Krebs von den Lungen 6 (KL-6) is a mucin-1 glycoprotein produced by type II pneumocytes. High levels of KL-6 in blood may be found in patients with lung fibrosis. In Asia this biomarker is used for diagnosis and prognosis in interstitial lung diseases (ILD). There is a lack of information regarding KL-6 cut-off point for diagnosis and prognosis in European population. The aim of this study was to establish the cut-off point for serum KL-6 associated with the presence of ILD in the Spanish population.
MethodsProspective study including subjects who underwent chest HRCT, PFTs and autoimmune blood analysis. Two groups were created: non-ILD subjects and ILD patients. Serum KL-6 concentrations were measured using a Lumipulse KL-6 reagent assay and the optimal cut-off value was evaluated by a ROC analysis. Data on demographics and smoking history was also collected.
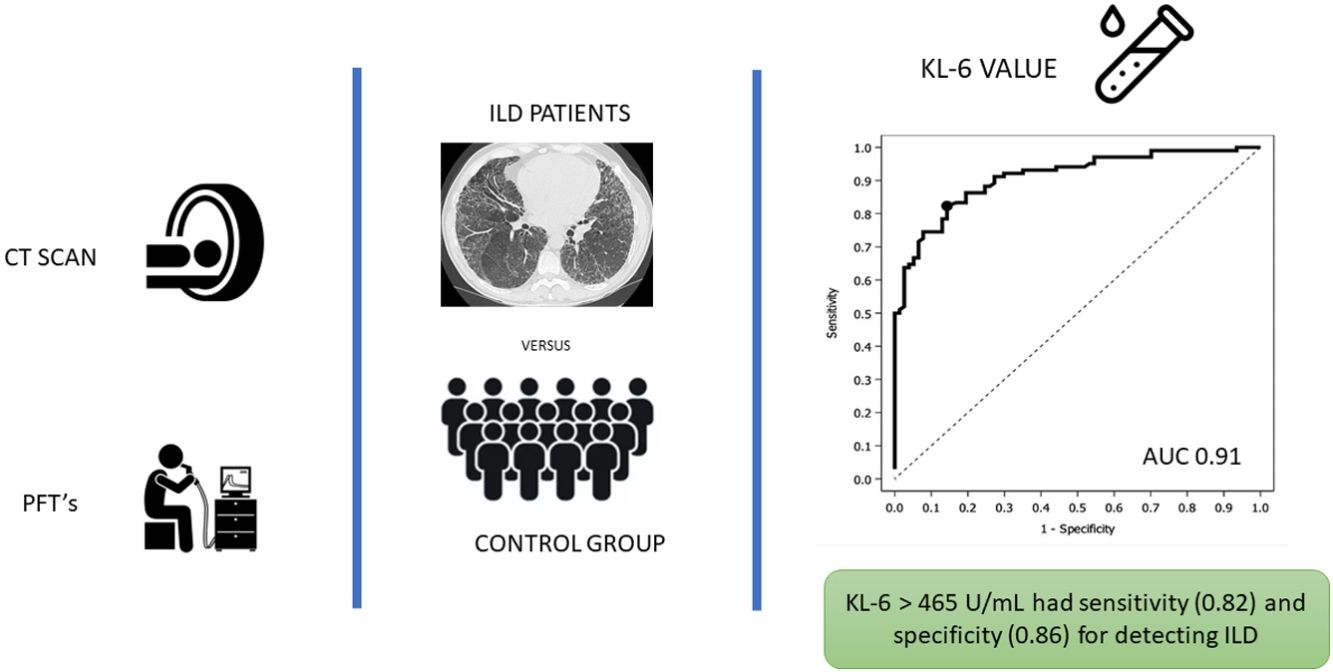
ResultsOne hundred seventy-nine patients were included, 102 with ILD. Median serum KL-6 values overall were 762U/mL, 1080 (±787)U/mL for the ILD group vs 340 (±152)U/mL for the non-ILD group (p<0.0001). The main radiological pattern was NSIP (43%). ROC analysis showed greater specificity (86%) and sensitivity (82%) for KL-6 465U/mL for detecting ILD patients. The multivariate logistic regression model pointed to the male sex, higher KL-6 values, lower FVC and low DLCO values as independent factors associated with ILD.
ConclusionSerum KL-6 values greater than 465U/mL have excellent sensitivity and specificity for detecting ILD in our Spanish cohort. Multicentre studies are needed to validate our results.
Interstitial lung disease (ILD) refers to a heterogenous group of diseases that mainly affect the alveolar–interstitial space and that share common features, such as the presence of lung fibrosis.1 Diagnosis is based on clinical, radiological and, if necessary, histological findings. Follow-up consists mainly of clinical evaluation, pulmonary function tests (PFTs), and chest high-resolution computed tomography (HRCT). However, there is a growing interest in serum biomarkers as a non-invasive means of early detection and follow-up of patients with ILD.
