To analyze the reliability and validity of the Spanish version of the OSA-18 quality of life questionnaire in children with apnea-hypopnea syndrome (SAHS).
MethodChildren with suspected SAHS were studied with polysomnography (PSG) before and after adenotonsillectomy (AA). Age, gender, clinical data, PSG, anthropometric data, and Mallampati and Brodsky scales were analyzed. OSA-18 was administered at baseline and 3–6months post AA. After translation and backtranslation by bilingual professionals, the internal consistency, reliability, construct validity, concurrent validity, predictive validity and sensitivity to change of the questionnaire was assessed.
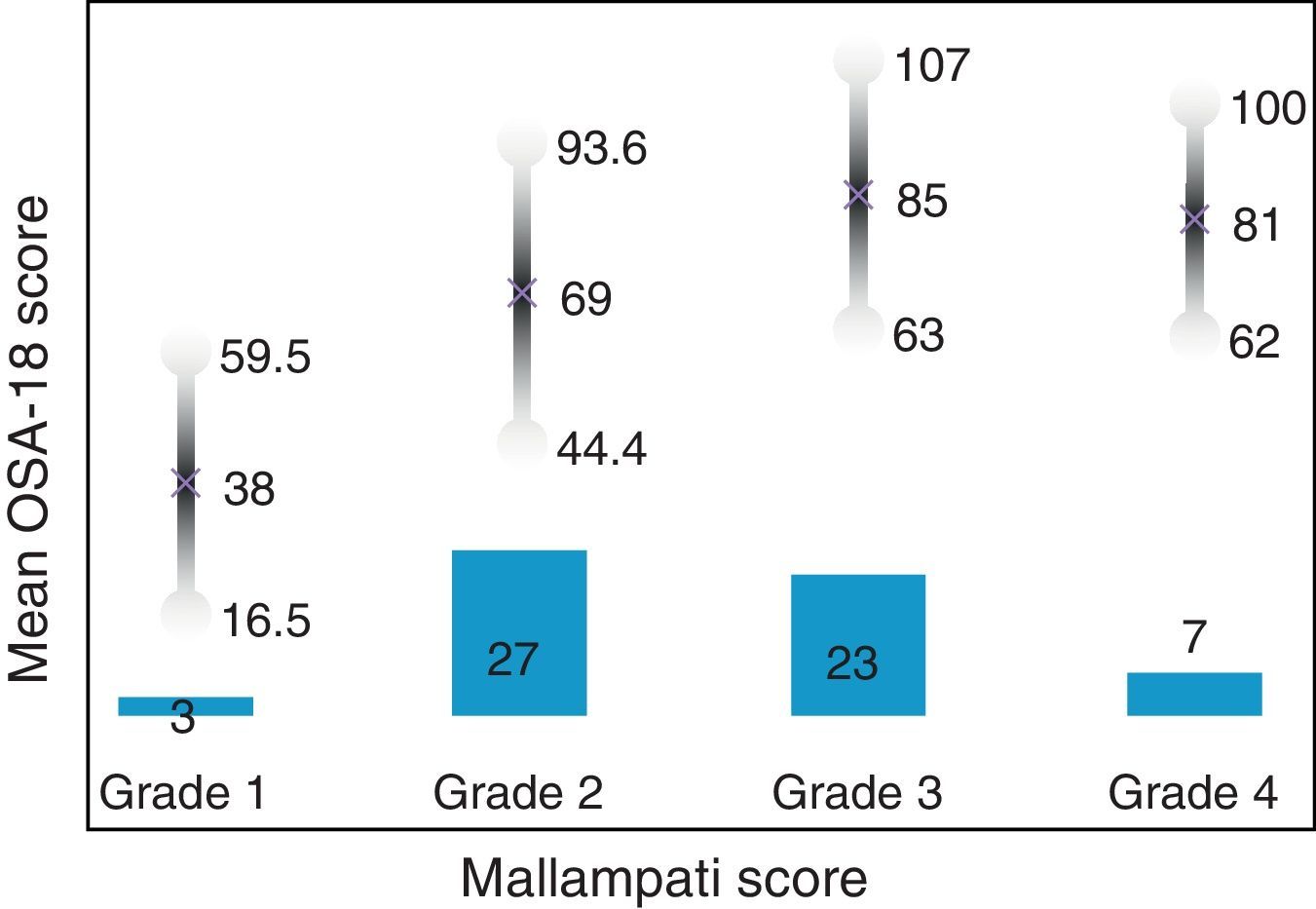
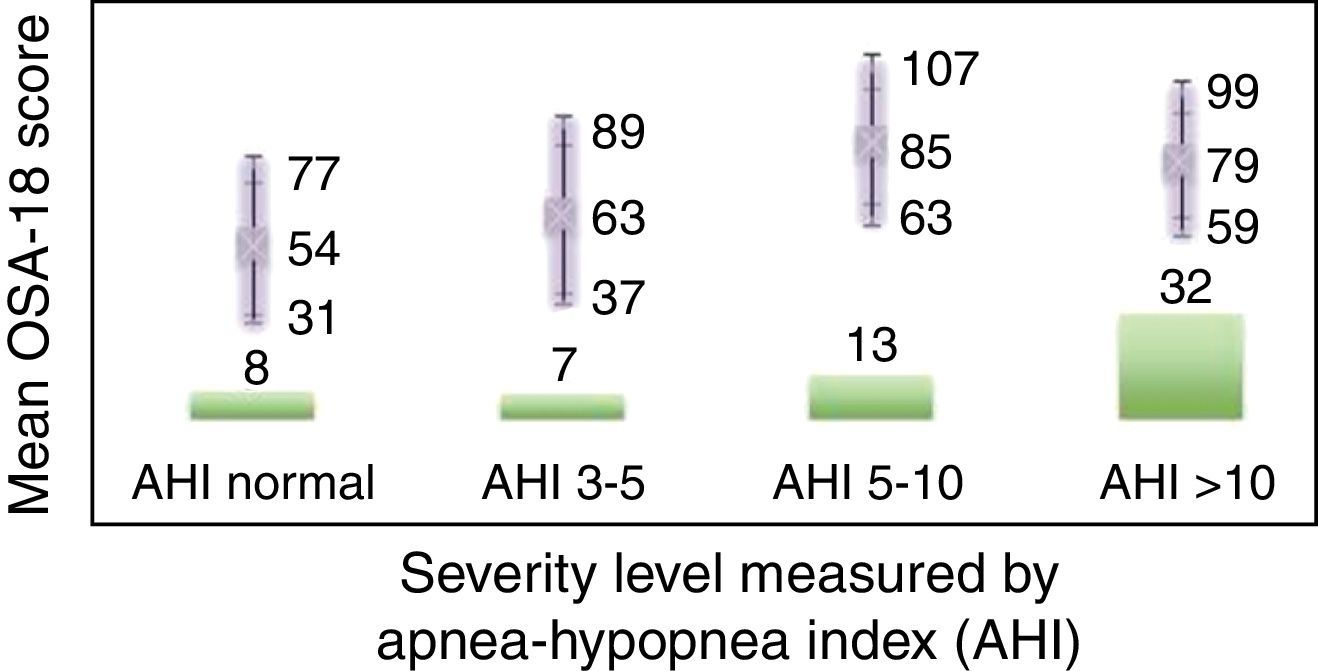
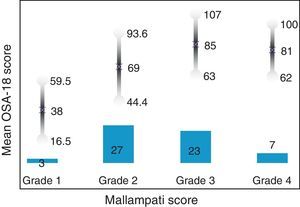
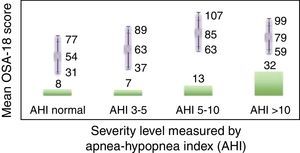
ResultsIn total, 45 boys and 15 girls were evaluated, showing BMI 18±4, neck 28±5, Brodsky (0: 7%; <25%: 12%; 25%–50%: 27%; >50 to <75%: 45%; >75%: 6%), AHI 12±7 pre AA. Global Cronbach alpha was 0.91. Correlations between domains were significant except for emotional aspects, although the total scores correlated with all domains (0.50–0.90). The factorial analysis was virtually identical to the original structure. The total scores showed good correlation for concurrent validity (0.2–0.45). With regard to predictive validity, the questionnaire adequately differentiated levels of severity according to Mallampati (ANOVA P=.002) and apnea-hypopnea index (ANOVA P=.006). Test-retest reliability was excellent, as was sensitivity to change, both in the total scores (P<.001) and in each domain (P<.001).
ConclusionsThe Spanish adaptation of the OSA-18 and its psychometric characteristics suggest that the Spanish version is equivalent to the original and can be used in Spanish-speaking countries.
