One-third of the world-wide population currently presents latent tuberculosis infection (LTI). In Spain, TB is situated as the third disease of mandatory notification. The standard technique for the diagnosis of ITL is the tuberculin test (PPD), although its most important drawback is its specificity since the proteins used are not specific for Mycobacterium tuberculosis. In recent years, research has been done and new diagnostic methods have been approved based on the in vitro quantification of the immune cell response, the so-called interferon gamma release assays (IGRA). Compared with PPD, the main difference is that IGRAs detect the release of interferon-gamma in response to specific tuberculous antigens. In the absence of a true reference test for the diagnosis of tuberculosis infection, it is difficult to establish the sensitivity and specificity of these new diagnostic techniques. IGRAs have been used in the detection of ITL in subjects with immune system alterations (HIV, EEI, IRC, rheumatologic diseases) with good results. They are also being extensively used in the study of contacts. In recent studies involving serial controls of said tests, they were observed to present conversions and reversions that occur after exposure to M. tuberculosis. Today and with the current knowledge, it seems that IGRAs can complement PPD, but not substitute them.
Un tercio de la población mundial presenta actualmente infección tuberculosa latente (ITL). En España la tuberculosis se sitúa como la tercera enfermedad de declaración obligatoria. La técnica habitual para el diagnóstico de ITL es la prueba de la tuberculina (PT), aunque su mayor problema es la especificidad, dado que las proteínas que utiliza no son específicas de Mycobacterium tuberculosis. En los últimos años se han investigado y aprobado nuevos métodos diagnósticos basados en la cuantificación in vitro de la respuesta inmune celular, los llamados interferon gamma release assays (IGRA). La diferencia fundamental con respecto a la PT es que detectan la liberación de interferón gamma en respuesta a antígenos tuberculosos específicos. En ausencia de una auténtica prueba de referencia para el diagnóstico de la infección tuberculosa es difícil establecer la sensibilidad y la especificidad de estas nuevas técnicas diagnósticas. Los IGRA han sido empleados en la detección de ITL en sujetos con alteración del sistema inmune (VIH, EEI, IRC, enfermedades reumatológicas) con buenos resultados. También están siendo muy utilizados en el estudio de contactos. En estudios recientes en los que se realizaron controles seriados sobre dichos test se observó que presentan conversiones y reversiones que ocurren después de la exposición a M. tuberculosis. A día de hoy y con los conocimientos actuales, parece que los IGRA pueden complementar la PT, pero no sustituirla.
According to the data of the World Health Organization (WHO),1 one-third of the world's population currently presents latent tuberculosis infection (LTI). In 2006, there were more than 9200000 new cases of tuberculosis (TB) the world over, with a prevalence of more than 14 million people, and almost 1.7 million deaths, which translates into a mortality of 18%. The WHO considers that the rate of world-wide incidence of TB reached its peak around 2002 and that afterwards it has stabilized or started to decline, but this fact is counteracted by the increase in population, which means that the actual number of new cases continues to rise. In 2005, in the region of Europe, the WHO was notified of 426717 cases of TB, with a rate of incidence of 48/100000 inhabitants, with a great difference between the different areas of the continent.
Tuberculosis in SpainAccording to the latest data published by the Spanish Epidemiological Vigilance network (Red de Vigilancia Epidemiológica), in mid-July 2009, 3340 new cases of TB had been notified.2
According to provisional data published by this national center, in 2009, 6070 cases of TB were registered. However, these numbers should be taken with a grain of salt as, despite the fact that TB is a disease requiring mandatory notification, it is estimated that at least one-third of cases are not reported.
Target PopulationThe data that are available about the natural history of TB suggest that in the first two years after the infection by Mycobacterium tuberculosis, between 5% and 10% of infected individuals develop tuberculosis disease.3 With a proper immune response by the infected individual, the bacillus can remain inactive for decades or even for a life-time. Consequently, the diagnosis and treatment of tuberculosis infection will be more effective if they are aimed at individuals at greater risk for the progression from infection to tuberculosis disease, including recently infected individuals and immunosuppressed patients.4
Diagnosis of Tuberculosis InfectionThe standard technique for diagnosing tuberculosis infection is the tuberculin test, which, after the injection of a purified protein derivative (PPD), shows evidence of a state of prior hypersensitivity of the organism against said substance. The tuberculin used in Europe is PPD RT-23. In the United States, there are two preparations, Aplisol and Tubersol, both with similar responses to RT-23. The main disadvantage of PPD is that the proteins used are not specific for M. tuberculosis, but are shared with other non-tuberculous mycobacteria and Mycobacterium bovis, which reduce the specificity of said test.
Immunological BasisIndividuals infected by M. tuberculosis react to PPD with a delayed hypersensitivity response mediated by cells (especially T lymphocytes), and after 48–72h an induration appears in the area of the injection. This hypersensitive response remains for life, although it may be reduced in the elderly, as well as in certain clinical alterations. Repeated PPD in a non-sensitized individual does not alone trigger the immune response.