The therapeutic and prognostic impact of ILD in connective tissue disease (CTD) is especially important given the high associated morbidity and mortality.2–4 In fact, for all patients with systemic sclerosis, a recent expert consensus5 recommends screening for ILD that includes HRCT, PFTs, and chest auscultation. Early detection is crucial since ILD may already be at an advanced stage by the time symptoms appear or PFT results are altered.
Krebs von den Lungen 6 (KL-6) is a mucin-1 glycoprotein produced by type II pneumocytes. High levels of alveolar KL-66 and blood KL-67 may be found in patients with lung fibrosis due to epithelial damage, pneumocyte regenerating activity, and increased vascular permeability.
KL-6 has been extensively studied in Asia,8 mainly in the Japanese population, where values are used in current clinical practice to diagnose9 and especially to evaluate ILD progression. KL-6 implications for idiopathic pulmonary fibrosis (IPF) diagnosis and prognosis10 has also been explored for fibrosing lung diseases associated with CTD,11,12 including systemic sclerosis.13
Nonetheless, there is a lack of information regarding KL-6 cut-off points, diagnostic and prognostic value for the European population. Therefore, the aim of this study was to establish the cut-off point for serum KL-6 associated with the presence of ILD in the Spanish population.
Material and MethodsStudy DesignOur prospective study, performed at Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) from September 2016 to July 2018, was approved by the Hospital Ethics Committee and complied with all clinical guidelines (protocol code: IIBSP-EPI-2018-108). Patients signed an informed consent before inclusion in the study. Results are reported following “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines.14
PopulationPatients from the respiratory medicine and rheumatology outpatients clinic were prospectively recruited. Inclusion criteria were age more than 18 years old, ability to perform PFTs and chest HRCT performed within the last 6 months before inclusion. In the ILD group, we included patients with interstitial lung involvement on chest HCRT following the Fleischner's society criteria.15 Note that this group was composed by all ILD aetiologies including CTD. The non-ILD group included healthy subjects that underwent a chest HCRT for other reasons (patients under study of pulmonary familiar fibrosis, cases of resolved post-infectious organizing pneumonia), patients with other respiratory conditions (e.g. asthma, bronchiectasis, COPD and pulmonary embolism) and CTD patients with no HRCT evidence of ILD. Exclusion criteria were the inability to perform PFTs and unsigned informed consent.
Patients were diagnosed following current guidelines and were managed independently for the purpose of the study. ILD cases were reviewed by a multidisciplinary decision committee.
Data CollectionAll included patients underwent complete blood analysis including serological antinuclear antibody (ANA) and extractable nuclear antigen (ENA) screening to test for autoimmunity and serum KL-6. PFTs, including spirometry, and diffusing capacity for carbon monoxide (DLCO) (helium dilution technique; Master Screen Body Diffusion, Jaeger) were also performed. PFT results and reference values were those recommended by the relevant European and Spanish societies.16–18 Data were also collected on demographics, smoking history, comorbidities, and pharmacological treatment. All subjects underwent HRCT that was reviewed by a radiologist with ILD expertise (TF). Fleischner Society recommendations were followed for imaging procedures and radiology pattern classification.19,20
KL-6 AnalysisSerum KL-6 concentrations, expressed in U/mL, were measured using a Lumipulse KL-6 reagent assay (Fujirebio Europe, NV) based on the chemiluminescent enzyme immunoassay (CLEIA) technology, following manufacturer instructions.
Statistical AnalysisContinuous data were summarized in terms of the number of observations, mean, standard deviation (SD), median, minimum, maximum, and first and third quartiles. Categorical data were summarized in terms of the number of subjects (N), frequency counts (n), and percentages (%). Statistical significance (p-value) in comparing the ILD and non-ILD groups was determined by the Student t-test for quantitative variables, Fisher's exact test for categorical variables, and the Mantel–Haenszel exact test for ordinal variables. Spearman's correlation coefficient was calculated to check for correlation between FVC and KL-6. p-Values for test multiplicity were not corrected.
The usefulness of serum KL-6 as a diagnostic biomarker for ILDs was evaluated by receiver operating characteristic (ROC) area under the curve (AUC) analysis. The optimal cut-off value to discriminate patients with ILD from healthy subjects or non-ILD patients was determined by Youden's J statistic, and the associated contingency table (cut-off value vs presence of ILD) summarized results for accuracy, disagreement, sensitivity, specificity, false positives, false negatives, positive predicted values, negative predicted values, Kappa index values, and McNemar's p-values. Also analyzed was a contingency table with a 5% false positive rate and 95% sensitivity. Logistic regression analysis was conducted to analyze how sex, FVC values, and KL-6 levels modified the ILD risk, and a conditional inference tree was computed using KL-6 and sex as predictors of ILD.