Adaptar al español y analizar la fiabilidad y la validez de la versión española del cuestionario OSA-18 de calidad de vida (CVRS) en el síndrome de apnea-hipopnea del sueño (SAHS) infantil.
MétodoSe incluyeron niños con sospecha de SAHS a los que se practicó polisomnografía (PSG) pre y post adenoamigdalectomía (AA). Se analizó: edad, género, clínica, PSG, datos antropométricos, grados de Brodsky y Mallampati. Se administró OSA-18 basal y entre 3-6meses post AA. Tras la traducción-retrotraducción por personas bilingües se evaluaron la consistencia interna, la fiabilidad, la validez de constructo, la validez concurrente, la validez predictiva y la sensibilidad a los cambios.
ResultadosSe evaluaron 45 niños y 15 niñas: IMC18±4, cuello 28±5, Brodsky (0: 7%; <25%: 12%; 25-50%: 27%; >50 a <75%: 45%; >75%: 6%), IAH: 12±7 pre AA. El alfa de Cronbach del global fue 0,91. Las correlaciones entre dominios fueron significativas salvo para aspectos emocionales, aunque el global se correlacionó con todos los dominios (0,50-0,90). El análisis factorial mostró una estructura prácticamente idéntica al original. En la validez concurrente, el global mostró buena correlación (0,2-0,45). En su validez predictiva, diferenció adecuadamente los niveles de gravedad según Mallampati (ANOVA p=0,002) e índice apnea-hipopnea (ANOVA p=0,006). La sensibilidad al cambio fue excelente, tanto en el global (p<0,001) como en cada dominio (p<0,001), así como la fiabilidad test-retest.
ConclusionesLa adaptación española del OSA-18 es comprensible y sus características psicométricas sugieren que la versión española es equivalente a la original y puede ser empleada en países de habla hispana.
