Given the number of changes that occur during late adolescence, it is possible that the behavior of asthma may also be different. The aim of our paper is to determine the prevalence of asthma in a population of late adolescents and its possible association with obesity, tobacco smoke exposure and family history of allergic disease.
Methods and subjectsIn a cross-sectional, population-based analytical study design, we selected a stratified random sample of subjects aged 15–18. By modeling risk through logistic regression, we assessed the relationship between asthma and the following covariables: gender, obesity, excess weight, family history of allergic disease and tobacco smoke exposure.
Results1600 subjects were included, and the following prevalences were identified: asthma 7.8%, obesity 9.6%, active smoking in the father 2 9.8%, active smoking in the mother 18.6% and active smoking habit in the study subjects 15.1%. In the final model, a significant association was found between asthma and the following variables: (1) asthma in the mother (adjusted OR [aOR]=2.95, 95% CI, 1.55–5.6), (2) history of allergic rhinitis (aOR=4.66, 95% CI, 2.63–8.25), and (3) male sex (aOR=1.48, 95% CI, 1.02–2.15). No association was seen with obesity or tobacco smoking of the parents.
ConclusionOur results suggest that maternal history of asthma, personal history of allergic rhinitis and male sex are related with asthma late adolescence, while smoking and being overweight are not.
Dada la serie de cambios que se presentan durante la adolescencia tardía, es posible que el comportamiento del asma sea diferente. Objetivo, determinar la prevalencia de asma en una población de adolescentes tardíos y su posible asociación con obesidad, exposición a humo de tabaco e historia familiar de enfermedad alérgica.
Métodos y sujetosEstudio transversal, analítico con base poblacional. Seleccionamos una muestra aleatoria estratificada de sujetos de 15 a 18 años de edad. Mediante modelaje de riesgos a través de regresión logística se evaluó la relación entre asma con las siguientes co-variables: sexo, obesidad, sobrepeso antecedente familiar de enfermedad alérgica y exposición a humo de tabaco.
ResultadosSe incluyeron 1.600 sujetos. Se encontraron las siguientes prevalencias: asma 7,8%, obesidad 9,6%, tabaquismo en el padre 29,8%, tabaquismo en la madre 18,6% y activo de los sujetos encuestados 15,1%. En el modelo final se encontró asociación significativa del asma con las siguientes variables: 1) asma en la madre (OR ajustado [ORa] = 2,95, IC95%, 1,55 a 5,6), 2) historia de rinitis alérgica (ORa = 4,66; IC95%, 2,63 a 8,25), y 3) el sexo masculino (ORa = 1,48; IC95%, 1,02 a 2,15), no así con la obesidad ni con el tabaquismo en cualquiera de los padres.
ConclusiónNuestros resultados sugieren que, con excepción de la historia materna de asma, la historia personal de rinitis alérgica y el sexo masculino, ni el tabaquismo, ni el exceso de peso están asociados con el asma en la adolescencia tardía.
The global incidence of asthma among adolescents is estimated at 12.6%, with considerable variations depending on the region of between 5.1% and 22%. In Mexico, the incidence varies from 1.2% to 14.9%, with tropical regions being the ones presenting the largest percentage values.1 Many risk factors have been implicated in the onset of asthma, the most frequent being the family history of allergic illness.2–5 However, it is the variability of the incidence of asthma that appears to indicate the presence of non-genetic factors associated with its appearance. Among these factors are: the breakdown of the family structure, food intake at birth, childhood history of respiratory infection, the presence of humidity in the home, low weight at birth.6–10 Two additional factors which have attracted particular attention in recent years are disorders characterized by overweight and exposure to cigarette smoke.
Both excess weight and obesity are considered very serious problems on a global scale, and recently they have gained in prevalence in the developing world.11 The frequency of both problems has greatly increased. In the USA, the incidence of excess weight doubled among children, and tripled among adolescents, after a period of almost 20 years.12 In our country, more than one-third of the adolescents suffer from excess weight or obesity.13 Recently many studies have been published with a view to explaining the link between asthma and obesity, but so far there has been some discrepancy between the results obtained.14–17 The role played by active or passive exposure to cigarette smoke in the incidence of asthma, like that of obesity, also appears to be unconsciousness.2,3,18–22
The vast majority of the research studies aimed at determining the incidence of asthma have centered their attention on late adolescence. This is a stage of life of physical, physiological, social and psychological change, as adolescents increasingly acquire the characteristics of an adult; it is functional activities that cause asthma and its associated features to behave differently. The aims of this study are twofold; in the first place to determine the incidence of asthma and its symptoms in a sample population of late adolescents; secondly, to determine whether obesity and exposure to cigarette smoke and a family history of allergies are among the factors associated with it.
Methods and SubjectsScenarioGuadalajara, which is situated in the center of the state of Jalisco, is the second most densely populated city in Mexico. According to a 2010 Household and Population Census, it has a population of 1495189.23
The framework of the sample is one hundred and twenty-one institutions of upper secondary education taking the full range of morning/evening shifts and grades into account, across both the private and public sectors; the schools in the city of Guadalajara are distributed across its seven administrative areas, with 51728 pupils in 1379 groups.
