relative risk of developing diabetic retinopathy of 0.21 compared to the others.14 This same number of repeats was also clearly more common in diabetics without nephropathy compared to those with.9 Several studies have shown the apparent importance of this polymorphism in inflammatory diseases. A study in patients with rheumatoid arthritis did not identify any differences compared to the general population in 2 polymorphisms in the NOS3 gene, although there was a significant difference in the NOS2 CCTTT repeat.15 This study, like ours, found an increase in the risk of rheumatoid arthritis, in this case more than 2-fold, if the number of repeats was less than 12.
No differences were found in the distribution of pentanucleotides in the most common types of PAH. This may be because of the sample size, but it may reflect reality. With the available data, the pathogenesis of PAH appears to be multifactorial, involving the complex interaction of several cell signals, rather than one in particular. Even type 2 receptor gene mutations in the bone morphogenetic proteins (BMPR2), one of the most important mechanisms in the development of hereditary PAH, do not represent a risk of more than 20% of developing the disease.16,17 The NO pathway is very important, and the theory may be put forward that in a situation that would favor PAH, including connective tissue disease, portal hypertension, etc., the presence of a polymorphism that changes one of the most potent pulmonary vasodilatory stimuli would increase the risk of developing the disease. The result of our study supports this possibility.
Except for a weak association with systolic PAP, no correlation was found between hemodynamic parameters and the number of pentanucleotides, despite the fact that lower NO production with poor vascular tone regulation may be a factor that would tend to raise pulmonary resistance. Since the number of positive vasodilator tests was very low (3 patients), no analysis could be made.
A better therapeutic response to phophodiesterase-5 inhibitor in patients with fewer pentanucleotides could have been assumed a priori, since NO production is reduced, but this was not confirmed by the study data. This type of drug has been shown to be effective in the treatment of PAH in numerous trials.18,19 Although the 25 patients who took these drugs were analyzed, the study was not designed for this purpose, since different types of PAH were included and somewhat more than half of the cases received combined treatment after having failed on another drug. Possibly the only way to evaluate this appropriately would be to use a much more homogeneous sample and on first-line treatment, since the number of confounding variables in our study makes it difficult to draw reasonably certain conclusions. It may also be speculated that if NO synthesis is markedly reduced, even when the destruction of its effector, GMP-c, is inhibited in part, in many cases the concentration achieved would not be sufficient to achieve relevant results. More reliable data may be obtained with riociguat, a drug not yet on the market. This compound stimulates guanylate-cyclase directly20 without depending on NO concentration to function.
No differences were found in the distribution of the polymorphism between patients who died and those who survived. The two types with better prognosis (congenital and thromboembolic heart disease; some the latter had undergone endarterectomy) and lung transplant recipients were excluded, so that patients with a more similar prognosis were studied from the start. The ideal situation would have been to stratify the analysis depending on the severity of the disease at the time of diagnosis, but the sample size made this practically impossible. The follow-up period of 2 years was perhaps too short to obtain significant results.
In conclusion, this study found fewer CCTTT pentanucleotide repeats in the promoter region of the NOS2 gene in patients with different types of PAH compared to the general population. Less than 12 repeats in both alleles significantly increased the risk of developing the disease. Since NOS2 transcriptional activity appears to increase with the number of pentanucleotides, this polymorphism may possibly have some type of pathogenic role in the development of the disease and may be interesting to study in patients with diseases associated with PAH.
FundingThis study has been partially financed by a research grant from Actelion.
Conflict of InterestsAdolfo Baloira has received research grants from Actelion and has acted as a consultant for Actelion, GSK, Pfizer, Lilly, Bayer and Ferrer.
The other authors declare no conflict of interests.
Please cite this article as: Baloira Villar A, Pousada Fernández G, Vilariño Pombo C, Núñez Fernández M, Cifrián Martínez J, Valverde Pérez D. Estudio de la repetición del pentanucleótido CCTTT en el gen de la sintasa inducible del óxido nítrico en pacientes con hipertensión arterial pulmonar. Arch Bronconeumol. 2014;50:141–145.