Sleep apnea-hypopnea syndrome (SAHS) in childhood is a very common disease affecting 2%–4% of children between 2 and 6 years of age.1,2 Tonsil and adenoid hypertrophy, craniofacial malformations, obesity, neurological diseases, such as infantile cerebral palsy, neuromuscular diseases and gastroesophageal reflux are some of the risk factors most commonly encountered in childhood.2 Pediatric SAHS is associated with significant morbidity mainly involving the central nervous system, causing neurocognitive (memory, general intelligence, executive function, etc.) and behavioral disorders, and affecting the cardiovascular system, leading to autosomal dysfunction, cardiac arrhythmias, arterial hypertension,3 ventricular remodelling,4 and endothelial involvement,5,6 and producing endocrine metabolic comorbidities.7,8
Chronic diseases such as SAHS can alter a patient's sense of well-being by affecting their psychosocial environment. These changes can be measured and quantified by way of health-related quality of life (HRQoL) questionnaires. Specific validated questionnaires are required for these purposes, as these tools are more sensitive to alterations following therapeutic interventions, and can be used to discern changes.9–11 Subjective perception of improvement after treatment (particularly when continuous positive airway pressure is required) is associated with improved patient adherence.12 A recent metaanalysis found that children with SAHS have a poorer health status than healthy children.13 As in adults, generic questionnaires have been used in this population, for example the Glasgow Children's Benefit Inventory,14 the Youth Quality of Life Instrument15 and others.13
The first questionnaires aimed at children and teenagers have only recently been developed. This population has particular features that differ from adults, so particular attention must be paid to evaluating the psychometric properties of these instruments.16 In childhood SAHS, questionnaires have so far evaluated HRQoL from the parents’ or caregivers’ point of view. Moreover, they have all been designed for administration in an English-speaking cultural context. If they are to be used in Spain, they must be translated and adapted to our culture.17–20 This must be done in a stepwise fashion: first the concept being measured must be shown to exist in the culture to which the questionnaire is being adapted, then the questionnaire must be translated from the original language to that of the target population, the metrics of the adapted questionnaire must be assessed, and its psychometric properties (validity and reliability) must be measured.21
Very few specific questionnaires have been designed for childhood SAHS, but the OSA-18, published in 2000 by Franco et al.,22 has been used in several studies for evaluating post-surgical changes, and has been proven to be reliable and sensitive to post-treatment changes.23–25
While many tools have been developed to measure sleep disturbances and disorders in adults, no specific validated questionnaire in Spanish for childhood SAHS has yet been published. Consequently, the aim of our study was to analyze the reliability and validity of the Spanish version of the HRQoL OSA-18 questionnaire for use in the initial evaluation and follow-up of children with SAHS.
MethodThe general guidelines of the International Test Commission26 and the guidelines proposed for adapting HRQoL questionnaires27 were followed to adapt the OSA-18. The adaptation process was based on the method of translation-backtranslation by professionals and a pilot study with patients at various stages.28
Study PeriodJanuary 2014–March 2015.
First Phase: Translation into SpanishAfter receiving permission from the authors of the OSA-18, the questionnaire was translated into Spanish by 2 bilingual experts whose mother tongue was Spanish. The 2 versions were unified by consensus between the translators and the researchers. This version was initially presented to the parents and caregivers of SAHS children to determine if the questions were clearly understood, and some aspects were modified to facilitate comprehension. The final text was given to an expert, English mother tongue, bilingual translator, for backtranslation, and after the relevant corrections were made, the translation into Spanish was finalized. The translators rated the difficulty of the translation and the backtranslation of each item on a scale of 1 (minimum)–10 (maximum). The researchers also scored rated each item in the Spanish version in terms of natural fluency and correctness on a scale of 1 (minimum)–10 (maximum). Finally, conceptual equivalence after backtranslation was assessed in 3 categories: A (fully equivalent), B (quite equivalent, some unconvincing expressions), and C (unconvincing equivalence).
Second Phase: Questionnaire ValidationTo determine whether the translated questionnaire met the same psychometric conditions as the original version, the reliability or internal consistency of each scale was evaluated using Cronbach's alpha, which measures the average correlation coefficient between each item and the scale as a whole, and between each item and the sum of items. Consistency was considered satisfactory when inter-group alpha was greater than 0.4 and the alpha of individual items was greater or equal to 0.8 (measuring a single dimension). The cohesion of the instrument was evaluated from the correlation between subscales and between each subscale and the total. To analyze concurrent validity (construct), we compared Pearson or Spearman correlation values (depending on the normality of the distribution of the variables) for the different domains of the questionnaire, overall and by subscale, with the apnea-hypopnea index (AHI) and other respiratory and anthropometric variables. Convergent validity correlated each item with its domain, and the divergent validity correlated each item with other domains. Predictive validity was analyzed using the Student t-test for independent samples to compare patient groups with different levels of SAHS severity, according to the AHI cutoff point. A concordance analysis was performed to evaluate the test-retest reliability, using the intraclass correlation coefficient (ICC) of the questionnaire administered initially and after 1-week, by items and overall score, in the same conditions. Concordance was considered good when ICC values were greater than 0.71 and moderate for values between 0.51 and 0.70.29
A descriptive analysis of the various study variables was performed after studying their distribution to determine the appropriate parametric and non-parametric statistic test, and the “ceiling” effect (percentage of responses with maximum score), the “floor” effect (percentage of responses with minimum score), and the percentage of unanswered items and the percentage of non-applicable items were evaluated.