Technique UsedThe Mantoux technique5 entails the intradermal injection of 2 units of PPD RT-23 tuberculin (0.1ml) with a 27-gauge needle on the inside of the forearm in an area where there are no skin lesions. In order for the technique to be correct, a papule measuring 6–10mm in diameter should be produced at the time of the injection. This is the most common PPD method.
Reading and InterpretationThe results are read 72h after the injection by measuring the cross-sectional diameter of the induration following the longitudinal axis of the forearm. The measurement is taken in millimeters. In cases where there is no induration and instead only erythema, it is interpreted as 0mm.
According to the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), the following indurations are considered positive6:
- -
In non-vaccinated persons, ≥5mm.
- -
In persons vaccinated with BCG, this poses the problem of discerning whether a tuberculin induration is due to a tuberculosis infection or instead to a response to the antigens that are shared with the BCG vaccine (M. bovis BCG). In this situation, certain clinical conditions are taken into account, considering the PPD positive with a diameter ≥5mm if, in addition to being vaccinated, the subjects either live with or maintain frequent contact with bacilliferous patients, present chest radiography with lesions suggesting former TB that had never been treated, are infected by HIV or are patients with pneumoconiosis.
- -
In the rest of subjects vaccinated with BCG, the results are determined to be an infection and not a secondary reaction to the vaccine if the size of the induration is >15mm. In general, the vaccine reaction does not usually cause indurations of more than 14mm, and it is considered that the greater their size and the longer the time passed since the vaccination, the more likely it is due to a tuberculous infection and not a vaccine reaction (the studies in this direction indicate that the interference of BCG on tuberculin reaction can be limited some 10–15 years after vaccination).4
The greater the probability of being infected or of developing the disease (for instance, in cases of recent contact or those infected with HIV), less should the vaccination history influence the interpretation of the test. In patients infected by HIV, a PPD <5mm does not exclude the diagnosis of infection as it could be due to the situation of anergy caused by the compromised immune response.
Tuberculin reactions with vesicles or necrosis in the inoculation area are also considered indicative of tuberculosis infection, regardless of the size of the induration or the vaccination history.
In cases of contact studies, the interpretation is rather simplified, as the vaccination history should not be contemplated and an induration ≥5mm is considered indicative of tuberculosis infection.
Table 1 summarizes the situations that can cause false positives of the test.
False Positives of the Tuberculin Test.
| 1. Individuals vaccinated with BCG |
| 2. Infection by opportunistic environmental mycobacteria (OEM) |
| 3. Individuals not sensitized to Mycobacterium tuberculosis who receive blood transfusions from sensitized persons |
| 4. Rupture of a vessel or infection in the area of the injection |
There are certain circumstances depending on the individual that may trigger false negatives with PPD, such as a concurrent viral infection, vaccinations with live viruses, immunosuppressive situations or treatment with drugs that diminish the immune response.
Table 2 summarizes the situations that could lead to false negatives of the test.
False Negatives of the Tuberculin Test.
| Related with the individual tested with PPD |
| 1. Viral infections: HIV, chicken pox, measles, parotiditis |
| 2. Bacterial infections: typhoid fever, brucellosis, leprosy, whooping cough, pleural, and disseminated tuberculosis |
| 3. Fungal infections: blastomycosis |
| 4. Vaccinations with living virus: measles, parotiditis, chicken pox |
| 5. Metabolic alterations: chronic renal failure |
| 6. Alterations in the protein state: severe protein depletion, afibrinogenemia |
| 7. Diseases of the lymphoid organs: lymphomas, chronic lymphocytic leukemia, sarcoidosis |
| 8. Drugs: corticoids and other immunosuppressants |
| 9. Age: newborns and seniors |
| 10. Situations of stress: surgery, burns, mental disease, graft versus host reaction |
| Related with the tuberculin used |
| 1. Inadequate storage (exposure to light and heat) |
| 2. Inappropriate dilutions |
| 3. Chemical denaturalization |
| 4. Contamination |
| 5. Adsorption (partial control with Tween 80) |
| Related with the method of administration |
| 1. Injection of sufficient quantity |
| 2. Subcutaneous injection |
| 3. Late administration once extracted from the vial |
| 4. Superficial injection with rupture of the vesicle formed and loss of |
| liquid |
| 5. Injection in an inflamed and vascularized area, diffusing the liquid |
| Related with the reading |
| 1. Inexperience of the reader |
| 2. Improper reading |
In some individuals, basically in older people infected years beforehand or in persons vaccinated in childhood, an initial PPD may be negative and a repeat test 7–10 days later may show positive. This “booster” phenomenon implicates that the first PPD can have a memory effect in the immune system, which, after a second test, produces the immune response. The definitive result of the test is considered that of the second reading.