Study DesignBy means of an analytical transversal study with population-based samples, the sample population was of adolescents aged 15–18 enrolled in upper secondary-school education. Prior to the study, data were obtained from the Jalisco Institute of Public Transparency and Information corresponding to the school census of the year 2009, as well as the names and addresses of the schools located in Guadalajara. The sample was obtained using a probabilistic three-stage, stratified cluster-sampling procedure. In the first stage, each administrative division was considered as one stratum in which a subsample of schools was calculated. In the second stage, all the grades of each school selected were considered as strata. From each stratum at least one group was selected at random (considered as a cluster), from which a list of the names of the students was obtained. Finally, in the third stage, by means of an alphabetically ordered list of names, a progressive and unrepeatable number was assigned to each student; and the sampling was performed by means of random computer-generated numbers. The number of subjects selected for each grade was in the same proportion by which each grade contributed to the total number of pupils at the school concerned.
With a view to identifying possible errors in the questionnaires, insufficient expertise on the part of those carrying out the surveys, a lack of material or insufficient resources, problems in the selection of subjects, etc., a pilot test was conducted with 20 adolescents selected at random for whom the results would not form part of the final analysis.
MeasurementsFrom February to June 2009, a visit was made to the schools attended by the selected adolescents. Their participation was requested and they were asked to sign a written informed consent form. The instrument used for the detection of asthma and its symptoms was the one developed by the group The International Study of Asthma and Allergies in Childhood (ISAAC). The central questions in the questionnaires designed for the detection of allergic rhinitis and atopic dermatitis, from the same methodology, were also used. Furthermore, the subjects were questioned concerning the family history of asthma and allergies as well as the present smoking habit either on the part of the adolescents who are subjects in the study themselves or either of their parents.
Weight and height were measured under standardized conditions; each measurement was taken twice on the same day, with the average being used for the final analysis. Weight was measured on SECA® precision scales, Model 752. For height measurements a SECA® height rod Model 206 was used.
DefinitionsBody Mass Index (BMI) was defined as weight (in kilograms) divided by the square of the height (in meters). Classification was based on the growth tables specific to sex and age as provided by the Center for Disease Control and Prevention (CDC). The subject was considered to be overweight when the BMI was >85%, and obese when it was >95%; excess weight was the presence of overweight or obesity. According to the WHO late adolescence is the period of life between 14 and 18 years.
EthicsThis research was reviewed and approved by the Medical and Ethical Research Committee at the Dr. Juan I. Menchaca Hospital Civil in Guadalajara. The teachers at each educational center approved the participation of their students in the study; furthermore, the adolescents themselves were free at every moment to opt out, if they so chose.
Statistical AnalysisTo identify the frequency of the symptoms of asthma, its incidence was calculated on the basis of intervals of confidence of 95% for proportions. To compare proportions the Chi square test or the Fisher exact test was required. In the mean comparison for independent groups, the Student t test was used and the adjusted risks were evaluated by means of a logistical regression analysis. A value of ≤0.05 was considered significant. The analyses were carried out by means of the SPSS programme (Statistical Package for the Social Sciences) for Windows, version 18.0.
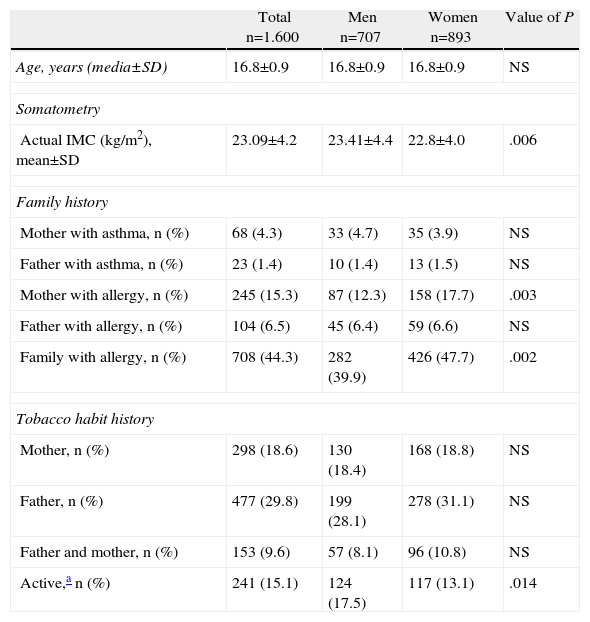
Results1600 adolescents were included in the study, ranging in age from 15 to 18, and the level of participation was 100%; 55.8% of the subjects were women. Table 1 shows the comparison of certain demographic aspects, the allergic antecedents and the smoking habit by gender. Approximately 44% of the subjects had a family member with some sort of allergic condition. A history of allergy in the mother was considerably more frequent in the female subjects than in the male subjects. 15.1% of the adolescents were active smokers.