All statistical analyses were performed using SAS® Version 9.4 TS1M5 (SAS Institute Inc., Cary, NC, USA) in a secure and validated environment.
ResultsA total of 179 patients were included, 102 with ILD. Mean (SD) age was 65.4 (12.9) years and 58% (n=104) were women. The main characteristics of the ILD and non-ILD groups are summarized in Table 1. There were statistically significant differences between the ILD and non-ILD groups regarding age and sex, but not smoking history.
Patient Demographic, Clinical, and Functional Respiratory Test Characteristics.
| Total (n=179) | ILD (n=102) | Non-ILD (n=77) | p-Value | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 75 (41.9%) | 55 (53.9%) | 20 (26.0%) | 0.0002a |
| Female | 104 (58.1%) | 47 (46.1%) | 57 (74.0%) | |
| Age, years | ||||
| Mean (SD) | 65.4 (12.9) | 69.2 (11.7) | 60.4 (12.7) | <0.0001b |
| Smoking, n (%) | ||||
| Never | 99 (53.3%) | 52 (51.0%) | 47 (61.0%) | 0.3341c |
| Formerly | 58 (32.4%) | 37 (36.3%) | 21 (27.3%) | |
| Currently | 22 (12.3%) | 13 (12.7%) | 9 (11.7%) | |
| FVC (L) | ||||
| Mean (SD) | 3.2 (1.0) | 2.9 (0.9) | 3.6 (1.0) | <0.0001b |
| FVC, % | ||||
| Mean (SD) | 91.8 (21.7) | 82.2 (19.7) | 105.0 (16.7) | <0.0001b |
| FVC <70%, n (%) | ||||
| No | 146 (83.0%) | 75 (73.5%) | 71 (95.9%) | |
| Yes | 30 (17.0%) | 27 (26.5%) | 3 (4.1%) | <0.0001a |
| DLCO, mL/min/mmHg | ||||
| Mean (SD) | 16.4 (6.2) | 14.4 (5.7) | 19.3 (5.6) | <0.0001b |
| DLCO initial value, % | ||||
| Mean (SD) | 72.7 (20.9) | 64.6 (19.3) | 83.8 (17.8) | <0.0001b |
| KL-6, U/mL | ||||
| Mean (SD) | 762.0 (704.7) | 1080.1 (787.7) | 340.7 (152.0) | <0.0001b |
| Initial treatment | ||||
| No | 146 (83.4%) | 81 (80.2%) | 65 (87.8%) | 0.2192a |
| Yes | 29 (16.6%) | 20 (19.8%) | 9 (12.2%) | 0.2192a |
DLCO: lung diffusing capacity for carbon monoxide; FVC: forced vital capacity; ILD: interstitial lung disease; KL-6: Krebs von den Lungen; SD: standard deviation.
Diagnoses (after multidisciplinary committee discussion) were as follows: IPF, 7 (6.9%); fibrotic non-specific interstitial pneumonitis (NSIP), 9 (8.9%); and fibrotic hypersensitivity pneumonitis, 9 (8.9%). Systemic sclerosis and Sjögren syndrome were the most prevalent CTDs in the ILD group patients: 16 (15.7%) and 14 (13.7%), respectively (see all ILD etiologies/diagnoses in Supplementary material Table 1).
The main radiological pattern was NSIP in 77 patients (43%), followed by usual interstitial pneumonia (UIP) in 57 (32%). Sub-analysis by radiological pattern showed statistically significant differences between the UIP and non-UIP groups for FVC <70%, DLCO, sex, and age but not for baseline KL-6 (see Supplementary material Table 2).
Mean (SD) FVC was 91.8% (21.7%) and mean (SD) DLCO was 72.7% (21%). Statistically significant difference was observed for FVC and DLCO between the ILD group and the non-ILD group (p<0.01). Group sub-analysis according to FVC >70% vs <70% revealed differences in KL-6, DLCO, UIP vs non-UIP radiology patterns, and sex. No differences were found with respect to age or smoking history (Table 2).