Third Phase: Sensitivity to ChangeIn a third phase, changes in quality of life in response to treatment were evaluated using the OSA-18, comparing the values of all questionnaire domains before and after treatment, using a Student's t-test for repeated measures.
Population- •
Inclusion criteria Children aged 2–14 years referred to the Sleep Unit, with a diagnosis of SAHS on polysomnography (PSG).
- •
Exclusion criteria Inability to complete follow-up for reasons of remoteness from the hospital, immunosuppression (severely undernourished children with primary immunodeficiency and AIDS), parents’ refusal to participate in the study, previous history of airway surgery or other type of intervention, such as cauterization or thermoplasty.
The diagnostic protocol consisted of anthropometric measurements [weight, height, body mass index (BMI), BMI percentile, neck circumference], SAHS-related symptom questionnaire,30 Mallampati score,31 degree of tonsil hypertrophy according to the Brodsky scale, nocturnal PSG monitored in the hospital and baseline administration of the OSA-18 questionnaires to the parents or caregivers on the same day as the test. After PSG, children considered candidates for surgery were referred to the ENT department. Patients were evaluated in a repeat PSG between 3 and 6 months after surgery to evaluate changes in respiratory parameters after treatment. Anthropometric data and symptom questionnaires were collected the same day, and the OSA-18 questionnaire was repeated.
Polysomnography StudyPatients were diagnosed using an Alice 5 PSG system (Philips Respironics) with EEG, EOG, nasal flow (pressure probe), chin EMG, chest and abdomen movements, ECG and tibial EMG in both legs. Respiratory events were defined according to criteria from the consensus document on sleep apnea-hypopnea syndrome in children.1 Polygraphic recordings were interpreted and corrected manually by the same observer. Severity was classified as mild SAHS: AHI<5, moderate SAHS: AHI 5–10, and severe SAHS: AHI>10.1
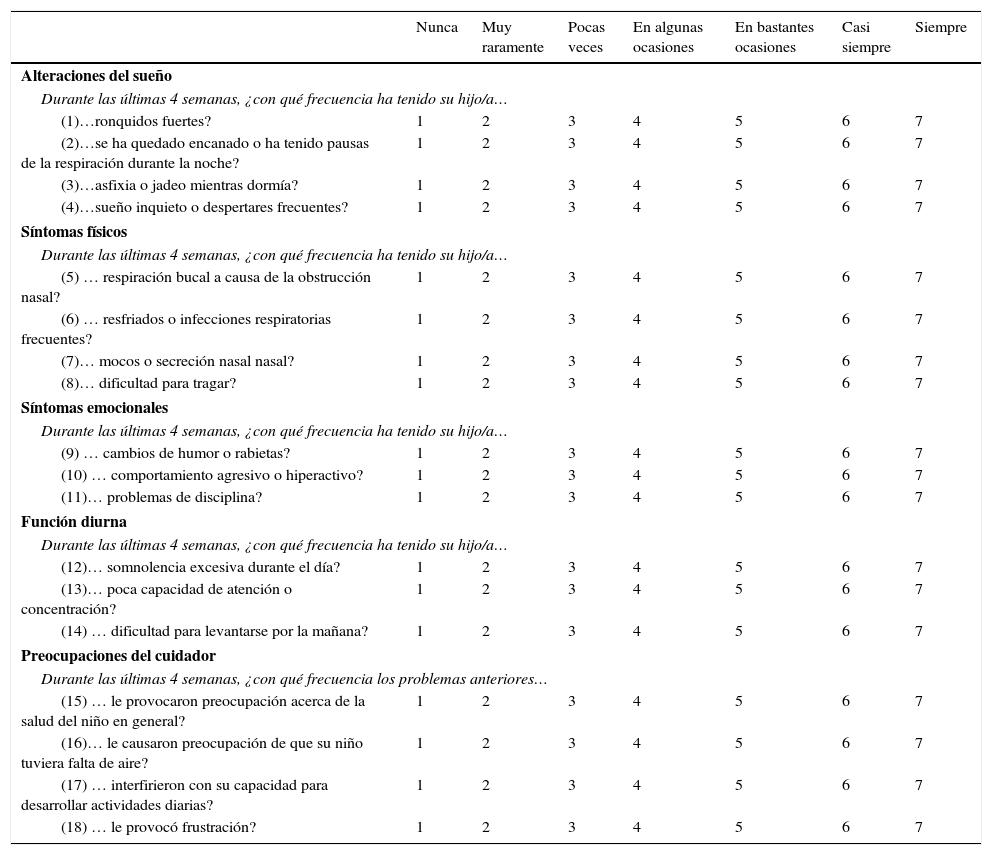
OSA-18 QuestionnaireThe OSA-18 questionnaire contains 18 items grouped in 5 domains, each of which is scored on a 7-point ordinal scale.
The OSA-18 domains are scored as follows:
- (a)
Sleep disturbance (4 items scored from 4 to 28)
- (b)
Physical suffering (4 items scored from 4 to 28)
- (c)
Emotional distress (3 items scored from 3 to 21)
- (d)
Daytime problems (3 items scored from 3 to 21)
- (e)
Parental or caregiver concern (4 items scored from 4 to 28)
The total OSA-18 score can range from 18 to 126. The OSA-18 questionnaire is used to classify the impact on quality of life as mild (score less than 60), moderate (score from 60 to 80), and severe (score higher than 80).
