Acute respiratory failure due to COVID-19 pneumonia often requires a comprehensive approach that includes non-pharmacological strategies such as non-invasive support (including positive pressure modes, high flow therapy or awake proning) in addition to oxygen therapy, with the primary goal of avoiding endotracheal intubation.
Clinical issues such as determining the optimal time to initiate non-invasive support, choosing the most appropriate modality (based not only on the acute clinical picture but also on comorbidities), establishing criteria for recognition of treatment failure and strategies to follow in this setting (including palliative care), or implementing de-escalation procedures when improvement occurs are of paramount importance in the ongoing management of severe COVID-19 cases. Organizational issues, such as the most appropriate setting for management and monitoring of the severe COVID-19 patient or protective measures to prevent virus spread to healthcare workers in the presence of aerosol-generating procedures, should also be considered.
While many early clinical guidelines during the pandemic were based on previous experience with acute respiratory distress syndrome, the landscape has evolved since then. Today, we have a wealth of high-quality studies that support evidence-based recommendations to address these complex issues. This document, the result of a collaborative effort between four leading scientific societies (SEDAR, SEMES, SEMICYUC, SEPAR), draws on the experience of 25 experts in the field to synthesize knowledge to address pertinent clinical questions and refine the approach to patient care in the face of the challenges posed by severe COVID-19 infection.
Acute respiratory failure (ARF) secondary to SARS-CoV2 infection (COVID-19) may require a variety of non-invasive respiratory support (NIRS) strategies in addition to conventional oxygen therapy (COT), including high-flow therapy (HFT), continuous positive airway pressure (CPAP), non-invasive mechanical ventilation (NIV) or awake proning (AP). During the early phase of the pandemic, guidelines were published based on previous experience in non-COVID patients.1 Four years later, the recommendations should be updated based on the evidence. To achieve this goal, four scientific societies (SEDAR, SEMES, SEMICYUC, SEPAR) with a total of 25 experts in the field participated in this document.
Despite the same societies published previously a specific document about ARF in non-COVID patients,2 some concepts of the current recommendations may be applicable, with some exceptions, to other infectious entities, such as viral or bacterial pneumonia with single organ failure. However, there are some discrepancies regarding the NIRS strategy to be used, with HFT being the modality of choice in non-COVID ARF.3 Obviously, these recommendations do not apply to other etiologies of ARF such as cardiogenic acute pulmonary edema.
The full document is available in the online supplement.
- 1.
What should be the clinical picture to consider initiation of NIRS?
COT was clearly the most important initial supportive technique in ARF after COVID-19.4 However, this technique was not sufficient in many patients.5 One of the key questions is the ideal time to initiate NIRS. The intense inspiratory during spontaneous ventilation in patients with ARF may generate elevated transpulmonary pressures and ultimately exacerbate self-induced lung injury (P-SILI).6 Therefore, initiation of NIRS could have a preventive effect.
During the first wave, some societies recommended initiating NIRS from a cut-off FiO2 of 0.4 under COT in addition to clinical criteria.7 The stratification system proposed in Italy, with four categories of patients, was one of the most followed.8 In this classification,9 the indication for initiation corresponded to the third degree of ARF severity. The German position paper suggested initiating HFT when PaO2≤55mm Hg and FR≥30/min on room air.10 In the English guidelines, the proposed criteria for initiating CPAP and O2 were the inability to maintain SpO2 between 92 and 94% at an FiO2 between 0.4 and 0.6.11 With these recommendations, the experts proposed two different scenarios for starting NIRS: early start (PaO2: FiO2<300 or SpO2<93% at O2>5L/min or SpO2<94% at FiO2 0.4%) or late start (SpO2<92% under O2 at 15L).12
Two retrospective studies13,14 compared the use of early versus late HFT and found significant differences in the rate of intubation, which was lower in the early group. Of course, both studies may have a selection bias, since patients in the late onset groups were selected if they presented with respiratory progression on COT. The only high-quality randomized trial designed to answer the question of early HFT showed no benefit of early administration on final prognosis.15
Non-targeted randomized controlled trials showed heterogeneity in defining the criteria for initiating NIRS. For example, the RECOVERY trial defined the clinical condition for randomization as patients with an FiO2 requirement equal to or greater than 0.4 and an SpO2≤94%,16 whereas the HENIVOT study17 required a PaO2/FiO2≤200 as a criterion for initiating NIRS. The PaO2/FiO2 index may not reflect the severity of exchange because it does not take into account baseline PaCO2, which is often decreased in patients with ARF secondary to COVID-19.18
Therefore, there is insufficient evidence to support early initiation of NIRS (mainly HFT) as a preventive measure against deterioration of respiratory status in patients with severe ARF secondary to COVID-19.
