There is still uncertainty about which aspects of cigarette smoking influence the risk of Chronic Obstructive Pulmonary Disease (COPD). The aim of this study was to estimate the COPD risk as related to duration of use, intensity of use, lifetime tobacco consumption, age of smoking initiation and years of abstinence.
MethodsWe conducted an analytical cross-sectional study based on data from the EPISCAN-II study (n=9092). All participants underwent a face-to-face interview and post-bronchodilator spirometry was performed. COPD was defined as post-bronchodilator FEV1/FVC<70%. Parametric and nonparametric logistic regression models with generalized additive models were used.
Results8819 persons were included; 858 with COPD and 7961 without COPD. The COPD risk increased with smoking duration up to ≥50 years [OR 3.5 (95% CI: 2.3–5.4)], with smoking intensity up to ≥39cig/day [OR 10.1 (95% CI: 5.3–18.4)] and with lifetime tobacco consumption up to >29 pack-years [OR 3.8 (95% CI: 3.1–4.8)]. The COPD risk for those who started smoking at 22 or later was 0.9 (95% CI: 0.6–1.4). The risk of COPD decreased with increasing years of cessation. In comparison with both never smokers and current smokers, the lowest risk of COPD was found after 15–25 years of abstinence.
ConclusionCOPD risk increases with duration, intensity, and lifetime tobacco consumption and decreases importantly with years of abstinence. Age at smoking initiation shows no effect. After 15–25 years of cessation, COPD risk could be equal to that of a never smoker. This work suggests that the time it takes to develop COPD in a smoker is about 30 years.
According to the Global Burden of Disease, in 2019, 3.28 million people died from Chronic Obstructive Pulmonary Disease (COPD), ranking as the third leading cause of death worldwide.1,2 In Spain, COPD is considered the fifth leading cause of death in men and the seventh in women.3 In 2017, the prevalence of COPD in Spain was estimated at 11.8% in population aged 40 years and over.4
Tobacco consumption is the main risk factor for COPD5 and usually COPD caused by it is initiated by lung injury and abnormal lung repair processes.6 Aspects related to tobacco consumption such as duration, intensity, accumulated consumption, age of initiation or years of abstinence can influence the risk of developing this disease. Many of these variables have been extensively studied to analyze their influence on the risk of lung cancer. The two most studied aspects were smoking duration and intensity7–12 and most studies observed that smoking duration has the greatest influence on the development of lung cancer.8,9,13
Currently, it is not known whether smoking duration is more relevant than smoking intensity on the COPD risk. The available literature is limited and most research has focused on dividing the groups as current smokers or former smokers. Recent publications and editorials14–16 have highlighted the need for clarity to: (a) better explain how smoking influences COPD risk to have a better understanding of the molecular mechanisms and, (b) design effective tobacco control policies and interventions for smokers. The few studies available are cross-sectional studies focused on analyzing the impact of aspects such as smoking duration on COPD14,15 and a case–control study with a small sample size focused on analyzing the relationship between age at onset or smoking duration and COPD.17 Therefore, the relationship and influence of several aspects related to tobacco consumption are not clear.
The objectives of this study are: (a) to estimate, with categorical and nonparametric models, the risk of COPD in current smokers compared to never smokers, related to smoking duration, smoking intensity, lifetime tobacco consumption and age at smoking initiation and (b) to assess how the COPD risk is reduced with the increase of years of cessation.
MethodsStudy designAn analytical cross-sectional study based on data from the EPISCAN-II study was conducted. This countrywide representative study was funded by GlaxoSmithKline-GSK to estimate COPD prevalence in Spain. The EPISCAN-II study was carried out in the Spanish population aged 40 years and older (n=9092) with twenty hospitals taking part from 17 Spanish regions. Therefore, it is a population-based multicenter study. Recruitment took place between April 2017 and February 2019. The EPISCAN-II protocol was previously published18 and registered at https://clinicaltrials.gov under no. NCT03028207 and at www.gsk-clinicalstudyregister.com/study/205932.
DefinitionsAll EPISCAN-II participants underwent forced spirometry according to the guidelines of the SEPAR Society. After about 15–30min of forced spirometry, a bronchodilator test was performed by inhaling 400μg of salbutamol. A 12% increase associated with a gain of 200ml was considered as criteria for bronchodilation. COPD cases were defined as those participants with forced expiratory volume in one second/forced vital capacity (FEV1/FVC) score<0.7 in post-bronchodilator spirometry (post-BD) and who did not have a previous diagnosis of COPD. All COPD cases were not previously diagnosed (incident cases). Participants without COPD were defined as people with a FEV1/FVC score≥0.7 and who did not have a previous diagnosis of COPD. A total of 219 participants classified by the post-BD as COPD subjects and 54 as non-COPD subjects were excluded in the present study because they had a previous diagnosis of COPD (prevalent COPD cases). This exclusion was due to avoid any change of tobacco consumption which might have happened in prevalent cases following COPD diagnosis and therefore give a biased information on the role of their smoking characteristics on COPD onset.