So-called tuberculin conversion is the situation in which an individual with PPD considered negative is then later positive. This represents the acquisition of a recent tuberculous infection and is defined as “converter”. Functionally, it is defined as an individual who goes from having a tuberculin test of <5mm to having one of ≥5mm, with a difference of at least 5mm in less than 2 years.
PPD, as all diagnostic tests, should only be used in persons in whom a possible therapeutic intervention could be based on the results.3 In TB, there are only two possibilities for therapeutic intervention: treatment of the patients and chemoprophylaxis, or preventive treatment of infected patients with a high risk for developing TB.
Table 3 shows the indications for PPD according to SEPAR.6
Indications for the Tuberculin Test.
| 1. Individuals with clinical suspicion of tuberculosis disease |
| 2. Individuals with high risk of progression from tuberculosis infection to disease due to medical conditions: HIV, IVDA, immunosuppressant treatment, silicosis, diabetes mellitus, malignant hematological diseases, chronic renal failure, alcoholism, malnutrition, gastrectomy, solid organ recipient |
| 3. Individuals with social risk for developing tuberculosis: health-care professionals, prison workers, teachers, laboratory personnel, immigrants from countries with high rates of infection |
| 4. Individuals with non-evolutionary lesions on thoracic radiography suggestive of tuberculosis |
| 5. Epidemiological studies |
| 6. Individuals at risk for recent infection; contact with TB |
In the general asymptomatic population, its use is not recommended as a screening method.
In recent years, new diagnostic methods have been researched and approved based on the in vitro quantification of the cellular immune response. These methods, generically referred in the literature as interferon gamma release assays (IGRA), detect the release of interferon gamma in response to specific tuberculous antigens.7
Immune Response to Tuberculosis InfectionInterferon gamma is an important molecule for the control of tuberculosis infection, and its participation is essential in the immune response that protects against said microorganism. This cytokine, produced by the CD4+, CD8+, and NK T lymphocytes, activates the infected macrophages, with the consequent release of IL-1 and TNF-α, limiting the growth and the multiplication of the mycobacteria. The individuals with deficiencies in the receptors or in the genes that codify the synthesis of this molecule are more susceptible to having mycobacterial infections with greater frequency and with greater severity.
IGRA Determination TechniquesThere are two commercial techniques for the in vitro diagnosis of tuberculosis infection: QuantiFERON-TB-Gold In Tube (Cellestis®, Victoria, Australia)8 and T-SPOT.TB (Oxford Immunotec®, Oxford, United Kingdom).9 The first generation of QuantiFERON-TB, approved by the Food and Drug Administration (FDA) in the year 2001, detected the release of interferon gamma in response to PPD. In 2004, the FDA approved the second generation of this diagnostic test, known as QuantiFERON-TB Gold, which, unlike the first generation did not use PPD as mycobacterial antigens, but instead used synthetic peptides that simulate more specific antigens, such as Early Secreted Antigenic Target-6 (ESAT-6) and Culture Filtrate Protein-10 (CFP-10). These two molecules are codified by the RD-1 region of the genome of M. tuberculosis and significantly increase the specificity compared with PPD. These antigens are absent in M. bovis BCG and in the majority of the non-tuberculous mycobacteria (except Mycobacterium kansasii, Mycobacterium marinum, Mycobacterium szulgai) (Table 4).
Specificity of ESAT-6 and CFP-10 in the Mycobacteria.
| Mycobacterium Tuberculosis Complex | Antigens | Environmental Mycobacteria | Antigens | ||
| ESAT-6 | CFP-10 | ESAT-6 | CFP-10 | ||
| M. tuberculosis | + | + | M. abscesus | − | − |
| M. africanum | + | + | M. avium | − | − |
| M. bovis | + | + | M. banderi | − | − |
| Strains contained in the vaccine | |||||
| Gothenburg | − | − | M. chelonae | − | − |
| Moreau | − | − | M. fortuitum | − | − |
| Tice | − | − | M. gordonae | − | − |
| Tokyo | − | − | M. intracellulare | − | − |
| Danish | − | − | M. kansasii | + | + |
| Glaxo | − | − | M. malmoense | − | − |
| Montreal | − | − | M. scrofulaceum | − | − |
| Pasteur | − | − | M. smegmatis | − | − |
| M. szulgai | + | + | |||
| M. terrae | − | − | |||
| M. xenopi | − | − | |||
Currently, the third generation of this test is on the market, known as QuantiFERON-TB Gold In Tube (QFT-GIT), which incorporates a third mycobacterial antigen: TB 7.7, and tubes specifically designed for taking blood samples for this test (Table 5).
Interpretation Criteria for T-SPOT.TB (T-Spot).
| Null Controla | Response to TBb | Response to Mitogenc | |
| Positived | ≤10 spots | ≥8 spots | Any |
| Borderlinee | ≤10 spots | 5, 6, or 7 spots | Any |
| Negativef | ≤10 spots | Any | ≤4 spots |
| Indeterminatee | >10 spots≤10 spots | <5 spots | Any<20 spots |
Based on Oxford Immunotec Limited©.