The criteria for starting NIRS would be the following:
- -
Moderate to severe dyspnea and evidence of increased work of breathing (use of accessory muscles or tachypnea>25rpm) or
- -
PaO2/FiO2<200 o (SpO2<92% at FiO2 of 0.4) o PaCO2>45mmHg and pH<7.35.19–21
- 2.
What should be the first line NIRS therapy?
- 2.1
Considering age and comorbidity
An expert consensus19 recommended the initial use of NIV only in the presence of global ARF (hypoxemia and hypercapnia) and in selected patients with increased work of breathing. It can be considered as a first option in patients with underlying respiratory comorbidity, secondary to chronic obstructive pulmonary disease (COPD), neuromuscular involvement or chest disease. In hypoxemic ARF, the use of CPAP would be the first choice in the presence of heart failure or acute pulmonary edema. In addition, CPAP has been used as one of the main treatment alternatives in selected patients with a therapeutic ceiling (no intubation order).22
There is no scientific evidence to support a device type based on age. However, it seems logical that HFT may be recommended in particularly frail patients or those with compromised baseline conditions due to better tolerability and fewer side effects. A retrospective study showed a reduction in mortality in the elderly population when the therapy was used in patients with PaO2/FiO2 between 200 and 300).14
- 2.2
Based on clinics and pulmonary gas exchange
The pre-pandemic FLORALI study23 showed that in hypoxemic ARF, mortality and ventilator-free days were significantly lower in the HFT-treated group than in the NIV-treated group. The same strategy was also applied in the early phases of the pandemic.4 However, in the RECOVERY trial24 the use of COT versus HFT showed no difference in intubation or 30-day mortality (45.1 vs. 44%), whereas the CPAP-treated group had a significantly lower rate. There were no standardized criteria for the initiation of invasive mechanical ventilation. On the other hand, the results of another recent randomized trial25 showed that the use of HFT reduced the need for intubation and the time to recovery from mechanical ventilation compared with patients treated with COT. Finally, in a retrospective study involving more than 1000 patients, the use of HFT was an independent factor in reducing the need for mechanical ventilation and mortality.26
A recent metaanalysis27 concluded that, despite a significant increase in the PaO2/FiO2 ratio in the positive pressure group, intubation and length of hospital stay were similar between HFT and NIV.
Another interesting option is the combination of therapies (CPAP/HFT; NIV/HFT), which facilitate resting and feeding. CPAP/HFT early combination has been associated with lower intubation and mortality rates in a cohort of patients with a PaO2/FiO2 ratio≤100.28
Finally, pressure support ventilation (PSV) may worsen ventilator-induced lung injury, and its use was associated with increased mortality in retrospective studies conducted during the early phase of the pandemic.29
Therefore, initiation with continuous positive airway pressure (CPAP) is recommended. The option of PSV should only be considered in cases of global acute respiratory failure.16,30 HFT is a recommended alternative in hypoxemic patients without respiratory acidosis.31–34
Patients with more severe hypoxemia (PaO2/FiO2 less than 150mmHg) should be closely monitored when starting NIRS, as there is a higher risk of failure.17,35
- 3.
Parameterization and adaptation of NIRS
- 3.1
Parameter choice
In HFT, it is recommended to start therapy at the maximum tolerated flow with an adequate FiO2 to maintain SpO2>92%.31,36,37 Some studies have suggested using peak flow during spontaneous ventilation as a reference to titrate HFT.38 The temperature should be set in the range of 31–37°C, with preference given to higher values. It has also been shown that the use of a surgical mask not only reduces aerosol spread to the environment, but also improves oxygenation without increasing the risk of CO2 rebreathing.39
In CPAP, it is usually initiated at 10cmH2O, not exceeding 12–13cmH2O to avoid barotrauma or negative hemodynamic effects, and FiO2 to achieve SpO2>92% or PaO2≥60mmHg. An improvement in oxygenation of ≥15% or ≥30% is equivalent to lung recruitment, which can be confirmed by ultrasound.40
In NIV, PEEP between 10 and 12cmH2O (similar to CPAP) and moderate PSV is recommended, with a target VT of 4–6ml/kg41 avoiding overassistance.42
- 3.2
Interface selection
The interface with the best reported results would be the helmet with High Efficiency Particulate Air (HEPA) filters, both in CPAP mode and for NIV.43–46 If a helmet is not available, full-face or oronasal masks can be used, although they carry a higher risk of aerosol dispersion due to increased leakage.47
The use of interfaces with the leak port built into the mask itself is not recommended.48 Nasal masks should also be avoided.
For nasal cannulas, it is recommended to seal more than 50% of the nostril diameter.49
The use of oils with hyperoxygenated fatty acids is recommended to protect the skin.50
- 4.