Calculation of Sample SizeFor the initial study, a confidence or safety interval was calculated (1−α): 95%; accuracy (d): 3%; proportion (approximate value of parameter measured): 5%, giving an initial sample (n) of 80 children. After adjusting the sample size for drop-outs, with an expected proportion (R) of 15%, the total sample was calculated as 60 individuals.
To calculate the number for patients needed to determine test-retest reliability and sensitivity to change, we determined a sample size of 20 patients. The criterion used to estimate the sample size was derived from the statistical requirements needed to calculate the reliability coefficient, assuming an expected reliability coefficient of ≥0.85 with that sample and a confidence interval of ±0.10 for Zα2=1.96.
StatisticsA descriptive analysis of the quantitative variables was performed, expressed as mean and standard deviation, or median and range, depending on the type of distribution. Distributions were analyzed for normality using the Kolmogorov–Smirnov test. In addition to the calculations used for validation of the questionnaires, we used the ANOVA test for repeat measures or the Kruskal–Wallis and multiple means comparisons to evaluate changes in scores for each of the questionnaires. The minimum level required for all calculations was P<0.05. Statistical calculations were performed using the SPSS program, version 18.
Ethical AspectsInformed consent for participation in the study was obtained from all parents and caregivers. The study was performed in accordance with the basic principles of the Declaration of Helsinki, the Council of Europe Convention on Human Rights and Biomedicine, the UNESCO Universal Declaration on the human genome and human rights, and Spanish legal requirements in the area of biomedical research, personal data protection and bioethics.
ResultsSixty consecutive patients were included, and all parents agreed to participate. In total, 45 boys and 15 girls were evaluated, aged 6±3 years, with a BMI of 18±4kg/m2 and mean neck circumference of 28±5cm. Brodsky index was 0 in 7%, <25% in 12%, 25%–50% in 27%, 50%–75% in 45%, and >75% in 6%. Mean AHI after PSG was 12±7h−1.
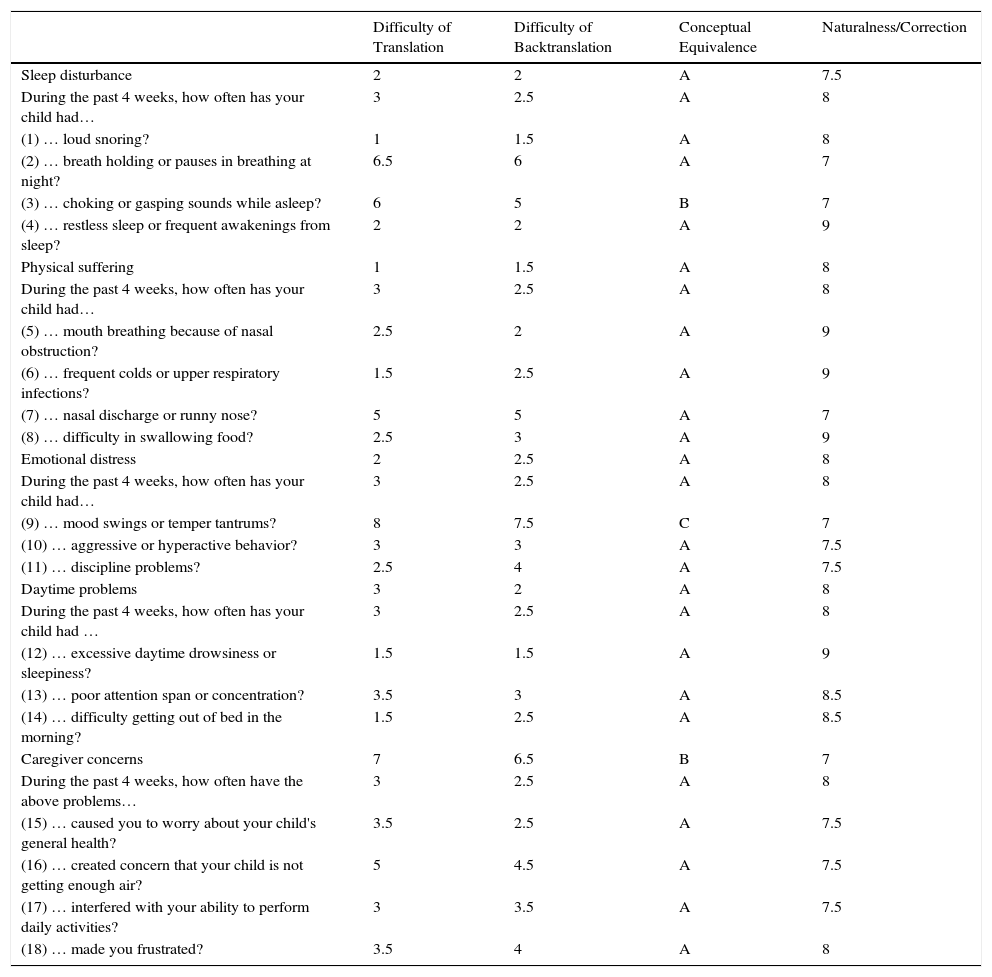
Translation difficulty was greater than 5 in 3 items (11%) and 1 domain (4%). After backtranslation, 2 items (7%) were considered type B and 1 was considered type C (4%). Questions surrounding equivalence (B and C) and expressions that were equivalent but lacked natural fluency or were grammatically incorrect were discussed by researchers and translators in 2 meetings. The final wording was agreed by consensus and included in the second version. The final version achieved scores >7 in all items and domains. The new version was administered to the parents of 10 children aged 2–14 years with a diagnosis of SAHS on PSG, and agreement was reached on the definitive version of the questionnaire (Table 1).