Characteristics of the Subjects.
| Total n=1.600 | Men n=707 | Women n=893 | Value of P | |
| Age, years (media±SD) | 16.8±0.9 | 16.8±0.9 | 16.8±0.9 | NS |
| Somatometry | ||||
| Actual IMC (kg/m2), mean±SD | 23.09±4.2 | 23.41±4.4 | 22.8±4.0 | .006 |
| Family history | ||||
| Mother with asthma, n (%) | 68 (4.3) | 33 (4.7) | 35 (3.9) | NS |
| Father with asthma, n (%) | 23 (1.4) | 10 (1.4) | 13 (1.5) | NS |
| Mother with allergy, n (%) | 245 (15.3) | 87 (12.3) | 158 (17.7) | .003 |
| Father with allergy, n (%) | 104 (6.5) | 45 (6.4) | 59 (6.6) | NS |
| Family with allergy, n (%) | 708 (44.3) | 282 (39.9) | 426 (47.7) | .002 |
| Tobacco habit history | ||||
| Mother, n (%) | 298 (18.6) | 130 (18.4) | 168 (18.8) | NS |
| Father, n (%) | 477 (29.8) | 199 (28.1) | 278 (31.1) | NS |
| Father and mother, n (%) | 153 (9.6) | 57 (8.1) | 96 (10.8) | NS |
| Active,a n (%) | 241 (15.1) | 124 (17.5) | 117 (13.1) | .014 |
SD, standard deviation; n, number of subjects with the characteristics in question; NS, non-significant.
Value of P obtained by Chi square or Student t.
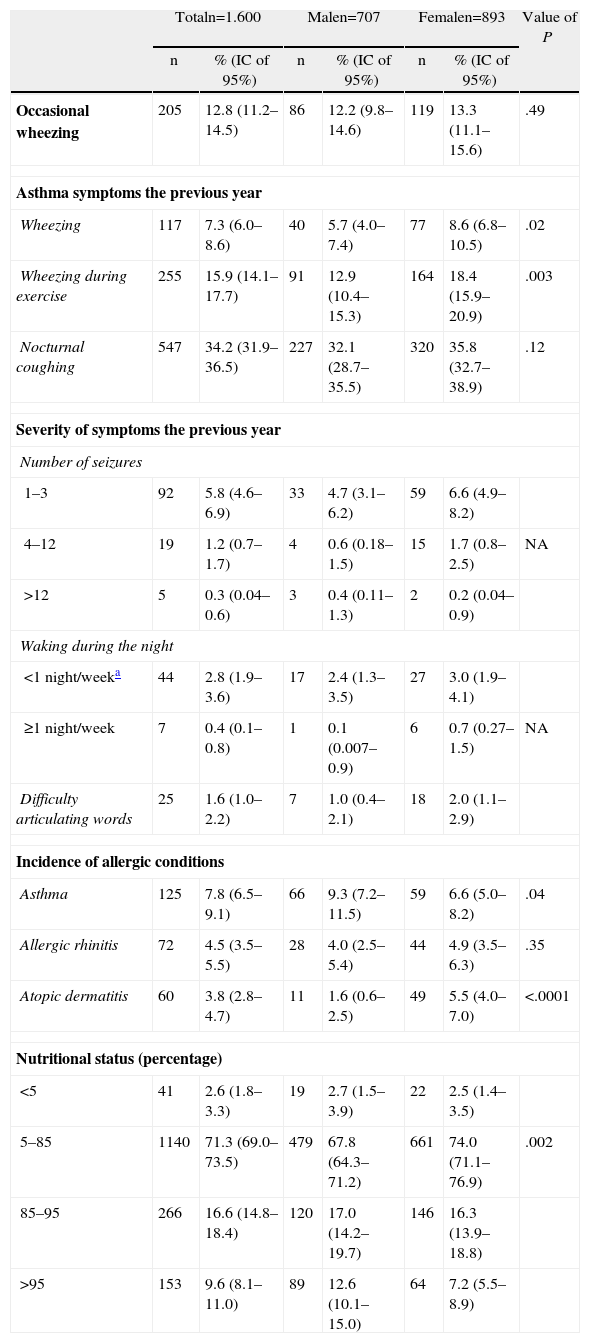
Table 2 shows the incidence o allergic conditions, which in all cases was lower than 10%. The symptoms of asthma were significantly more frequent in the female than in the male subjects. In general, severe asthma was present in fewer than 2% of the subjects. When the frequency of allergic conditions was compared by gender, asthma was found to be predominant in males and atopic dermatitis significantly predominant in females. More than a quarter of the subjects were overweight, with the males presenting a greater frequency of overweight and obesity.
Incidence of Symptoms, Allergic Conditions and Nutritional Status by Gender.