How should the response to NIRS be monitored?
Monitoring of NIRS should include a combination of clinical and oxygenation parameters. The important role of intermediate care units during the different phases of the pandemic should be highlighted.51
The criteria for a positive response to NIRS are the following.
- •
Improvement in clinical signs of work of breathing, with decreased respiratory rate (RF) and accessory muscle use, and improvement in dyspnea.
- •
Improvement of oxygenation:
- ∘
ROX index: The combination of SpO2 and RR ((SpO2/FiO2)/RR) has been proposed as an index that may be useful in predicting the success or failure of HFT treatment.52 The cut-off points described for predicting the success of HFT in COVID patients were very similar to those previously described in non-COVID patients.53,54
- ∘
Exhaled tidal volume should also be monitored in patients on NIV or CPAP. Persistent intense inspiratory effort7 and high exhaled tidal volume (>9–9.5ml/kg ideal body weight) have been associated with NIRS failure.41,55
- ∘
- 5.
When should we consider NIRS failure?
The definition of NIRS failure and when it occurs in hypoxemic ARF in COVID-19 is controversial. The risk-benefit trade-off between avoiding unnecessary intubation and delaying intubation needs to be carefully considered. Therefore, it may be useful to adopt the criteria defined in the most relevant clinical trials.16,17,56 Failure of NIRS should be defined as the presence of 2 or more of the criteria in Table 1.
Criteria for NIRS failure.
| Absence of improvement or worsening of symptoms or signs on admission, including oxygenation data and increased respiratory rate |
| Appearance of signs of respiratory muscle fatigue or the use of accessory muscles |
| Presence of acidosis, both respiratory and metabolic |
| Inability to properly clear respiratory secretions |
| Signs of hemodynamic instability, including hyperlactacidemia |
| Deterioration of the level of consciousness or presence of seizures |
| Intolerance to the device, especially in mask wearers. |
The presence of two or more of the conditions suggests NIRS failure.
Studies agree that the final decision to intubate for NIRS failure should be at the discretion of the clinician and that objective criteria should simply guide the decision. It is also advisable to closely monitor patients treated with NIRS for more than 72h who do not improve or show signs of late deterioration, as well as those who show acute deterioration after a previously stable situation, to proceed with intubation and invasive ventilation.57,58
- 6.
What should be done if NIRS fails?
Although NIRS failure often is followed by tracheal intubation and initiation of invasive mechanical ventilation (IMV), in the event of failure of treatment with HFT, rescue treatment with CPAP or NIV may be considered in selected cases (reversible condition and do-not-intubate orders), although mortality is very high.59
Finally, if NIRS fails in patients with a therapeutic ceiling, it should be replaced by COT and symptomatic management of dyspnea should be optimized. These last steps should ideally be carried out in collaboration with hospital palliative care teams.
- 7.
What is the role of awake prone positioning in non-intubated patients?
Prone positioning has been shown to improve oxygenation and mortality in intubated patients with moderate to severe acute respiratory distress syndrome (ARDS).60,61 Since the publication of the PROSEVA study, it should be considered the standard of care in the management of ARDS.62 The prone position in ARDS patients favors lung recruitment, improving homogeneity and reducing lung stress and strain. The homogeneous distribution of ventilation reduces overdistension of the ventral areas. Improved ventilation of the dorsal regions also improves the V/Q ratio by reducing the shunt.61
The same mechanisms described for the prone position of the patient in IMV also occur in awake pronated patients with NIRS (mainly HFT).63 However, the experience is much more limited. The only pre-pandemic study published included 20 patients, of whom 9 (45%) required intubation; of the 11 non-intubated patients, 8 received HFT and awake prone, and 6 of these required escalation to NIV.64 Since the early stages of the pandemic, AP has been recommended by several scientific societies for its potential benefits, but without clear evidence.19,65 The results of a meta-trial involving more than 1000 patients showed that the AP in patients with COVID-19 requiring HFT reduced the need for intubation.56 In addition, a recent systematic review and meta-analysis confirmed that the AP reduces the need for intubation in patients with acute hypoxemic respiratory failure secondary to COVID-19.66 AP may be beneficial in patients with COVID-19-related hypoxemic acute respiratory failure who require admission to the ICU and receive NIRS treatment.
- 8.
NIRS de-escalation protocols
Patients with global ARF: There are few randomized trials evaluating different NIRS weaning strategies, and none specifically in COVID-19. No relevant differences have been shown between a sudden NIV withdraw and a progressive weaning strategy.67,68 However, it is suggested a gradual withdrawal of NIV sessions69 and/or set PSV70 in difficult-to-wean patients.