Translation and Backtranslation of OSA-18 Questionnaire.22
| Difficulty of Translation | Difficulty of Backtranslation | Conceptual Equivalence | Naturalness/Correction | |
|---|---|---|---|---|
| Sleep disturbance | 2 | 2 | A | 7.5 |
| During the past 4 weeks, how often has your child had… | 3 | 2.5 | A | 8 |
| (1) … loud snoring? | 1 | 1.5 | A | 8 |
| (2) … breath holding or pauses in breathing at night? | 6.5 | 6 | A | 7 |
| (3) … choking or gasping sounds while asleep? | 6 | 5 | B | 7 |
| (4) … restless sleep or frequent awakenings from sleep? | 2 | 2 | A | 9 |
| Physical suffering | 1 | 1.5 | A | 8 |
| During the past 4 weeks, how often has your child had… | 3 | 2.5 | A | 8 |
| (5) … mouth breathing because of nasal obstruction? | 2.5 | 2 | A | 9 |
| (6) … frequent colds or upper respiratory infections? | 1.5 | 2.5 | A | 9 |
| (7) … nasal discharge or runny nose? | 5 | 5 | A | 7 |
| (8) … difficulty in swallowing food? | 2.5 | 3 | A | 9 |
| Emotional distress | 2 | 2.5 | A | 8 |
| During the past 4 weeks, how often has your child had… | 3 | 2.5 | A | 8 |
| (9) … mood swings or temper tantrums? | 8 | 7.5 | C | 7 |
| (10) … aggressive or hyperactive behavior? | 3 | 3 | A | 7.5 |
| (11) … discipline problems? | 2.5 | 4 | A | 7.5 |
| Daytime problems | 3 | 2 | A | 8 |
| During the past 4 weeks, how often has your child had … | 3 | 2.5 | A | 8 |
| (12) … excessive daytime drowsiness or sleepiness? | 1.5 | 1.5 | A | 9 |
| (13) … poor attention span or concentration? | 3.5 | 3 | A | 8.5 |
| (14) … difficulty getting out of bed in the morning? | 1.5 | 2.5 | A | 8.5 |
| Caregiver concerns | 7 | 6.5 | B | 7 |
| During the past 4 weeks, how often have the above problems… | 3 | 2.5 | A | 8 |
| (15) … caused you to worry about your child's general health? | 3.5 | 2.5 | A | 7.5 |
| (16) … created concern that your child is not getting enough air? | 5 | 4.5 | A | 7.5 |
| (17) … interfered with your ability to perform daily activities? | 3 | 3.5 | A | 7.5 |
| (18) … made you frustrated? | 3.5 | 4 | A | 8 |
NB: transcription of original OSA-18 questionnaire published in 2000 by Franco et al.22
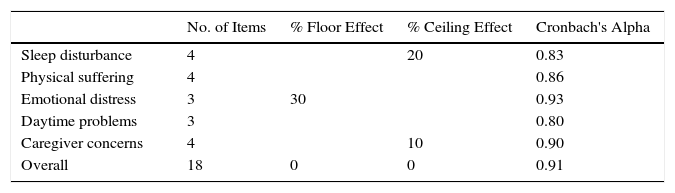
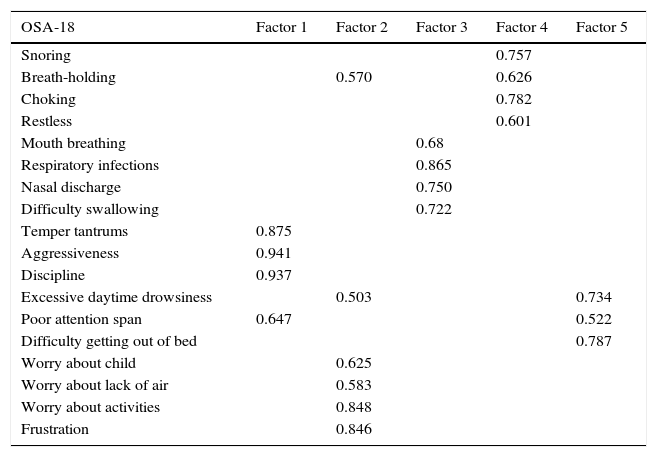
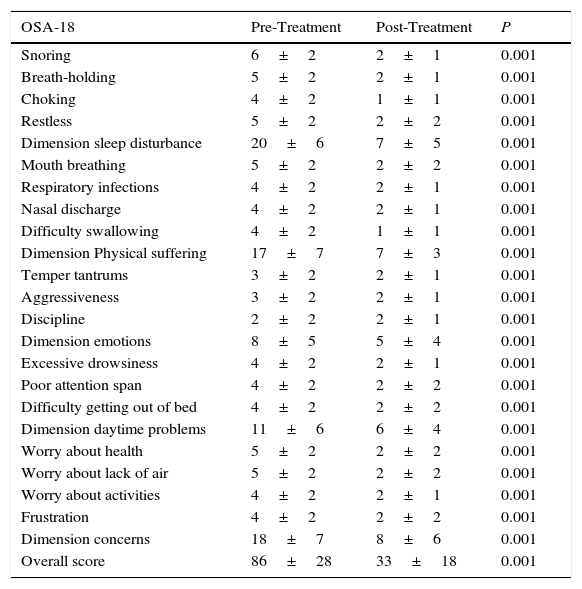
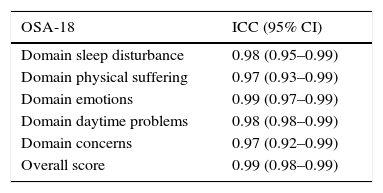
Internal consistency or reliability analyzed using Cronbach's alpha was 0.91, demonstrating excellent reliability for the overall questionnaire and for each domain, with no significant variation after exclusion of each item from a certain domain, or after exclusion of 1 domain from the overall score (Table 2). Correlations between domains were significant except in the case of emotional aspects, although the overall score correlated with all domains. Items showed a normal distribution, with no ceiling effect in any domain (maximum 10% of patients in D5), and no floor effect, except in D3 (30% of patients). When the overall questionnaire was analyzed, both the floor and the ceiling effects were 0%. The value of the overall questionnaire correlated significantly with all domains. The construct validity determined by factor analysis of principal components with varimax rotation and forcing a structure of 5 components corresponding to the 5 questionnaire dimensions is shown in Table 3 (showing saturations>0.5 only). We found that the structure of the Spanish version is practically