The number of spots resulting from the incubation of peripheral blood mononuclear cells (PBMC) in a culture medium without antigens.
Number of spots resulting from the stimulation of PBMC with two separate groups of the ESAT-6 and CFP-10 peptides, except the null control.
- 1.
QFT-GIT: In this technique, 3 specific tubes are used that come with the reagent kit (one of the tubes includes the ESAT-6, CFP-10, and TB 7.7 specific tuberculous antigens, called the problem tube; another contains phytohemagglutinin, which is the positive control tube; and the third contains no reagents, which is the negative control tube). A total of 3ml of blood is required (1ml per tube) and the blood is extracted directly into the tubes themselves. Later, after having shaken the tubes, they are incubated for 18–22h at 37°C, after which time the tubes are centrifuged and the plasma obtained is used for the enzyme immunoassay that detects and quantifies the interferon gamma released by the lymphocytes of the patient. This step can be done either manually or totally automatically. After incubation, the plasma may be stored for several weeks without affecting the results, which, if necessary, would facilitate the organization of the work load of the laboratory. The technique uses specific software for the emission of the results.
- 2.
T-SPOT.TB: In order to carry out this technique, 8–10ml of heparinized blood is used. In the laboratory, and following the indications of the manufacturer, the mononuclear layer is separated, in which, after washing and later cell count, the number of cells will be adjusted to a quantity of 250000cells/ml. This quantity will be used as inoculum in the 4 wells that are included in this test (2 wells with the ESAT-6 and CFP-10 antigens, and the other 2 for the positive and negative controls). The plate is incubated for 18–22h at 37°C in a CO2 incubator, after which time the immunospot is done, allowing for the quantification of the number of interferon producing cells (observed as spots, each spot representing the footprint of an individual T-lymphocyte interferon secretor). An interpretation algorithm developed by the manufacturer facilitates the emission of the results. Technically, T-SPOT.TB requires more blood, more preparation time and is more painstaking than QFT-GIT. Furthermore, it does not allow for working with the samples in a differed manner. The guidelines recommended by the manufacturers of these tests for their interpretation are shown in Tables 5 and 6.8,9
Table 6.Interpretation Criteria for QuantiFERON-TB Gold In Tube (QFT-GIT) Test.
Interpretation Null Controla Response to TBb Mitogenc Positived ≤8.0 ≥0.35IU/ml and ≥25% of the null control Any Negativee ≤8.0 <0.35IU/ml or <25% of the null control ≥0.5 Indeterminatef ≤8.0 <0.35IU/ml or <25% of the null control <0.5 >8.0 Any Any Based on Cellestis Limited. QuantiFERON-TB Gold©.
The new in vitro diagnostic techniques for tuberculosis infection offer important advantages over PPD: they do not present interferences with the BCG vaccine, the subjectivity of the interpretation is avoided, an appointment for reading the results is avoided and they incorporate a positive control that provides valuable information for interpreting an apparently negative test as either a true negative or indeterminate as a result of technical errors or due to immunosuppression.
Sensitivity and SpecificityWith the lack of a true reference test for the diagnosis of tuberculous infection, it is difficult to establish the sensitivity and the specificity of these new diagnostic techniques.
In order to resolve the problem of sensitivity, three strategies have been used: (1) evaluate the patients who have active TB and therefore should be infected; (2) evaluate the individuals who have been in contact with tuberculous patients and stratify them according to degree of exposure; (3) analyze the agreement between the IGRA and PPD determination tests.10,11
Clinical Performance of the IGRA Determination Techniques in Immunocompetent PatientsAn important facet of the transmission of TB is that the risk for infection is mainly determined by the frequency, duration and the proximity of the contact with the person diagnosed with tuberculosis. Therefore, for a new technique to be considered more sensitive and more specific than PPD, it should be more closely related with the level of exposure and should not take into regard the state of vaccination (BCG). This theory has been used to compare the degree of precision of the IGRA determination techniques with PPD in contact studies,11,12 which have concluded that these new techniques correlate equally well or even better than PPD, regardless of BCG.
In one of the largest studies published to date that included 535 subjects,12 the results of QTF-GIT and T-SPOT.TB were not interfered with by the history of vaccination with BCG, although this did occur with the tuberculin test, which demonstrates the greater specificity of the former. In addition, IGRA also correlated better with the exposure to TB disease.13–18 In one of the studies carried out in exposed children,18 a dose-response relationship was found between the bacilli load in the sputum and the positivity of the IGRA. Those who had been in contact with the patients with a greater bacilli load in sputum more frequently had positive IGRA and tuberculin tests.19–22
Clinical Performance of QTF-GIT and T-SPOT.TB in Immunosuppressed PatientsTuberculosis and HIVTB has become the most important cause of co-infection in the patients infected by HIV, a situation that affects approximately 13 million people in the world.1 In Africa, TB is the main cause of death in patients infected with HIV, and in addition it is the most frequent disease in patients with AIDS who are being treated with antiretroviral drugs. The detection of LTI is crucial in persons infected with HIV because they have a higher rate of progression to tuberculosis disease than non-infected persons, even if they are in antiretroviral treatment.