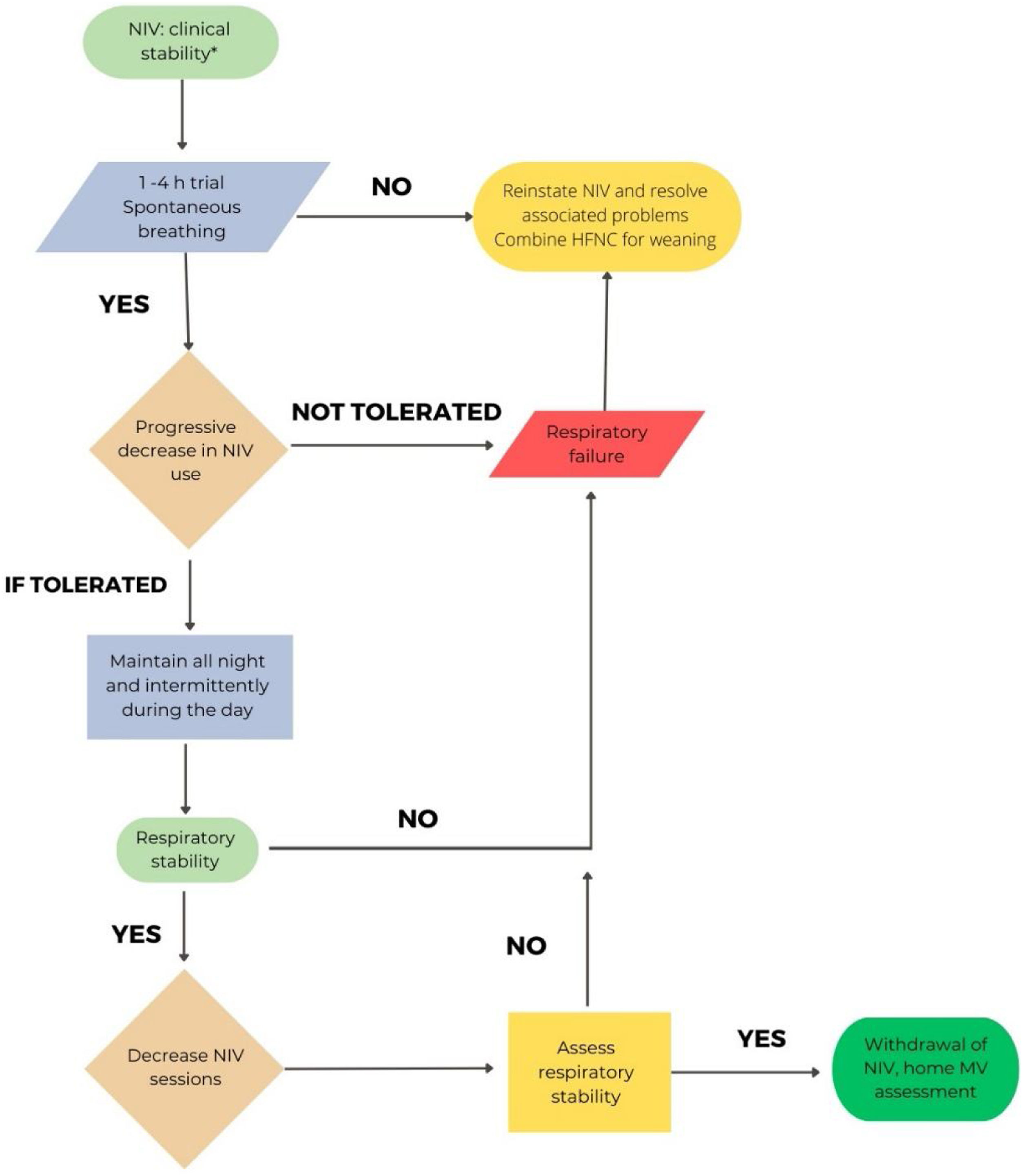
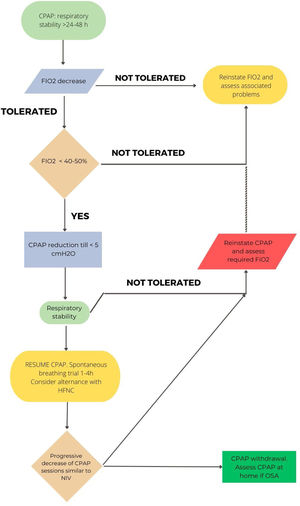
Considering the different characteristics of COVID-19 involvement in patients with hypercapnic failure (mainly COPD),71 the proposed withdrawal may be more conservative than in situations of exacerbation due to other etiologies. Fig. 1 shows the proposal for hypercapnic ARF.