Patients Characteristics According to FVC <70%.
| FVC >70%(n=146) | FVC <70%(n=30) | p-Value | |
|---|---|---|---|
| Sex | |||
| Male | 56 (38.4%) | 18 (60.0%) | 0.0413a |
| Female | 90 (61.6%) | 12 (40.0%) | |
| Age | |||
| Mean (SD) | 64.5 (13.0) | 68.5 (12.4) | 0.1243b |
| Tobacco history | |||
| Never | 80 (54.8%) | 16 (53.3%) | 0.7773c |
| Former smoker | 46 (31.5%) | 12 (40.0%) | |
| Smoker | 20 (13.7%) | 2 (6.7%) | |
| DLCO (%) initial | |||
| Mean (SD) | 76.4 (19.2) | 49.4 (15.2) | <0.0001b |
| KL-6 value | |||
| Mean (SD) | 653.2 (547.2) | 1340.5 (1058.4) | 0.0015b |
| HRCT pattern | |||
| Non-UIP | 59 (78.7%) | 15 (55.6%) | 0.0261a |
| UIP | 16 (21.3%) | 12 (44.4%) | |
| UIP | 16 (21.3%) | 12 (44.4%) | |
DLCO: lung diffusing capacity for carbon monoxide; FVC: forced vital capacity; HRCT: high-resolution computed tomography; KL-6: Krebs von den Lungen; SD: standard deviation.
Mean (SD) serum KL-6 values overall were 762 (704)U/mL, and were 1080 (787)U/mL for the ILD group vs 340 (152)U/mL for the non-ILD group (p<0.0001).