| Totaln=1.600 | Malen=707 | Femalen=893 | Value of P | ||||
| n | % (IC of 95%) | n | % (IC of 95%) | n | % (IC of 95%) | ||
| Occasional wheezing | 205 | 12.8 (11.2–14.5) | 86 | 12.2 (9.8–14.6) | 119 | 13.3 (11.1–15.6) | .49 |
| Asthma symptoms the previous year | |||||||
| Wheezing | 117 | 7.3 (6.0–8.6) | 40 | 5.7 (4.0–7.4) | 77 | 8.6 (6.8–10.5) | .02 |
| Wheezing during exercise | 255 | 15.9 (14.1–17.7) | 91 | 12.9 (10.4–15.3) | 164 | 18.4 (15.9–20.9) | .003 |
| Nocturnal coughing | 547 | 34.2 (31.9–36.5) | 227 | 32.1 (28.7–35.5) | 320 | 35.8 (32.7–38.9) | .12 |
| Severity of symptoms the previous year | |||||||
| Number of seizures | |||||||
| 1–3 | 92 | 5.8 (4.6–6.9) | 33 | 4.7 (3.1–6.2) | 59 | 6.6 (4.9–8.2) | |
| 4–12 | 19 | 1.2 (0.7–1.7) | 4 | 0.6 (0.18–1.5) | 15 | 1.7 (0.8–2.5) | NA |
| >12 | 5 | 0.3 (0.04–0.6) | 3 | 0.4 (0.11–1.3) | 2 | 0.2 (0.04–0.9) | |
| Waking during the night | |||||||
| <1 night/weeka | 44 | 2.8 (1.9–3.6) | 17 | 2.4 (1.3–3.5) | 27 | 3.0 (1.9–4.1) | |
| ≥1 night/week | 7 | 0.4 (0.1–0.8) | 1 | 0.1 (0.007–0.9) | 6 | 0.7 (0.27–1.5) | NA |
| Difficulty articulating words | 25 | 1.6 (1.0–2.2) | 7 | 1.0 (0.4–2.1) | 18 | 2.0 (1.1–2.9) | |
| Incidence of allergic conditions | |||||||
| Asthma | 125 | 7.8 (6.5–9.1) | 66 | 9.3 (7.2–11.5) | 59 | 6.6 (5.0–8.2) | .04 |
| Allergic rhinitis | 72 | 4.5 (3.5–5.5) | 28 | 4.0 (2.5–5.4) | 44 | 4.9 (3.5–6.3) | .35 |
| Atopic dermatitis | 60 | 3.8 (2.8–4.7) | 11 | 1.6 (0.6–2.5) | 49 | 5.5 (4.0–7.0) | <.0001 |
| Nutritional status (percentage) | |||||||
| <5 | 41 | 2.6 (1.8–3.3) | 19 | 2.7 (1.5–3.9) | 22 | 2.5 (1.4–3.5) | |
| 5–85 | 1140 | 71.3 (69.0–73.5) | 479 | 67.8 (64.3–71.2) | 661 | 74.0 (71.1–76.9) | .002 |
| 85–95 | 266 | 16.6 (14.8–18.4) | 120 | 17.0 (14.2–19.7) | 146 | 16.3 (13.9–18.8) | |
| >95 | 153 | 9.6 (8.1–11.0) | 89 | 12.6 (10.1–15.0) | 64 | 7.2 (5.5–8.9) | |
95% CI, 95% intervals of confidence; n, number of subjects; NA, not applicable or was not calculated.
Value of P obtained by Chi square test, the Yates correction or the Fisher exact test.
Intervals of confidence of 95% for values near 0 calculated by means of the Fleiss quadratic method.
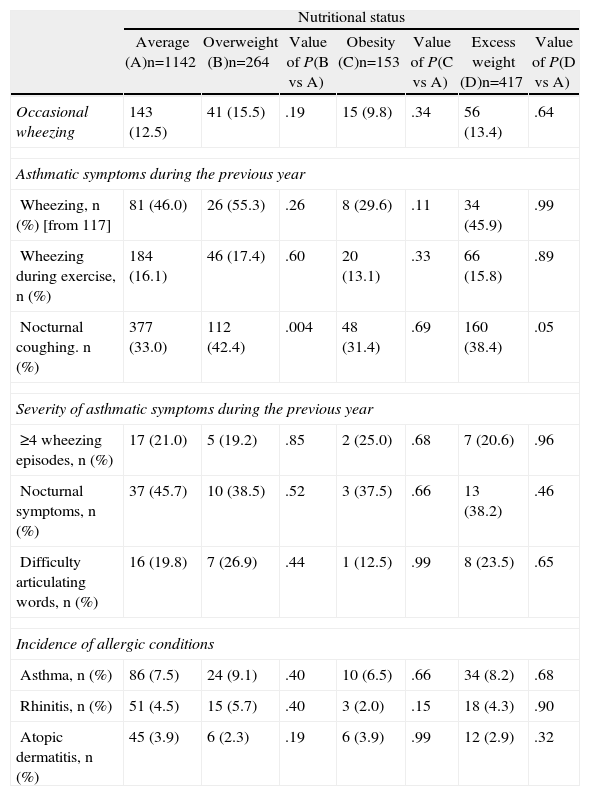
When the presence of symptoms of asthma during the previous year was measured against the nutritional status of the adolescents (Table 3), a significant association was found between the presence of overweight (P=.004) and weight excess with nocturnal coughing (P=.05), but in the case of obesity there was no such correlation.