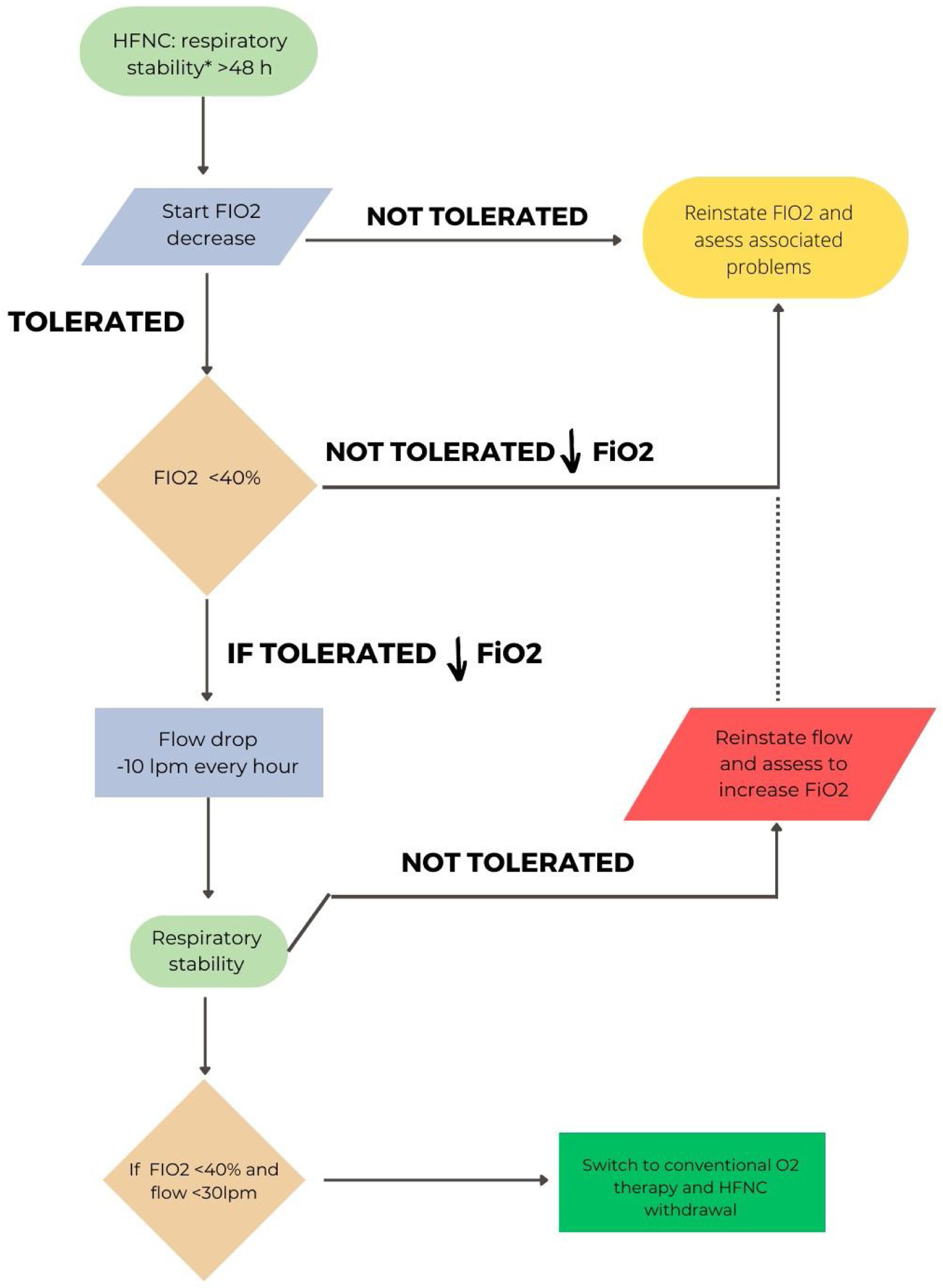
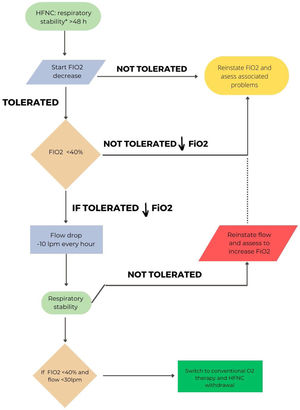
In hypoxemic ARF, the approach will be different depending on whether HFT or CPAP have been used. A pragmatic strategy could be to gradually reduce FiO2 with the aim of maintaining SpO2 between 92 and 98%.3,18 Once an FiO2 of 40% or less is reached, flow reduction would be initiated.72 An ongoing study is comparing sequential FiO2 and flow reduction strategies or vice versa.73Fig. 2 shows the proposal for withdrawal for patients with HFT. A special situation would be the combined use of CPAP/HFT, in which a progressive increase of HFT periods and a progressive decrease in FiO2 would be recommended. When FiO2 is below 50%, a reduction in CPAP level can be considered.