The diagnosis of LTI in patients infected by HIV has been traditionally based on PPD, which, in addition to the disadvantages mentioned before, adds in these patients an important rate of anergy.23,24
In comparison with PPD, the data on the current generation of IGRA determination techniques suggest greater specificity,25 fewer false positives due to previous vaccination with BCG26 and a greater sensitivity in populations with a low incidence of TB.27 However, there are few data describing the performance of IGRA in persons infected by HIV, whose immune alterations can affect the performance of these tests that are based on the activation of lymphocytes.28
As for the diagnostic performance of IGRA in those infected by HIV, a series of studies have added consistency to the greater sensitivity of T-SPOT.TB over PPD. A recent study23 found that T-SPOT.TB was more sensitive than PPD and correlated better with active TB.
With regards to the level of CD4, several authors29 found a number of indeterminate results that were related with patient CD4 levels. Thus, the rate of indeterminate results in patients with CD4≥100 cells/mm3 was 3%, while for the patients with CD4<100 cells/mm3 it was 24%. If we take into account the viral load, the number of indeterminate results increases proportionally as it increases. Thus, for example, in patients with undetectable viral load, 15% of indeterminate results were obtained. In the patients considered category 2 (1log−4logRNA HIV copies/ml) 17% indeterminate results were obtained; in category 3 (4log−7logRNA HIV-1 copies/ml) it was 28%.30,31
Inflammatory Bowel DiseaseInflammatory bowel disease (IBD) includes a heterogeneous group of disorders that run their course with chronic inflammation of the gastrointestinal mucosa. Up to one-third of patients with IBD have a severe form of the disease that requires the use of immunomodulatory drugs or biological agents for the control of the inappropriate response of the immune system in these patients. Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine that plays an important role in the pathogenesis of IBD. Infliximab (IFX), an IgG1 monoclonal anti-body that specifically binds with TNF-α, has been demonstrated to be effective for inducing and maintaining remission, both in Crohn's disease as well as in ulcerous colitis.
In 2001, the FDA reported on 70 cases of TB out of the 147000 patients treated with IFX throughout the world, both for IBD as well as for rheumatoid arthritis. Two-thirds of the cases were found in Europe, and Spain was the European country with the greatest number of affected patients.32 The majority had an extrapulmonary location, with disseminated disease in 24% of cases. This type of tuberculosis disease is associated with immunosuppression, which suggests that TNF-α plays an important role in the response of the host against TB, which includes the formation of granulomas.33 Given that the majority of the cases appeared after starting treatment with IFX, that the prevalence of TB in their countries was low and that they had not recently been exposed to patients diagnosed with TB, they concluded that TB was caused by the reactivation of an LTI.34 These findings uphold the recommendation for adopting measures to rule out the existence of LTI, previous to the establishment of treatment with anti-TNF-α antibodies. In our country, the Spanish Group for Crohn's Disease and Ulcerous Colitis (Grupo Español para la Enfermedad de Crohn y la Colitis Ulcerosa—GETECCU) published their own recommendations35 in 2003, which were revised in 2006,36 according to which patients diagnosed with LTI should receive treatment with isoniazid before initiating treatment with biological drugs. The results obtained to date show an important reduction in TB cases within this population.37–39
Rheumatologic DiseasesPatients with chronic inflammatory rheumatic disease have a high risk for TB, which has increased even more after the incorporation of biological therapy. In most cases, the disease is caused as a consequence of the reactivation of a latent infection; therefore, its incidence varies considerably depending on the prevalence of infection in the area studied.
The practice of systematic screening, both for infection as well as for tuberculosis disease prior to the start of biological therapy, has led to a lower incidence of TB in this population. The experience of the Spanish cohort of patients treated with biological agents (BIOBADASER) of the Spanish Rheumatology Society shows a reduction in TB cases since the implementation of the official recommendations in 2002 when compared with previous years (117 vs 522 cases/100000 persons-year, respectively). However, we must also highlight an important increase in the use of etanercept (human protein made up of receptor p75 of the tumor necrosis factor and the Fc portion of the human IgG1) during the second period of the study, in detriment to IFX, which could explain part of the reduction in incidence. Even when assuming the effectiveness of these measures, the incidence of TB in that patient collective continues to be almost five times higher than that of the general Spanish population. Several causes could explain this fact: inadequate application of the protocols, lack of compliance with the treatment for the tuberculosis infection, exogenous reinfection, and the limitations of PPD for diagnosing latent infection in the clinical context of these patients.