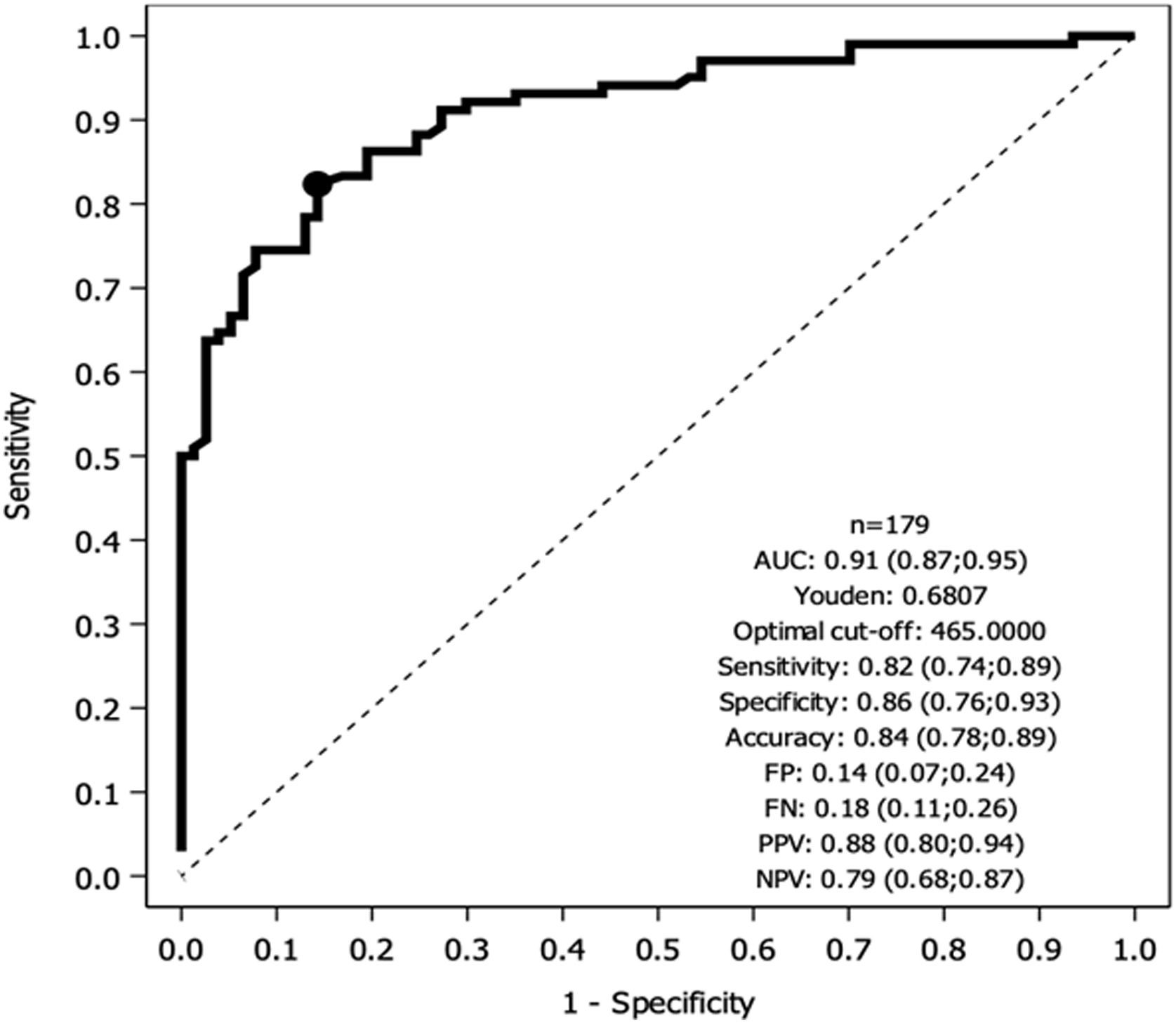
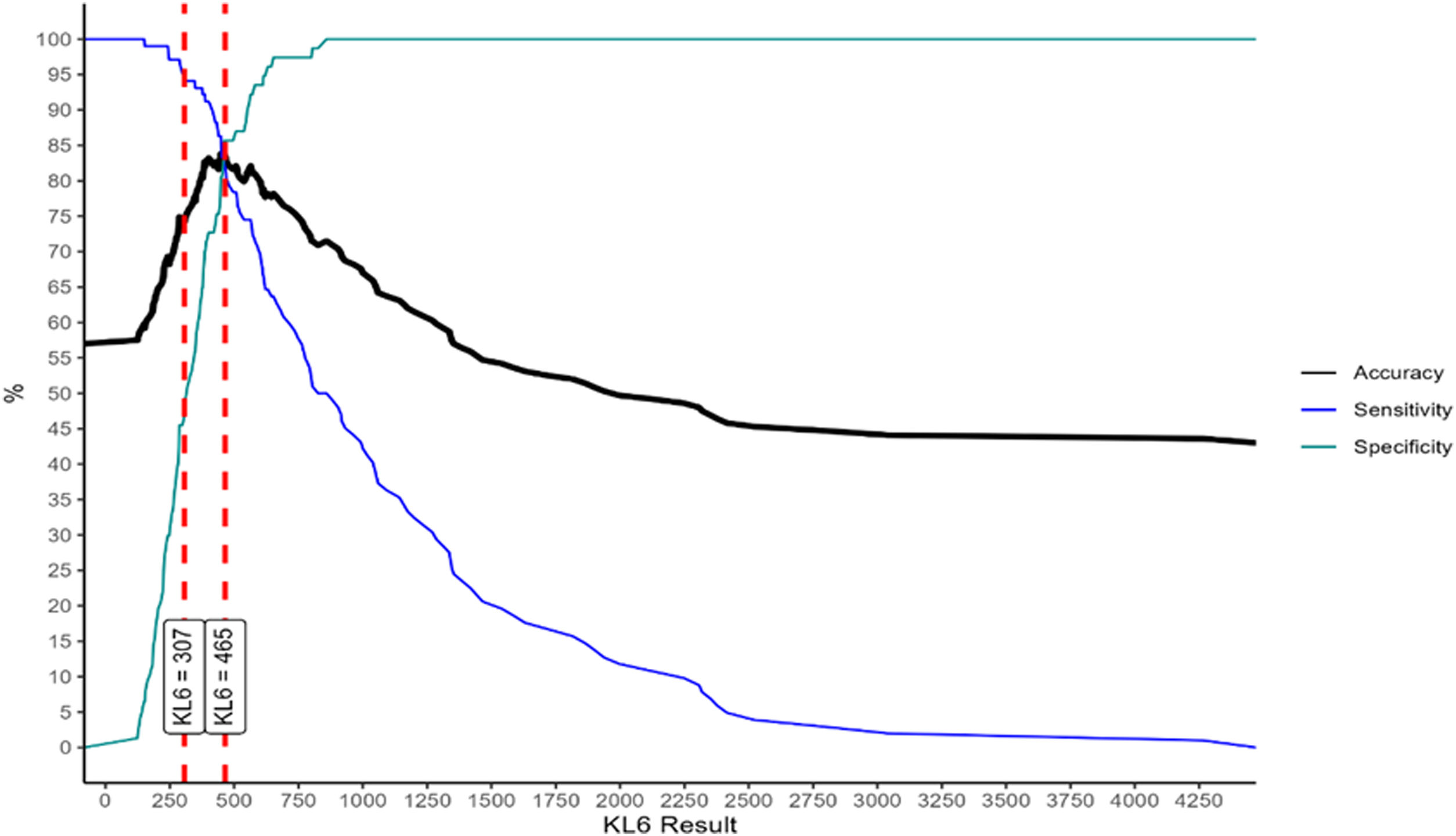
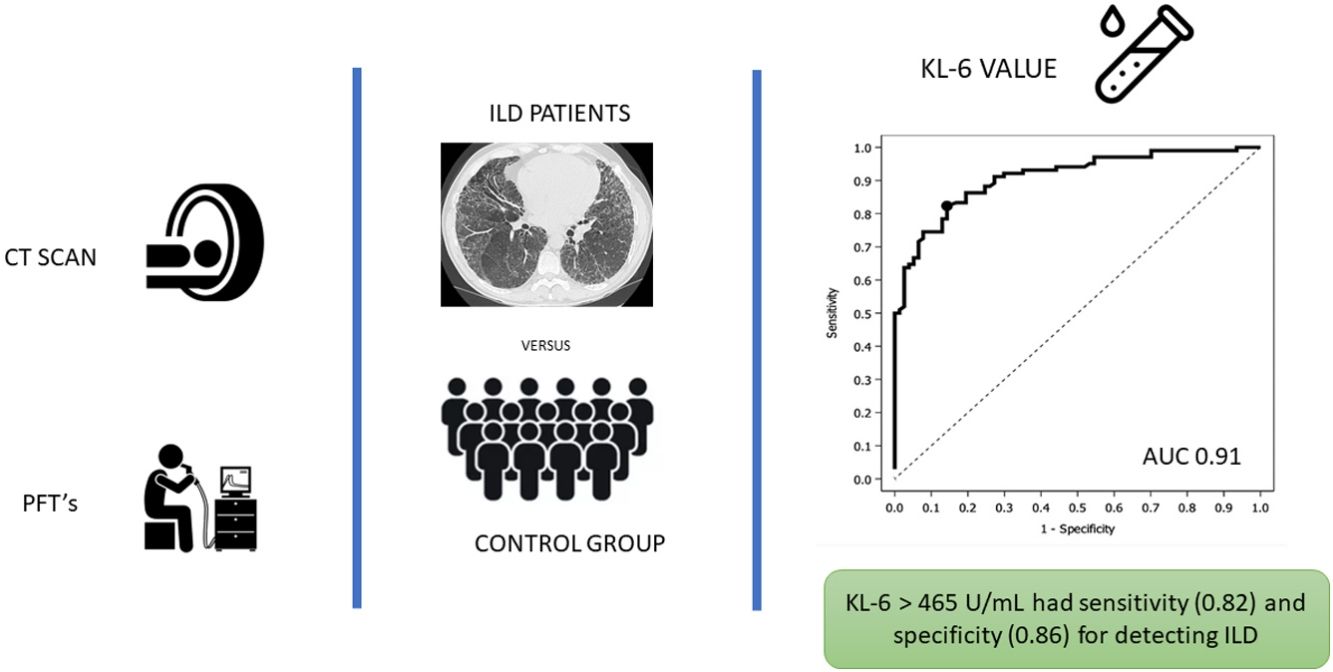
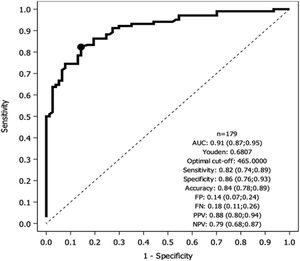
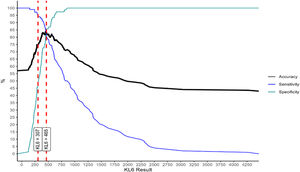
Fig. 1 depicts results of the ROC analysis using AUC, showing greater specificity (86%) and sensitivity (82%) for KL-6 ≥465U/mL for patients with ILD. ROC analysis to determine the optimal cut-off value for screening purposes showed 95% sensitivity for KL-6 ≥307U/mL. Fig. 2 depicts sensitivity and specificity values according to KL-6 values and the chosen cut-off points.
In order to establish a better correlation between KL-6 values and PFT we performed a secondary ROC analysis to identify ILD based on FVC and DLCO ranges. An optimal KL-6 cut-off point was 449U/mL for FVC greater than 80% group (sensitivity 83%, specificity 81%) and 942U/mL for FVC between 50 and 80%. As for DLCO, the optimal cut-off was 424U/mL for DLCO greater than 80% (sensitivity 82%, specificity 74%), 469U/mL for DLCO between 50 and 80% and 489U/mL for DLCO <50% (see Supplementary material Figs. 1 and 2).
Sub-analyses of ILD, ILD-CTD, non-ILD, and non-ILD-CTD groups revealed no statistically significant differences in KL-6 values between the ILD and ILD-CTD groups, or between the non-ILD and non-ILD-CTD groups.
The multivariate logistic regression model pointed to male sex, higher KL-6 values, lower FVC and low DLCO values as independent factors associated with ILD (Table 3).
Prognostic Factors for Interstitial Lung Disease.
| N=152 | Logistic Multivariate Model | |||
|---|---|---|---|---|
| OR | 95% CILower Limit | 95% CIUpper Limit | Wald Testp-Value | |
| Gender (male vs female) | 2.57 | 1.37 | 4.83 | 0.0032 |
| FVC (%) | 0.94 | 0.90 | 0.98 | 0.0036 |
| DLCO (%) | 0.96 | 0.93 | 0.99 | 0.0169 |
| KL-6 (for each increase of 100 units) | 2.33 | 1.57 | 3.46 | <0.0001 |
OR: odds ratio; CI: confidence interval; DLCO: lung diffusing capacity for carbon monoxide; FVC: forced vital capacity; KL-6: Krebs von den Lungen.