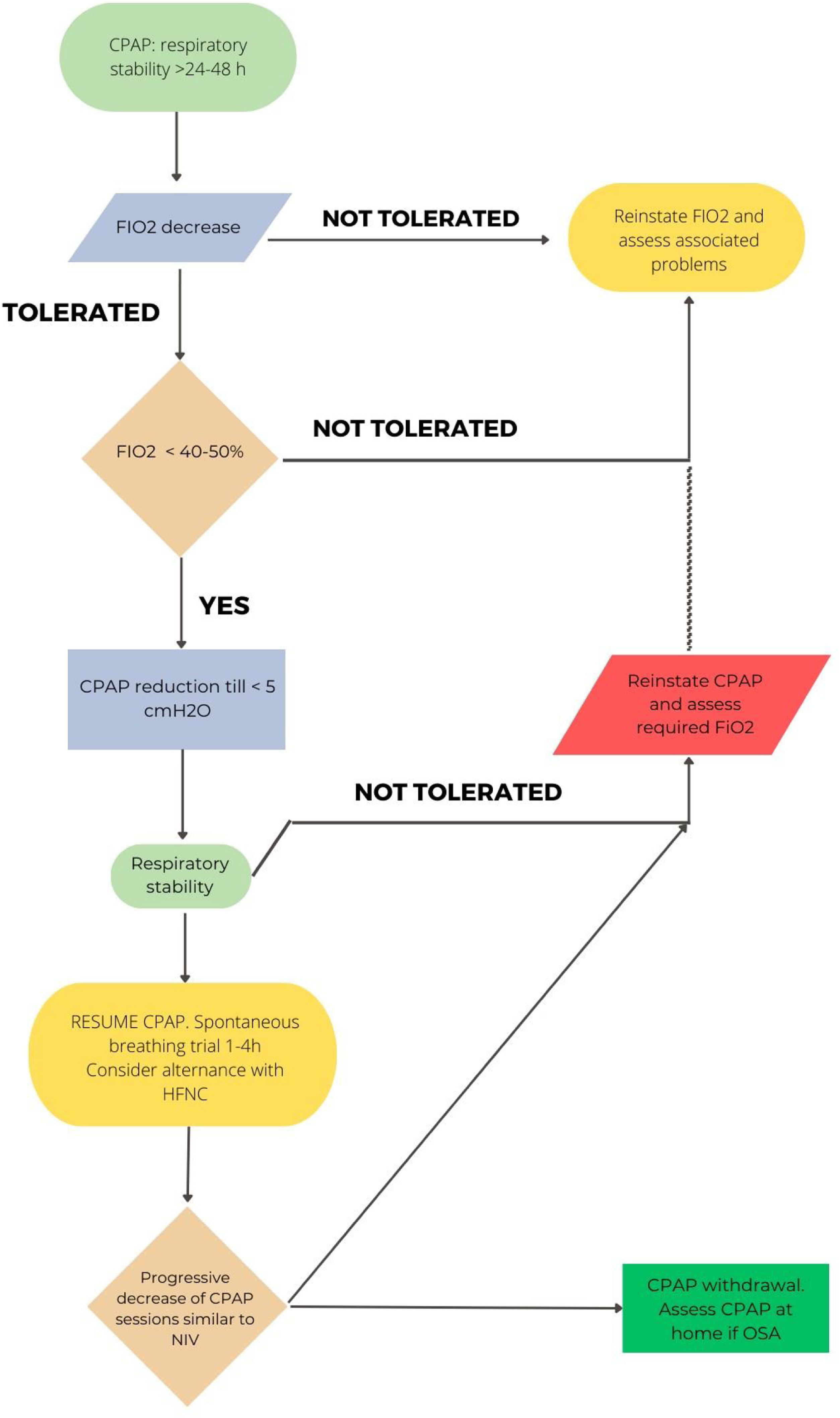
In CPAP patients, when clinical stability is achieved (RR<25rpm, absence of accessory muscle use, good level of consciousness) with a CPAP level<5cmH2O and FiO2<50%, a CPAP weaning trial could be considered. If PaO2/FiO2>240 under COT and FiO2<0.4 is maintained for 24hours, the patient can be considered as “successfully weaned”.74,75Fig. 3 reflects this strategy.
- 9.
Other supportive measures in the NIV patient: nutrition, hydration and skin protection
Interface pressure ulcers (IPU) occur in 5–50% of patients after the first 2h of NIV therapy, particularly at the bridge of the nose, and using oronasal masks. On the other hand, hospitalized patients with COVID-19 have a high prevalence of malnutrition (14–70%), which worsens their prognosis. Table 2 reflects the supportive recommendations for both conditions.76–85
- 10.