Incidence of Asthmatic Symptoms, Asthma, Allergic Rhinitis and Atopic Dermatitis According to Nutritional Status.
| Nutritional status | |||||||
| Average (A)n=1142 | Overweight (B)n=264 | Value of P(B vs A) | Obesity (C)n=153 | Value of P(C vs A) | Excess weight (D)n=417 | Value of P(D vs A) | |
| Occasional wheezing | 143 (12.5) | 41 (15.5) | .19 | 15 (9.8) | .34 | 56 (13.4) | .64 |
| Asthmatic symptoms during the previous year | |||||||
| Wheezing, n (%) [from 117] | 81 (46.0) | 26 (55.3) | .26 | 8 (29.6) | .11 | 34 (45.9) | .99 |
| Wheezing during exercise, n (%) | 184 (16.1) | 46 (17.4) | .60 | 20 (13.1) | .33 | 66 (15.8) | .89 |
| Nocturnal coughing. n (%) | 377 (33.0) | 112 (42.4) | .004 | 48 (31.4) | .69 | 160 (38.4) | .05 |
| Severity of asthmatic symptoms during the previous year | |||||||
| ≥4 wheezing episodes, n (%) | 17 (21.0) | 5 (19.2) | .85 | 2 (25.0) | .68 | 7 (20.6) | .96 |
| Nocturnal symptoms, n (%) | 37 (45.7) | 10 (38.5) | .52 | 3 (37.5) | .66 | 13 (38.2) | .46 |
| Difficulty articulating words, n (%) | 16 (19.8) | 7 (26.9) | .44 | 1 (12.5) | .99 | 8 (23.5) | .65 |
| Incidence of allergic conditions | |||||||
| Asthma, n (%) | 86 (7.5) | 24 (9.1) | .40 | 10 (6.5) | .66 | 34 (8.2) | .68 |
| Rhinitis, n (%) | 51 (4.5) | 15 (5.7) | .40 | 3 (2.0) | .15 | 18 (4.3) | .90 |
| Atopic dermatitis, n (%) | 45 (3.9) | 6 (2.3) | .19 | 6 (3.9) | .99 | 12 (2.9) | .32 |
Value of P obtained by Chi square.
Group A (average nutritional status) was the control group.
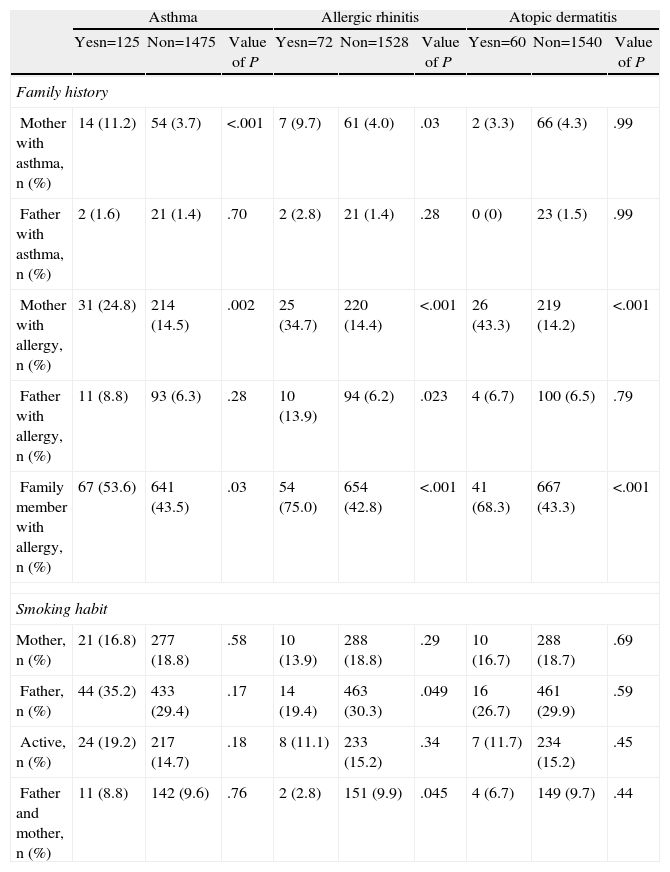
The association of the incidence of asthma and other atopic illnesses with hereditary and exposure to cigarette smoke is shown in Table 4. In the case of asthma, both the history of allergic illness and asthma in the mother and the history of atopic disorders in the family were associated with it. Regarding exposure to tobacco smoke, the frequency of cases of adolescents with asthma confirmed by a doctor was not found to be associated with active smoking or with the fact of living with smokers.
Influence of Heredity and Exposure to Snuff Smoke in the Prevalence of Allergic Diseases.