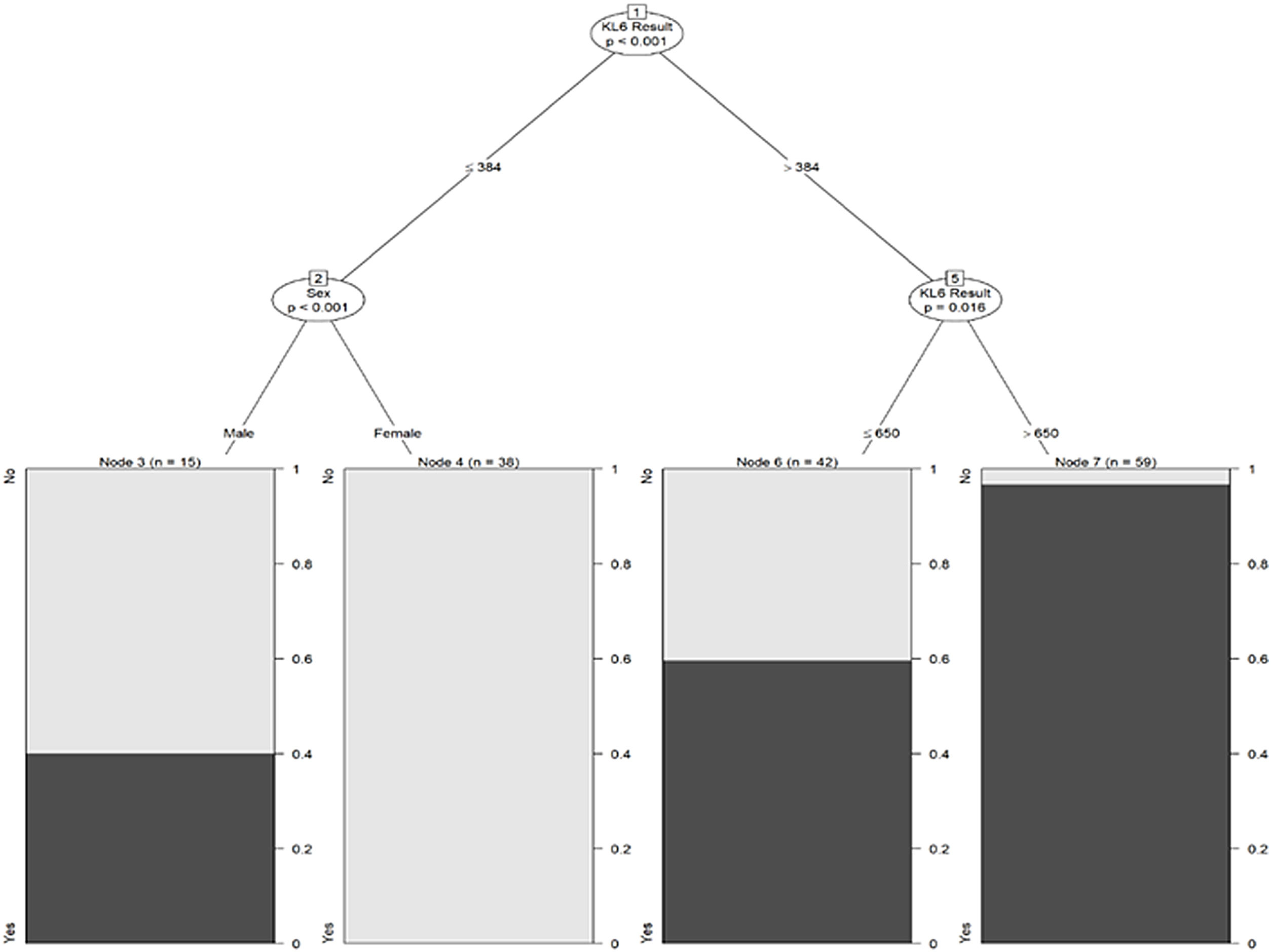
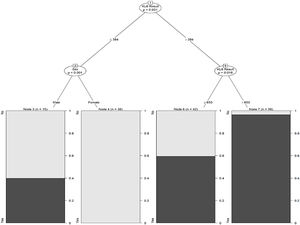
Finally, Fig. 3 depicts the conditional inference tree model to facilitate ILD prediction/identification, starting with an initial KL-6 value of 364U/mL and then including sex and a second KL-6 value of 650U/mL. In men compared to women, the probability of ILD increased by 40% when KL-6 was below 364U/mL. Considering only the KL-6 value, the probability increased by 95% from 650U/mL onwards.