Results With IGRA in Inflammatory Bowel Disease and in Rheumatic DiseasesThe experience with IGRA in the diagnosis of LTI in the disease mediated by inflammatory mechanisms is still rather limited and comes from small-scale cross-sectional studies centered on evaluating the agreement between PPD and IGRAs, without correlating the results with the risk factors for tuberculosis infection. They conclude that the agreement between PPD and IGRA is low and that the disagreeing results (PPD+, IGRA−) are due to previous vaccination with BCG.37–41
As for the correlation between IGRA and TB risk factors, in a recent study of 142 patients with disease mediated by inflammatory mechanism,37 the IGRAs were more closely related with risk factors for LTI than tuberculin. Moreover, the positivity of PPD correlated with the previous vaccination with BCG, not true for IGRA. Martin et al.39 compared the two IGRA tests in patients with rheumatoid arthritis and they observed that both QTF-GIT as well as T-SPOT.TB correlated with TB risk factors.
Based on current evidence, it can be concluded that, in patients with inflammatory disease mediated by immune mechanisms who follow an immunosuppressant treatment, the specificity of IGRA is greater than PPD.
Chronic Renal FailurePatients with chronic renal failure (CRF) who require hemodialysis or peritoneal dialysis are an example of a population that characteristically manifests cutaneous anergy to PPD antigens and that has a high risk for developing active TB42–45. This risk is approximately 10–25 times greater for the reactivation of an LTI in comparison with the general population.46–48 What is more, hemodialysis units are places where TB could be disseminated more easily.49
It is known that CRF is associated with numerous alterations of the immune system, the majority related with the alteration in cell immunity.50,51 Among them are the reduced proliferative response of the lymphocytes, the deficiency of interleukin-2, peripheral B lymphocyte deficiency and increased cell apoptosis.52–54 A recent study of 203 patients with CRF undergoing hemodialysis55 compared three diagnostic methods for TB infection (PPD, T-SPOT.TB, and expert evaluation). PPD showed a very low sensitivity and only presented positivity in one out of every five patients. T-SPOT.TB was positive in approximately three out of every four patients with risk factors for LTI. The sensitivity, backed by the panel of experts who confirmed the LTI cases, was nearly 75%.
Regarding peritoneal dialysis, the study by Palomar et al.56 states that these new techniques measure a type of immune response different from that involved in the delayed hypersensitivity response to PPD, and, unlike what happens in the contact study, in immunodepressed patients recent tuberculous infection is as important as remote infection. The study concludes that IGRAs complement PPD, as performing both simultaneously increases the diagnostic probability of TB.
The Value of QTF-GIT and T-SPOT.TB in the Prediction of the Development of Tuberculosis DiseaseFor a person with positive PPD, the risk for developing active TB is estimated at 5%–10%.57 However, there are very few longitudinal studies that allow us to make conclusions about the capability of IGRAs for predicting the risk of developing active TB.
In a study carried out in Germany with the participation of 601 contacts close to people with positive smears and positive cultures for M. tuberculosis, QFT-GIT performed better in the prediction of active TB58 than PPD, using a cut-point of 5mm. Five (2.3%) of the 219 contacts with indurations ≥5mm on PPD developed TB, while six (14.6%) of the 41 contacts with positive results for QFT-GIT developed the disease (P=.003). However, an unusually large proportion (59%) of the contacts had an induration (PPD) between 5 and 9mm. The proportion of those considered positive due to PPD with a cut-point of 10mm who developed active TB (5 out of 90 [5.6%]) was similar to the positive proportion of QFT-GIT (6 out of 41 [14.6%], P=.1). In addition, only 2 of the 6 contacts with positive QFT-GIT results that developed active TB had the diagnosis confirmed by culture. In another study, the sensitivity for predicting later active TB was not significantly different for the two tests.59
Another study of 339 immigrants in the Netherlands gave the results that PPD and QFT-GIT have a similar value for predicting active TB.60 There was a two-year follow-up of the contacts whose PPD was ≥5mm between 0 and 3 months after the diagnosis of the index patients. Nine (3.1%) out of 288 contacts with PPD ≥10mm developed active TB, compared with 7 (3.8%) out of 184 with PPD ≥15mm, 5 (2.8%) out of 178 with a positive QFT-GIT result and 6 (3.3%) out of 181 with a positive T-SPOT.TB developed the disease. The sensitivity for detecting the development of active TB over the course of the follow-up period was 100% for PPD with a cut-point of 10mm, 88% for a PPD with a cut-point of 15mm, 63% for QFT-GIT and 75% for T-SPOT.TB. Despite the fact that PPD with a cut-point of 10mm identified the greatest number of contacts who developed active TB (9 out of 9 [100%]), and QFT-GIT identified the least number of contacts that developed active TB (5 out of 9 [63%]), the sensitivities of the two tests were not statistically different (P=.08).