| Asthma | Allergic rhinitis | Atopic dermatitis | |||||||
| Yesn=125 | Non=1475 | Value of P | Yesn=72 | Non=1528 | Value of P | Yesn=60 | Non=1540 | Value of P | |
| Family history | |||||||||
| Mother with asthma, n (%) | 14 (11.2) | 54 (3.7) | <.001 | 7 (9.7) | 61 (4.0) | .03 | 2 (3.3) | 66 (4.3) | .99 |
| Father with asthma, n (%) | 2 (1.6) | 21 (1.4) | .70 | 2 (2.8) | 21 (1.4) | .28 | 0 (0) | 23 (1.5) | .99 |
| Mother with allergy, n (%) | 31 (24.8) | 214 (14.5) | .002 | 25 (34.7) | 220 (14.4) | <.001 | 26 (43.3) | 219 (14.2) | <.001 |
| Father with allergy, n (%) | 11 (8.8) | 93 (6.3) | .28 | 10 (13.9) | 94 (6.2) | .023 | 4 (6.7) | 100 (6.5) | .79 |
| Family member with allergy, n (%) | 67 (53.6) | 641 (43.5) | .03 | 54 (75.0) | 654 (42.8) | <.001 | 41 (68.3) | 667 (43.3) | <.001 |
| Smoking habit | |||||||||
| Mother, n (%) | 21 (16.8) | 277 (18.8) | .58 | 10 (13.9) | 288 (18.8) | .29 | 10 (16.7) | 288 (18.7) | .69 |
| Father, n (%) | 44 (35.2) | 433 (29.4) | .17 | 14 (19.4) | 463 (30.3) | .049 | 16 (26.7) | 461 (29.9) | .59 |
| Active, n (%) | 24 (19.2) | 217 (14.7) | .18 | 8 (11.1) | 233 (15.2) | .34 | 7 (11.7) | 234 (15.2) | .45 |
| Father and mother, n (%) | 11 (8.8) | 142 (9.6) | .76 | 2 (2.8) | 151 (9.9) | .045 | 4 (6.7) | 149 (9.7) | .44 |
n: number of subjects.
Value obtained my means of Chi square test, Yates correction or Fisher exact test
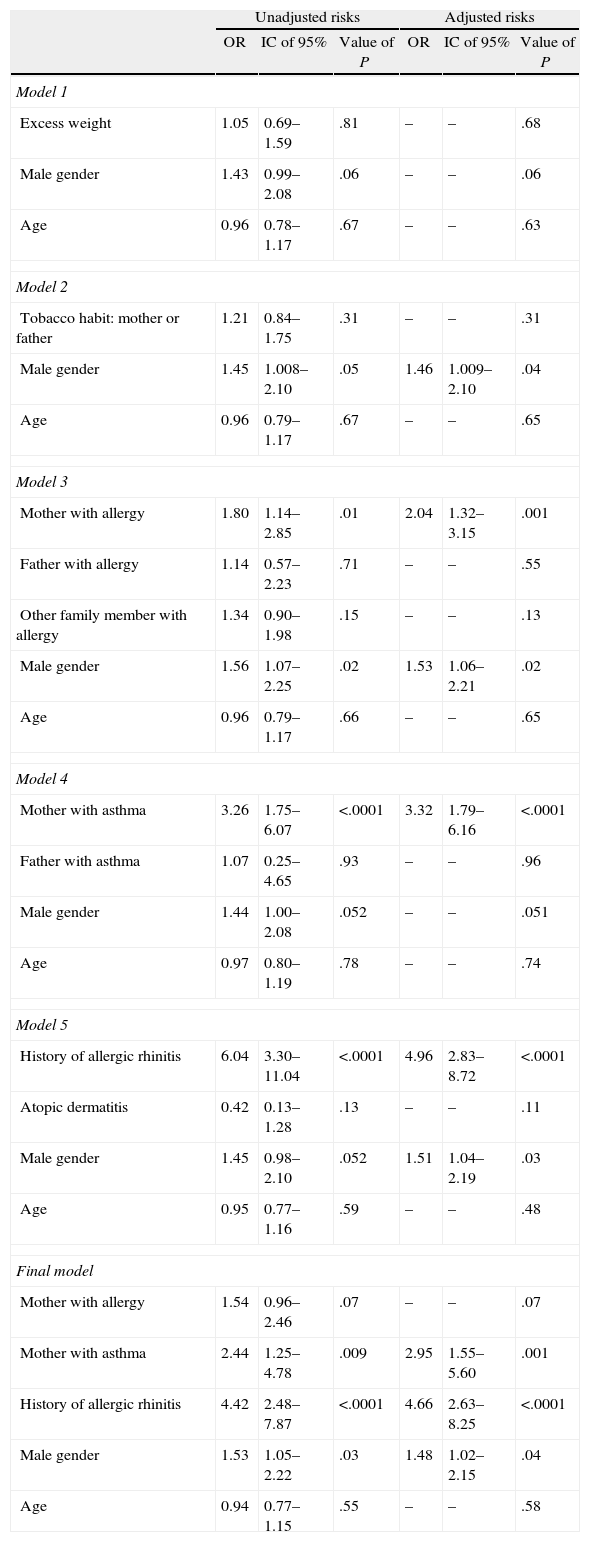
Table 5 shows the different models of logistical regression for the incidence of asthma as a dependent variable. In the model which included excess weight, male sex and age, no significant association was found. The subsequent models, 2 and 3, made it possible to show that the male sex was significantly associated with asthma, but they also showed the maternal history of allergy to be an associated factor. A fourth model showed that the presence of asthma in the mother was associated with asthma. The final model was constructed from the covariables that had been identified previously, and shows that the maternal history of allergy, a personal history of allergic rhinitis and the male sex remained significantly associated with asthma.