Information retrieval, variables and proceduresInformation regarding sociodemographic variables, smoking consumption and comorbidities was collected in a face-to-face interview performed by trained personnel. The sociodemographic variables collected were sex, age, highest educational level achieved (primary education or less, secondary education, university studies, other), living situation (lives alone, does not live alone) and employment situation (working, unemployed, household duties, student, retired, other situations). Based on the question “What is your situation with regard to tobacco?”, participants were classified as current smokers, former smokers (if they had not smoked for at least 6 months) and never smokers. Current smokers and former smokers were asked about the number of cigarettes consumed per day (cig/day) and the age of smoking initiation. Former smokers were asked about the age at smoking cessation. Smoking duration and pack-years were calculated for current smokers and former smokers, and years of abstinence for former smokers. Information regarding comorbidities was collected through the Charlson index and the COTE index.
Statistical analysisLogistic regression models were used in which the dependent variable was having COPD or not. To analyze the risk of COPD in current smokers compared to never smokers, former smokers were excluded from this analysis (n=3112) to avoid any possible bias due to the years of abstinence. The independent variables were: smoking duration, smoking intensity, lifetime tobacco consumption (calculated in pack-years), and age of smoking initiation. Smoking duration was calculated from the age at smoking initiation and age at the time of the interview. It was divided into 6 categories (0, 1–20, 21–30, 31–40, 41–50 and >50 years) and adjusted for sex and cig/day. Smoking intensity was measured as the number of cig/day, was classified into 6 categories (0, 1–9, 10–19, 20–29, 30–39, 20–39, and >39cig/day) and was adjusted for sex and age. A stratified analysis of smoking duration and intensity was performed. Lifetime tobacco consumption was calculated from number of cig/day and smoking duration. Based on the number of pack-years smoked, current smokers were divided into 3 tertiles: light smokers (who smoked 1<13 pack-years), moderate smokers (who smoked 13–29 pack-years) and heavy smokers (who smoked>29 pack-years). Lifetime tobacco consumption was adjusted by sex. The age of smoking initiation was divided into 5 categories (<15, 15–16, 17–18, 19–21 and >21 years) and was adjusted by sex and cig/day. For age of onset, being<15 years old was the reference category and for the rest of the models it was being a never smoker.
To analyze COPD risk in former smokers, two logistic regression models were performed, one using as the reference category never smokers and the other using as reference category current smokers. In these models, years of abstinence were included as an independent variable. In the model using never smokers as reference, years of abstinence were divided into 5 categories (1–2, 3–5, 6–10, 11–15 and >15 years of abstinence). In the model using current smokers as reference, years of abstinence were categorized into: 1–2, 3–5, 6–10, 11–15 and >15 years of abstinence. Both models were adjusted for sex, age, and cig/day.
The results are presented as odds ratios (OR) accompanied by 95% confidence intervals (95% CI). The statistical analysis was performed using R software v.4.1.2.
Six nonparametric generalized additive models (GAM) were applied for the variables of smoking duration (adjusted for sex and cig/day), smoking intensity (adjusted for sex and age), lifetime tobacco consumption (adjusted for sex), age of onset (adjusted for sex and cig/day) and years of abstinence (adjusted for sex, age and cig/day). The reason for using age as adjustment variables in only some models but not in others was to avoid correlation with the independent variables including time (i.e. duration of smoking or pack-years), which would have implied a risk of collinearity. The reasons for adjustment of each variable are presented in Appendix 1 of the supplementary material. In the models for duration, intensity and lifetime tobacco consumption, the reference category was never smoker. For age at onset, the reference category was 17 years, and for years of abstinence, in one model was never smoker and in the other was current smoker. The results are expressed using figures describing how COPD risk is modified with these different exposures. The figures were performed with the R package mgcv v.1.8-38.