What measures should be taken to prevent the spread of the virus to healthcare workers?
Supportive strategies for skin protection and nutrition in COVID-19 patients.76–85
| Skin protection | Nutrition. |
|---|---|
| • Check the skin every 4h to keep it clean and dry and to keep the mask in a normal position, avoiding excessive tightening.• In case of prolonged NIRS, either with CPAP or NIV, total face mask (TFM) or helmet should be considered.• When IPU occur with the oronasal mask, change to a TFM or helmet.• Repeated application of hyperoxygenated fatty acids.• If possible, allow for “skin breaks” after 12 hours of NIV. | • Early detection of malnutrition in high-risk patients: elderly patients and/or patients with • 2 or more chronic diseases• Start feeding in the first 48h.• For critically ill patients, start with half the calculated calories and increase every three days until the target (70–80% of requirement) is reached.• In the NIV/CPAP patient with poor tolerance to interruptions, parenteral nutrition should be considered.• If the enteral route is used, the helmet is preferable as it allows nasogastric tube placement.• If a nasogastric tube is used, continuous feeding with a normocaloric-hyperproteic formula is recommended rather than a bolus.• In the HFT patient and during the weaning phase of NIV, oral enteral nutrition, including supplementation, should be prioritized to achieve nutritional goals. |
The aerosol generating procedures (AGP) with higher risk of transmission of SARS-CoV-2 infection to healthcare workers are endotracheal intubation, NIV and nebulization. Other AGP, such as secretion aspiration or bronchoscopy, have not shown such conclusive results.86
Intubation is considered a high-risk practice for COVID-19 transmission. Table 3 reflects the strategy to reduce the SARS-CoV-2 transmission during endotracheal intubation.87,88
Strategy for avoiding aerosol dispersion during endotracheal intubation.
| • Avoid delays or multiple attempts (the most experience professional should perform the intubation).• Pre-oxygenation prior to intubation using manual mask ventilation and an airway mask bag unit should be performed with a HEPA filter placed between the mask and bag.• Pre-oxygenation with HFT may reduce hypoxemia during intubation but increases expiratory air dispersion.• Consider the use of a video laryngoscope as this will allow greater distance between the clinician and the airway.• Consider the use of a fibrobronchoscope if a difficult airway is anticipated.• Rapid sequence intubation. |
Aspiration of secretions in patients with an artificial airway is considered a high-risk APG89 and although their ability to infect healthcare workers with COVID-19 has not been demonstrated,86 the use of closed versus open suction systems is recommended.90
Regarding the spontaneous breathing test before extubation, it is suggested to perform the test with the CPAP/PSV method instead of the T-tube to avoid disconnection of the patient. If the T-tube test is used, a filter should be placed on the expiratory limb.88
It is recommended that the extubation procedure is performed by two professionals, with the ventilator connected to the orotracheal tube and the closed suction system aspirating while the orotracheal tube is removed.88
During NIV, exhaled air is dispersed up to 1m around the patient.91,92 This distance may be greater if the mask is not properly fitted91 and is proportional to the set pressures.88 In addition, the use of intentionally leak systems with single-limb circuits has been shown to increase exhaled air dispersion.92 Oral masks are preferable to nasal masks. If there is no good seal with the oral mask, the use of full-face masks or helmet may be considered.
Although aerosol therapy has not been shown to increase the likelihood of infection risk to healthcare workers, it is recommended that protective measures be taken.93–95 The dispersion of particles into the environment is related to the transmission capacity, and jet systems are therefore discouraged because of their higher dispersion.96
For patients with spontaneous breathing or HFT, inhaled therapy with an MDI device and spacer chamber is recommended.95 For patients requiring aerosol therapy, vibrating mesh devices with mouthpiece and filter are recommended,97 although the deposited drug doses may be higher, requiring dose adjustment. If inhaled therapy is used with NIV, an adapter in inspiratory limb is recommended (in metered-dose inhalers). Vibrating mesh systems should be fitted to the elbow of the mask when nebulizing.93–95,98,99
Finally, the following general measures should be taken:
- •
Design of a dual circuit (clean and dirty area), separating COVID-positive from COVID-negative patients.
- •
Use of PPE, FFP2 or FFP3 individual masks, hydroalcoholic gel and safety distance.
- •
Implement physical segregation barriers, equipment and surface hygiene and respiratory protection measures.
- •
Adaptation of working conditions: use of common areas, organization of workstations and shifts.100,101
- 11.