DiscussionThe serum KL-6 cut-off value for ILD diagnosis in the European population has not been elucidated. In this prospective study, we found that 465U/mL was the optimal KL-6 cut-off for the presence of ILD, while a KL-6 cut-off of 307U/mL showed greater sensitivity for screening purpose. As reported in other series,7,21 serum KL-6 levels are independently correlated with the presence of ILD. Our population showed a wide range of KL-6 values. This variability might be attributed to the diverse ILD etiologies and the varying degrees of disease extension and activity at the time of inclusion.
High variability in KL-6 cut-off values for ILD diagnostic purposes has been observed in different populations. In Asian cohorts, mainly Japanese, the optimal cut-off is around 500U/mL,22–25 a value that differs from those reported in the few studies of KL-6 that exist for European populations. A small-scale retrospective Irish study with 34 patients26 reported an optimal KL-6 cut-off of 459U/mL, similar to that of our cohort. In contrast, for a retrospective French series of 75 patients, aimed at detecting ILD in patients with systemic sclerosis, a higher KL-6 value of 872U/mL was reported, with specificity of 95%.27 Horimasu et al.28 performed a landmark large-scale study (453 subjects) that compared serum KL-6 levels in German and Japanese ILD patients and healthy subjects, and also evaluated MUC1 polymorphisms that could affect KL-6 levels; the authors reported an optimal cut-off value of 659U/mL for the German subjects and 461U/mL for the Japanese subjects, concluding, after a genotype analysis, that this cut-off difference was partially due to different distributions of the MUC1 rs4072037 genotypes. Moreover, and as was the case in our study, KL-6 was found to be an independent factor for ILD presence and was not correlated with age or smoking.
The findings of Horimasu et al.28 reinforce the hypothesis of variations between populations and also point to differences reflecting different genetic bases. This would suggest that KL-6 values are not absolute and that KL-6 cut-off values may need to be established and validated for specific populations.
Another finding for our cohort was an optimal KL-6 cut-off for ILD screening purposes of 307U/mL with up to 95% sensitivity. This high sensitivity was kept in a subgroup analysis with preserve PFT, which highlights KL-6 utility for early stages of disease. Early diagnosis of ILD is currently a challenge, particularly for ILD-CTD, as diagnostic delay29–31 has a significant impact on survival.32 Further investigations are needed to facilitate early detection aimed at changing the natural course of this disease.
Drawing on the logistic regression analysis, we created a conditional inference tree based on KL-6 values and sex, as potentially useful to predict ILD. A recent study also used a decision tree to predict ILD, but based on smoking history and age as well as KL-6 levels.33 We suggest that our simple screening tool, based on KL-6 and sex, may be useful in clinical practice for ILD screening purposes.
Regarding radiology findings, NSIP was the main pattern (43%) followed by UIP (32%). In a subgroup analysis comparing the UIP and non-UIP groups, no statistically significant differences in serum KL-6 values were evident, a finding similar to other studies that found no KL-6 level differences between patients with UIP and NSIP.34,35
Our subgroup analysis for FVC pointed to higher KL-6 levels in patients with FVC <70%, corroborating the study by Horimasu et al.,28 who reported that serum KL-6 levels were inversely correlated with FVC and DLCO values. A correlation between KL-6 baseline levels and the annual changes of PFTs has also been found in another recent study.36
The main limitations of our study are its single-centre nature and the relatively small number of included patients. Furthermore, although radiology patterns were established, the degree of fibrosis was not quantified in the HRCT scans. As a strength, our study was prospective and included a control group.
ConclusionsOur data show that serum KL-6 values greater than 465U/mL have excellent sensitivity and specificity for detecting ILD. KL-6 values could be used to screen for ILD risk for instance in patients with CTD. Multicentre studies are needed to validate our results.
Author's ContributionsPMB, DC and IC conceived the idea and designed the methods. DC, SB and PMB collected the data. LMM and AM performed the KL-6 analysis. TF and MA contributed to the analysis and interpretation of data. All the authors reviewed the results and approved the final manuscript.
FundingThis study was supported by Fujirebio (product supply).
Conflicts of InterestD. Castillo reports personal fees and non-financial support from Roche, grants, personal fees and non-financial support from Boehringer-Ingelheim, grants from Fujirebio, personal fees from Veracyte, outside the submitted work. D. Castillo is part of the Editorial board of Archivos de Bronconeumologia and declare that they have remained outside the evaluation and decision-making process in relation to this article.
P. Millán-Billi, I. Castellví, L. Martinez-Martinez, A. Mariscal, S. Barril, Miriana D’Alessandro and T. Franquet declare no conflicts of interest.