Factors Associated with Late Adolescence.
| Unadjusted risks | Adjusted risks | |||||
| OR | IC of 95% | Value of P | OR | IC of 95% | Value of P | |
| Model 1 | ||||||
| Excess weight | 1.05 | 0.69–1.59 | .81 | – | – | .68 |
| Male gender | 1.43 | 0.99–2.08 | .06 | – | – | .06 |
| Age | 0.96 | 0.78–1.17 | .67 | – | – | .63 |
| Model 2 | ||||||
| Tobacco habit: mother or father | 1.21 | 0.84–1.75 | .31 | – | – | .31 |
| Male gender | 1.45 | 1.008–2.10 | .05 | 1.46 | 1.009–2.10 | .04 |
| Age | 0.96 | 0.79–1.17 | .67 | – | – | .65 |
| Model 3 | ||||||
| Mother with allergy | 1.80 | 1.14–2.85 | .01 | 2.04 | 1.32–3.15 | .001 |
| Father with allergy | 1.14 | 0.57–2.23 | .71 | – | – | .55 |
| Other family member with allergy | 1.34 | 0.90–1.98 | .15 | – | – | .13 |
| Male gender | 1.56 | 1.07–2.25 | .02 | 1.53 | 1.06–2.21 | .02 |
| Age | 0.96 | 0.79–1.17 | .66 | – | – | .65 |
| Model 4 | ||||||
| Mother with asthma | 3.26 | 1.75–6.07 | <.0001 | 3.32 | 1.79–6.16 | <.0001 |
| Father with asthma | 1.07 | 0.25–4.65 | .93 | – | – | .96 |
| Male gender | 1.44 | 1.00–2.08 | .052 | – | – | .051 |
| Age | 0.97 | 0.80–1.19 | .78 | – | – | .74 |
| Model 5 | ||||||
| History of allergic rhinitis | 6.04 | 3.30–11.04 | <.0001 | 4.96 | 2.83–8.72 | <.0001 |
| Atopic dermatitis | 0.42 | 0.13–1.28 | .13 | – | – | .11 |
| Male gender | 1.45 | 0.98–2.10 | .052 | 1.51 | 1.04–2.19 | .03 |
| Age | 0.95 | 0.77–1.16 | .59 | – | – | .48 |
| Final model | ||||||
| Mother with allergy | 1.54 | 0.96–2.46 | .07 | – | – | .07 |
| Mother with asthma | 2.44 | 1.25–4.78 | .009 | 2.95 | 1.55–5.60 | .001 |
| History of allergic rhinitis | 4.42 | 2.48–7.87 | <.0001 | 4.66 | 2.63–8.25 | <.0001 |
| Male gender | 1.53 | 1.05–2.22 | .03 | 1.48 | 1.02–2.15 | .04 |
| Age | 0.94 | 0.77–1.15 | .55 | – | – | .58 |
95% CI, 95% intervals of confidence; OR, Odd ratio.
Odds ratios obtained by logistical regression.
In the adjustment variables neither odds ratios nor intervals of confidence are shown.
This investigation shows that the incidence of asthma in late adolescence in the area in which the study took place is 7.8% (95% CI: 6.5%–9.1%). When this figure is compared with other parts of Mexico, we find variations, such as in the central region where it oscillates between 3.6% and 4.4%,4,24 while in the north east it reaches 6%.14 Recently the results were published corresponding to phase III of ISAAC, a study which included a population of 798685 adolescents, in which the global incidence of asthma was shown to be 12.6%, with geographical variations of between 5.1% and 22%, with the lowest frequencies being recorded for Africa, India and Eastern Europe, in sharp contrast with the higher figures for North America and Oceania. The highest incidences were recorded in English-speaking regions (19.9%). In the same study, the results for Mexico were between 1.2% and 14.9%, with the highest values being recorded for tropical regions.1 Other studies using different methods from ISAAC continue to show substantial differences in the incidence of asthma.2,20 In the case of the late adolescents assessed for our study, the presence of wheezing in the year preceding the study (7.3%) was close to the Mexican average (6.9%), but it was much lower than in the south east, where it varied between 13% and 14.9%.1 The risk factors involved in this case would appear to be the same as those for asthma. The risk factors invoked to explain the differences in incidences of asthma are numerous. Our study offers additional information from an often neglected group, concerning the relationship between asthma and some of these factors. With regard to the importance of gender in the evolution of asthma, it is known that during childhood asthma is more common in males than in females and in adulthood it is clearly predominant in women, but it is during early adolescence that the beginning of this reversal has been located25; in our population, asthma was common amongst men, with male gender remaining the single most significant factor associated with it. This same behavior has been documented in the United Arab Emirates,26 where the group of males aged between 13 and 19 was associated with the incidence of asthma, adjusted OR of 1.45 (95% CI 1.10–1.9, P=.008), in which the group of men aged between 13 and 19 presented a significantly greater incidence of asthma, adjusted OR of 1.45 (95% CI 1.10–1.90, P=.008). However, this did not occur with men over the age of 19, adjusted OR of 0.75 (95% CI 0.60–0.95, P=.02). On the opposite end of the scale, our results differ from those obtained in northeast Mexico in a study carried out with a similar age group, in which no significant correlation was found between asthma and gender OR of 1.02 (95% CI 0.80–1.30, P=NS).14 By means of a cross-sectional study carried out with adolescents of Argentinean origin aged between 15 and 18, no correlation was found either between gender and the incidence of asthma, OR of 1.16 (95% CI 0.94–1.45, P=.18).27 What we can deduce from this, at least as far as our region is concerned, is that the greater frequency of asthma among women than among men tends to be associated with the later stages of life. The factors that account for these differences have yet to be elucidated, but the role played by hormones would appear to be significant.