Indications for NIRS after weaning from mechanical ventilation
- 11.1
Following extubation
Positive evidences on the usefulness of NIRS have been reported mainly in patients with hypercapnic ARF.102 However, in recent years, studies conducted in patients with hypoxemic ARF, in specific groups at high risk of reintubation, have also shown its usefulness. Vaschetto et al.103 showed that early extubation followed by immediate NIV is safe and effective in selected patients (Table 4), with a reduction in the duration of mechanical ventilation and hospital stay.102,104
Groups at risk (hypoxemic ARF) in which NIV after extubation is recommended.102,104
| • Over 65 years of age.• APACHE II score greater than 12 (on the day of extubation).• Obesity with BMI>30.• Poor secretion management.• Difficult or prolonged weaning.• More than 1 comorbidity.• Heart failure as the primary cause of ARF.• Moderate to severe COPD.• Airway management problems.• Long-term mechanical ventilation. |
In COVID-19 patients, Cammarota et al. showed that early extubation followed by immediate use of NIV, shortened the duration of MV and reduced the rate of failure and reintubation.105 Given the few data available in COVID-19 patients, the recommendation would be to use NIRS (NIV or HFT) in patients of high-risk groups displayed in Table 4.
- 11.2
In tracheostomized patients
The conditioning of medical gases applied directly to the tracheostomy is supported by scientific evidence.106 The use of home humidification systems is recommended when the oxygen flow exceeds 4L/min.107 These recommendations are old and were developed before the technical development of HFT, which meant that COT was systematically applied to these patients.108 Recent studies have shown that high-flow systems, can help to reduce ventilator weaning and decannulation times.109,110 These studies do not compare the specific effect of HFT as it is applied across the board to all patients. There is no clear effect beyond gas conditioning.111 The tracheostomized patient therefore has the option of HFT supplementation directly through the tracheostomy tube.
ConclusionsSeveral years after the pandemic, the management of patients with COVID-19 pneumonia and ARF remains a challenge for supportive care with NIRS. Several recommendations, summarized in Table 5, have been agreed to address clinically relevant issues in daily practice.
Summary of recommendations.
| There is insufficient evidence that early initiation of NIRS (mainly HFT) is effective in preventing respiratory deterioration in patients with severe ARF secondary to COVID19 |
| NIRS onset criteria:- Moderate to severe dyspnea and evidence of increased work of breathing (accessory muscle use or tachypnoea>30rpm OR- PaO2/FiO2<200 or (SpO2<92% at FiO2 of 0.4) or PaCO2>45mmHg and pH<7.35 |
| In frail patients, HFT should be preferred due to better tolerability and fewer side effects. |
| Initiation with CPAP is recommended. The option of PSV should only be considered in those with global acute respiratory failure. HFT is a recommended alternative in hypoxemic patients without respiratory acidosis. |
| For HFT, it is recommended to start therapy at the maximum tolerated flow with an adequate FiO2 to maintain SpO2>92%.In the case of CPAP, it is usually initiated with values around 10 cmH20, without exceeding 12–13cmH2O to avoid barotrauma or negative hemodynamic effects, and FiO2 to achieve SpO2>93% or PaO2≥60mmHg.In the case of PSV NIV, PEEP between 10 and 12cmH2O and BP to target VT 4–6ml/kg and FiO2 to target SpO2 90–95% is suggested. |
| The helmet type is the interface of choice. If a helmet is not available, full face or oronasal masks can be used. The use of interfaces with a leak port in the mask is not recommended.It is recommended that nasal cannulas used for high flow therapy seal more than 50% of the nostril diameter. |
| Response to NIRS should be monitored by monitoring clinical signs of work of breathing and oxygenation using the ROX index. |
| Although failure of NIRS often results in the indication of tracheal intubation and initiation of invasive mechanical ventilation (IMV), in selected cases where there is potential for reversibility, rescue treatment with CPAP or NIV may be considered, although mortality is very high. If NIRS fails in patients with a therapeutic ceiling, it should be replaced by conventional oxygen therapy and symptomatic management of dyspnea should be optimized. |
| Prone positioning may be beneficial in patients with COVID-19-related acute hypoxemic respiratory failure who require admission to intensive care and receive NIRS treatment. |
| The strategy for weaning from NIRS should be progressive, with differences depending on the modality used. |
| Of particular importance is the strategy to prevent skin breakdown due to the interface.Consideration should be given to initiating enteral or parenteral nutrition within 48h. |
| Individual protection measures must be implemented for workers in contact with infected patients. Differentiated circuits for infected and non-infected patients should be organized.Vibrating mesh nebulizers should be preferred if aerosol therapy is required.Patient air filtration systems should be established in case of therapy with positive pressure systems. |
| The use of post-extubation NIRS is recommended in groups at risk of failure.In the tracheostomized patient, it is possible to provide HFT directly through the tracheostomy tube. |
The authors state that they have no conflict of interests