the same as that of the original questionnaire. Items are grouped similarly and saturate in the components to which they belong. Only 1 exception was observed: the item “poor attention span”, which in the original questionnaire belongs to the domain of daytime problems, saturates better in this questionnaire in the emotional domain, which is understandable given the characteristics of the item in question and the domain. In terms of concurrent validity, the overall analysis shows good correlation (0.2–0.45) between most of the variables and their correlating domains and between the domains and the variables they measure. Individually, D1 shows correlations with snoring, apneas, and choking of 0.32–0.46; D2 with nasal obstruction and nasal symptoms of 0.2–0.28; D3 shows the poorest correlations (e.g., with hyperactivity or shyness) but correlates well with school performance (0.35); D4 correlates with school performance and excessive sleepiness (0.2–0.3); and D5 correlates significantly with symptoms and school performance (0.24–0.35). When predictive validity was evaluated, the questionnaire differentiated well between levels of severity as measured by the Mallampati score (ANOVA P=0.002) and AHI (ANOVA P=0.006) (Figs. 1 and 2). Sensitivity to change was excellent, both overall (P<0.001) and in each domain (P<0.001) (Table 4). Test–retest repeatability was also excellent, both overall and in each domain (Table 5).
Construct Validitya
| OSA-18 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 |
|---|---|---|---|---|---|
| Snoring | 0.757 | ||||
| Breath-holding | 0.570 | 0.626 | |||
| Choking | 0.782 | ||||
| Restless | 0.601 | ||||
| Mouth breathing | 0.68 | ||||
| Respiratory infections | 0.865 | ||||
| Nasal discharge | 0.750 | ||||
| Difficulty swallowing | 0.722 | ||||
| Temper tantrums | 0.875 | ||||
| Aggressiveness | 0.941 | ||||
| Discipline | 0.937 | ||||
| Excessive daytime drowsiness | 0.503 | 0.734 | |||
| Poor attention span | 0.647 | 0.522 | |||
| Difficulty getting out of bed | 0.787 | ||||
| Worry about child | 0.625 | ||||
| Worry about lack of air | 0.583 | ||||
| Worry about activities | 0.848 | ||||
| Frustration | 0.846 |
aOnly saturations >0.50 are tabulated.
Sensitivity to Change.
| OSA-18 | Pre-Treatment | Post-Treatment | P |
|---|---|---|---|
| Snoring | 6±2 | 2±1 | 0.001 |
| Breath-holding | 5±2 | 2±1 | 0.001 |
| Choking | 4±2 | 1±1 | 0.001 |
| Restless | 5±2 | 2±2 | 0.001 |
| Dimension sleep disturbance | 20±6 | 7±5 | 0.001 |
| Mouth breathing | 5±2 | 2±2 | 0.001 |
| Respiratory infections | 4±2 | 2±1 | 0.001 |
| Nasal discharge | 4±2 | 2±1 | 0.001 |
| Difficulty swallowing | 4±2 | 1±1 | 0.001 |
| Dimension Physical suffering | 17±7 | 7±3 | 0.001 |
| Temper tantrums | 3±2 | 2±1 | 0.001 |
| Aggressiveness | 3±2 | 2±1 | 0.001 |
| Discipline | 2±2 | 2±1 | 0.001 |
| Dimension emotions | 8±5 | 5±4 | 0.001 |
| Excessive drowsiness | 4±2 | 2±1 | 0.001 |
| Poor attention span | 4±2 | 2±2 | 0.001 |
| Difficulty getting out of bed | 4±2 | 2±2 | 0.001 |
| Dimension daytime problems | 11±6 | 6±4 | 0.001 |
| Worry about health | 5±2 | 2±2 | 0.001 |
| Worry about lack of air | 5±2 | 2±2 | 0.001 |
| Worry about activities | 4±2 | 2±1 | 0.001 |
| Frustration | 4±2 | 2±2 | 0.001 |
| Dimension concerns | 18±7 | 8±6 | 0.001 |
| Overall score | 86±28 | 33±18 | 0.001 |
Test–Retest Repeatability.