Among the factors constantly associated with asthma is the family history of allergic and atopic disorders.2–5 In this study we show that maternal history is strongly associated with asthma, and this was also found to be the case with allergic rhinitis. From the genetic point of view there are several gene polymorphisms which explain why heredity on the mother's side has a greater impact than on the father's in the development of asthma: the presence of polymorphisms in the maternal antioxidant genes (GSTP1 and GSTM1, GSTT1) and the high affinity IgE receptor beta chains (FceRI-beta), as well as in the atopic alleles located in the 11q13 chromosome.28 The effect of family atopic disorders could not be evaluated in our study, so for the time being we are unable to say what role this factor plays in the development of asthma.
The harmful effects of exposure to active or passive smoking on a person's health have been documented on numerous occasions; but the role that it plays in the incidence of asthma is a matter of debate. In our study, one-third of the adolescent subjects lived with parents who smoked, but what was even more important was the high proportion of adolescents who were smokers themselves. Nevertheless, we show in this study that in no case was the incidence of smoking related to exposure to tobacco smoke; previous research has produced similar findings.2,18–20
Conversely, a multicentric study carried out with several cohorts of children, whose development was tracked from pregnancy to the age of 4 years old, found a significant correlation between postnatal exposure to tobacco smoke and an asthma diagnosis, OR of 1.69 (95% CI, 1.01–2.82)21; a similar link was found between the presence of more than one smoker at home and the development of wheezing during the 12 months prior to the study, OR of 1.27 (95% CI, 1.01–1.59).3 Hawkins and Berkman22 also found that the presence of a member of the family who smoked increased the likelihood of the children developing asthma.
It would seem that exposure to tobacco smoke is related with the intensity of the symptoms and the number of aggravations of the asthma, as was demonstrated by Mannino et al.,29 through the Third National Health and Nutrition Examination Survey, who showed that children exposed to large amounts of tobacco smoke were more likely to have moderate to severe asthma (OR 2.7, 95% CI, 1.1–1.68), and that they also suffered from a more significant diminishing of the lung function. On account of their consequences, disorders characterized by excess weight (overweight and obesity) are the most serious health problems in different parts of the world.11 In Mexico, the National Survey of Health and Nutrition (ENSANUT 2006) showed that more than 30% of adolescents (12–19 years of age) were either overweight or obese.13 In our sample population 25% presented excess weight.
These high rates of overweight or obesity, as observed in our population, led us to consider the possibility of a link between this factor and the incidence of asthma, but our results did not indicate that there was any such link. The information provided in this respect has thrown up conflicting results. In our country alone, Vázquez-Nava et al.14 in a sample of late adolescents found a link between asthma and obesity, OR of 1.47 (95% CI, 1.08–1.99; P=.01), but not between asthma and overweight, OR of 0.99 (95% CI, 0.69–1.42; P=non-significant). These same researchers, this time with children of 4–5 years old, found that asthma was associated neither with obesity (OR of 1.47; 95% CI: 1.16–0.64; to 2.08), nor with overweight (OR of 1.58; 95% CI: 0.78–3.19).15 In our opinion, the causes of this inconsistency lie in the way in which the studies are designed, the age group studied, the way in which the weight and height of the participants are measured, the cut-off points used to classify overweight and obesity, among other factors. One area in which a stronger link has been demonstrated between asthma and obesity is the severity of the asthmatic condition in the obese, a more severe intensity of the condition having been observed in obese sufferers.30
This study suffers from certain limitations. One of them has to do with the lack of confirmation of an asthma diagnosis by more objective means, such as clinical history or the performance of respiratory function tests. Another is recall bias, a condition which is very common in epidemiological studies based on questionnaires. This might explain, to a large extent, why such a low rate of wheezing has been observed among these subjects. Although the sample of adolescents surveyed only took into account those who come to the city of Guadalajara to study, we believe that the behavior of the variables studied are a clear reflection of the reality of this age group as a whole, as the rate of participation was high; a range of different administrative and geographical districts of the city were included, and it covered schools in both the public and private sector.
ConclusionsAs no previous results were available to us in relation to the epidemiological behavior of asthma in late adolescents, we find ourselves unable, for the time being, to evaluate the general tendency of one of the most common respiratory disorders. Our results will therefore serve as a departure point for these tendencies to be determined. With regard to the risk factors studied in this paper, the maternal history of asthma, the presence of allergic rhinitis, and being male were all significantly associated with the incidence of asthma.
Conflict of InterestsThe authors have no conflict of interest to declare.
Our gratitude to the authorities of the Institute for Transparency and Public Information of Jalisco, and teachers and students for their participation in this study. Tonatiuh Bedolla Pulido and Carlos Guzman Venegas, for their help in capturing the information and on-line calculation of BMI.
Please cite this article as: Bedolla-Barajas M, et al. Asma en adolescentes tardíos del occidente de México: prevalencia y factores asociados. Arch Bronconeumol. 2013;49:47–53.