In conclusion, IGRAs, for the prediction of the development of tuberculous disease, do not seem to provide important differences compared to PPD.
The Use of QTF-GIT and T-SPOT.TB in Contact StudiesUntil now, many studies have been based on the study of contacts in TB. Initially, these were based on PPD, but since the incorporation of IGRA, these new techniques have been the topic of research on numerous occasions related with the contact studies.18,61–66 In two of these studies,18,66 the greater the recent exposure (greater duration of the exposure or greater number of acid-fast bacilli in the source sputum samples) was more associated with the positive results for IGRA than with PPD, which suggests that IGRAs could be better than PPD in detecting recent infection. In these studies, people with less exposure were more likely to be positive to PPD than to IGRA, which suggests that PPD could have been better than detecting the infection by means of IGRA for the detection of old/remote infection that was produced before (and therefore was not caused as a result of) recent exposure.63
In another research study,61 the proximity of the recent exposure (meaning the same room, another room, another dwelling) is more associated with the results of PPD than with the QFT-GIT results.
In the case of IGRA with regard to the study of contacts, we can conclude that although there are suggestions that their positivity could indicate recent infection, this is not proven and more studies will be needed to confirm this.
Conversions and Reversions in IGRAThe studies carried out until now about IGRA did not do serial controls of said tests. In the study by Hill et al., which was performed by using the serial IGRA test in a series of contacts exposed to persons diagnosed with TB in a country with high prevalence (Gambia), it was observed that IGRA—more specifically ELISPOT—occasionally present conversions and reversions that occurred after the exposure to M. tuberculosis.67–71
In order to interpret the result of serial, we should first answer a series of questions:
- 1.
What is the reproducibility of the response of the T cells in a specific timeframe? (variations in the inter-individual response).
- 2.
What do we consider reversion and what cut-point should we use to define it?
- 3.
What is the clinical significance and the prognosis of a reversion?
- 4.
What is a conversion and what cut-point should be used to define it? How can it be distinguished from the non-specific variations of the T cell response over time?
- 5.
What is the prognosis of an IGRA conversion? Do individuals with clear conversions (e.g. a large increase in the interferon gamma response over time) have a greater risk for the progression to active disease than the individuals with weaker conversions or negative results?
Unfortunately, none of the studies published to date provide evidence on the cited questions, which seems to demonstrate that conversions and reversions are produced when the IGRA tests are serial and likewise when PPD are serial.68–71
These serial studies demonstrate at the same time that IGRAs are very dynamic tests and that the response of the T-cells, especially the weakly positive responses, tend to fluctuate over time, even in absence of specific treatment.
Although the data that are currently available are limited, they suggest that the positive results vary more than the negative. This is in part expectable because the positive results can vary in both directions, while the negative results can vary between 0 and the diagnostic cut-point. In short, these new diagnostic methods can be inherently prone towards conversions and reversions, and it still has not been determined whether this characteristic dynamism is an influence when the results are evaluated.
On the other hand, many of these serial tests have been carried out in countries with high incidences of TB. It is not clear whether similar findings will be found in countries where the exposure to TB is less frequent.
IGRA Reversions and PrognosisIn general, the reversions are less frequent when the response to interferon gamma is strong.
In contrast, IGRA reversions are more frequent when both tests disagree (for example, positive IGRA, negative PPD). The discordant results are almost always weakly positive and are usually just above the cut-point.
Why do IGRA reversions occur? Some may reflect healing of the tuberculosis infection (spontaneously or secondary to treatment). Other reversions are caused by biological variations between individuals positive to IGRA, and also can be produced due to the variability in the procedures carried out in the laboratory.
In daily practice, the individuals who had had a positive result to IGRA will not undergo the test again, as neither will those individuals positive for PPD.
In that case, are reversions important outside the setting of research studies? Probably not, until we have a better understanding of the significance and the prognosis of said reversions.
IGRA Conversions and Their SignificanceDespite that fact that a high rate of IGRA conversions have been documented in high-risk populations with a high incidence of TB, there is no agreement in how to define the conversions.
With the data that are available, we cannot answer the following questions:
What degree of increase in interferon gamma indicates a new infection? How much of this variation is due to the test itself or to the biological variability? Should the same cut-point be used to define LTI and conversions? Some studies show that if a “negative-to-positive” cut point is used to define conversions, these can be greater with IGRA than with PPD, which can indicate greater sensitivity to the conversions (not necessarily for the diagnosis of LTI). However, a part of these may be due to non-specific variations around the cut-point. None of the completed studies take into consideration said variations.