| OSA-18 | ICC (95% CI) |
|---|---|
| Domain sleep disturbance | 0.98 (0.95–0.99) |
| Domain physical suffering | 0.97 (0.93–0.99) |
| Domain emotions | 0.99 (0.97–0.99) |
| Domain daytime problems | 0.98 (0.98–0.99) |
| Domain concerns | 0.97 (0.92–0.99) |
| Overall score | 0.99 (0.98–0.99) |
ICC (95% CI): intraclass correlation coefficient with 95% confidence level.
The concept of health in children and teenagers does not only consider physical, psychological and social aspects, but also their ability to perform age-appropriate activities. In the case of children and teenagers, the dimensions that are generally explored are associated with their ability to perform activities of daily living (mobility and personal care), cognitive acquisition (memory, ability to concentrate and learn), emotions (positive and negative), self-perception, interpersonal relationships (with friends and family members), and relationships in their immediate environment (family cohesion, social support). Most HRQoL instruments for the pediatric population are designed on the psychometric model, measuring the ability of the individual to distinguish between stimuli of different intensity and recording the responses on a scale (usually a Likert-type scale).
Some units use the Pediatric Sleep Questionnaire, developed by the University of Michigan, to screen patients with childhood SAHS, to obviate the need for a PSG, or as a symptom questionnaire. The validated version in Spanish does not appear to be a true reflection of the original32,33 in terms of its daytime behavior and sleepiness dimension, which is unquestionably of the utmost importance in childhood SAHS.
The OSA-18 questionnaire has been adapted to other languages,34–36 and has demonstrated its utility in childhood SAHS. Some authors have used it as a screening tool to determine if it can substitute the PSG, but the questionnaire was not designed for this purpose.37
The OSA-18 has excellent internal consistency. Its predictive validity is good-to-excellent, although it would be improved if the severity groups were more balanced in number (there is a trend towards significant SAHS severity), and the concurrent validity is also good. The emotional domain is the most problematic in terms of psychometric characteristics. The construct is practically identical to that of the original questionnaire. Moreover, this questionnaire has been seen to be a very good predictive tool when compared with the Mallampati score and with SAHS severity measured by AHI.
In conclusion, the results suggest that the Spanish version of the OSA-18 questionnaire is equivalent to the original and is suitable for use in Spanish-speaking countries (Appendix A)
FundingFunded in part by a grant from the Valencian Foundation of Pulmonology (2013) and a SEPAR 2014 Grant.
Conflict of InterestsThe authors state that they have no conflict of interests.
| Nunca | Muy raramente | Pocas veces | En algunas ocasiones | En bastantes ocasiones | Casi siempre | Siempre | |
|---|---|---|---|---|---|---|---|
| Alteraciones del sueño | |||||||
| Durante las últimas 4 semanas, ¿con qué frecuencia ha tenido su hijo/a… | |||||||
| (1)…ronquidos fuertes? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (2)…se ha quedado encanado o ha tenido pausas de la respiración durante la noche? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (3)…asfixia o jadeo mientras dormía? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (4)…sueño inquieto o despertares frecuentes? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Síntomas físicos | |||||||
| Durante las últimas 4 semanas, ¿con qué frecuencia ha tenido su hijo/a… | |||||||
| (5) … respiración bucal a causa de la obstrucción nasal? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (6) … resfriados o infecciones respiratorias frecuentes? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (7)… mocos o secreción nasal nasal? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (8)… dificultad para tragar? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Síntomas emocionales | |||||||
| Durante las últimas 4 semanas, ¿con qué frecuencia ha tenido su hijo/a… | |||||||
| (9) … cambios de humor o rabietas? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (10) … comportamiento agresivo o hiperactivo? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (11)… problemas de disciplina? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Función diurna | |||||||
| Durante las últimas 4 semanas, ¿con qué frecuencia ha tenido su hijo/a… | |||||||
| (12)… somnolencia excesiva durante el día? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (13)… poca capacidad de atención o concentración? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (14) … dificultad para levantarse por la mañana? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Preocupaciones del cuidador | |||||||
| Durante las últimas 4 semanas, ¿con qué frecuencia los problemas anteriores… | |||||||
| (15) … le provocaron preocupación acerca de la salud del niño en general? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (16)… le causaron preocupación de que su niño tuviera falta de aire? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (17) … interfirieron con su capacidad para desarrollar actividades diarias? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| (18) … le provocó frustración? | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
Please cite this article as: Chiner E, Landete P, Sancho-Chust JN, Martínez-García MÁ, Pérez-Ferrer P, Pastor E, et al. Adaptación y validación al español del cuestionario de calidad de vida OSA-18 para la evaluación del síndrome de apnea-hipopnea de sueño infantil. Arch Bronconeumol. 2016;52:553–559.