Prognosis for IGRA Conversions and Potential Use of IGRA as a Predictive Test for the Development of Tuberculosis DiseaseThe risk for developing active TB has been established in various cohort studies using PPD as a reference test. Likewise, thanks to controlled clinical assays, we know that initiating prophylactic treatment in persons with positive PPD reduced the risk for active disease.72
Unfortunately, there are no equivalent data for IGRA. The data that we have available are limited to a small study73 that was carried out between the family contacts of index cases and obtained as a result an association between persons with a strong response of interferon gamma to stimulation with ESAT-6 and later progression to active TB. Despite this, the prognosis of a positive result to IGRA has still yet to be determined.
What is the prognosis of an IGRA conversion? The conversion implies recent infection (incident), and the prognosis is different than the other infection that already existed previously (prevalent). On the other hand, the prognosis of a “strong conversion” can be very different than that of a “weak conversion”. There are no data that respond directly to the question of the prognosis of the conversions, but up-and-coming data suggest that responses to both ESAT-6 and CFP-10 antigens closely correlate with in vivo replication and with the progression from infection to disease. Recently, Andersen et al.74 have suggested a hypothesis by which high and/or increasing interferon gamma levels produced in response to ESAT-6 by the T cells in recently infected persons can be a sign of incipient disease, and therefore can serve as a prognostic marker of the later development of the clinical disease in the near future.
Cost-Effectiveness AnalysisThe study of contacts of patients with TB is recommended as a means for detecting infected persons who may later develop the disease. It has been demonstrated that the treatment of infected persons, fundamentally with isoniazid, diminishes the development of future TB cases.75 The effectiveness and cost-effectiveness of these programs are very influenced by the precision when identifying the individuals infected with risk for developing tuberculosis disease.76
The guidelines referring to the use of IGRA depend on each country. Thus, for example, the Centers for Diseases Control (CDC) in the United States recommend substituting PPD with IGRA in all cases. Contrarily, in the United Kingdom, the National Institute of Health and Clinical Excellence (NICE) recommends the use of IGRA in combination with PPD, but only in the cases in which the tuberculin was positive.77
A recently published study78 comparing the two tests currently available (QTF-GIT and T-SPOT.TB) concludes that, for the study of contacts, joint PPD/IGRA strategies are more economical than the use of either T-SPOT.TB–QTF-GIT or PPD alone.
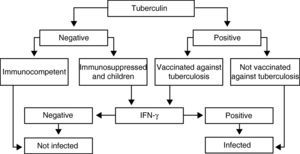
Recommendations (Fig. 1)The IGRA determination techniques in immunosuppressed patients and in children should be carried out when PPD is negative, as it could be a false negative as a consequence of immune alterations, and also when it is positive in persons who had previously been vaccinated with BCG, as it could be related with the vaccine itself.
ConclusionsThere are two meta-analyses10,79–88 that summarize the results that have been obtained to date with IGRA. The main conclusions are shown in Table 7.
Sensitivity of the Screening Tests.
| Sensitivity | PPD | QFT-GIT | T-SPOT.TB |
| No. of studies | >10 | 19 | 17 |
| No. of patients | 1238 | 988 | 837 |
| Sensitivity | 69.9% (0.67–0.72) | 81% (0.78–0.83) | 87.5% (0.85–0.90) |
| Heterogeneity | 81.3% | 77.5% | 75.6% |
| Specificity | PPD | QFT-GIT | T-SPOT.TB |
| No. of studies | 6 | 5 | 3 |
| No. of patients | 847 (no BCG) | 513 | 255 |
| Sensitivity | 97% | 99.2% (95% CI, 0.98–1.00) | 86.3% (95% CI, 0.81–0.90) |
| Indeterminate | QTF-GIT | T-SPOT.TB |
| No. of patients | 21,922 | 12,165 |
| Indeterminate | 2.14% (0.02–0.02)4.42% | 3.80% (0.03–0.04)6.12% |
IS: immunosuppressed.
As a summary, and taking into account current knowledge, the question is whether IGRA could substitute PPD for ruling out tuberculosis infection in patients who are about to receive treatment with agents that could affect immune system function. Where we stand today, it seems that they may be a compliment, but not a substitute. No data is available about the long-term development of TB that would allow us to make a decision on treatment based exclusively on the IGRA result. But that is not all, as the theoretical basis of IGRA also indicates that these techniques measure a type of immune response that is different to what is detected in the delayed hypersensitivity responses to PPD. Unlike what occurs in contact studies, in patients who are going to undergo immunosuppressant treatment, both recent as well as remote infections are important. In any case, IGRA represent a notable advance in the diagnosis of tuberculosis infection. Their place for screening persons at risk—among these candidates for treatment with agents against tumor necrosis factor—has still yet to be defined. In order to do so, longitudinal studies are necessary to provide solid evidence about the prognostic value of risk indicators for developing TB in the long-term.
Conflict of InterestThere is no conflict of interests.
The author's heart-felt thanks to José María García and Juan José Palacios for all their help during the completion of this paper.
Please cite this article as: Arias Guillén M. Avances en el diagnóstico de la infección tuberculosa. Arch Bronconeumol. 2011;47:521–30.