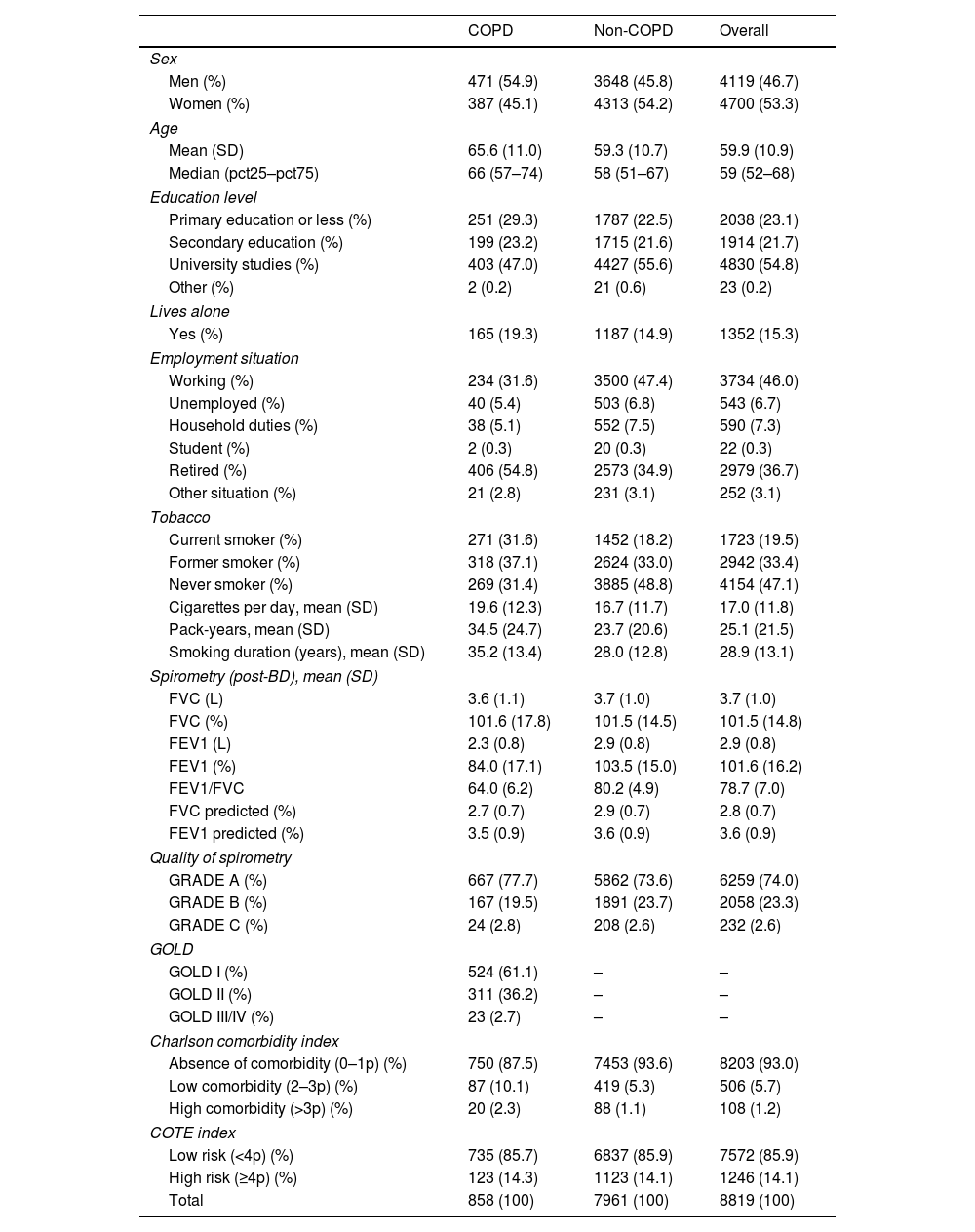
ResultsA total of 8819 subjects were included, of which 858 had COPD and 7961 did not have COPD. Their main characteristics are described in Table 1.
Demographic and clinical characteristics of participants.
| COPD | Non-COPD | Overall | |
|---|---|---|---|
| Sex | |||
| Men (%) | 471 (54.9) | 3648 (45.8) | 4119 (46.7) |
| Women (%) | 387 (45.1) | 4313 (54.2) | 4700 (53.3) |
| Age | |||
| Mean (SD) | 65.6 (11.0) | 59.3 (10.7) | 59.9 (10.9) |
| Median (pct25–pct75) | 66 (57–74) | 58 (51–67) | 59 (52–68) |
| Education level | |||
| Primary education or less (%) | 251 (29.3) | 1787 (22.5) | 2038 (23.1) |
| Secondary education (%) | 199 (23.2) | 1715 (21.6) | 1914 (21.7) |
| University studies (%) | 403 (47.0) | 4427 (55.6) | 4830 (54.8) |
| Other (%) | 2 (0.2) | 21 (0.6) | 23 (0.2) |
| Lives alone | |||
| Yes (%) | 165 (19.3) | 1187 (14.9) | 1352 (15.3) |
| Employment situation | |||
| Working (%) | 234 (31.6) | 3500 (47.4) | 3734 (46.0) |
| Unemployed (%) | 40 (5.4) | 503 (6.8) | 543 (6.7) |
| Household duties (%) | 38 (5.1) | 552 (7.5) | 590 (7.3) |
| Student (%) | 2 (0.3) | 20 (0.3) | 22 (0.3) |
| Retired (%) | 406 (54.8) | 2573 (34.9) | 2979 (36.7) |
| Other situation (%) | 21 (2.8) | 231 (3.1) | 252 (3.1) |
| Tobacco | |||
| Current smoker (%) | 271 (31.6) | 1452 (18.2) | 1723 (19.5) |
| Former smoker (%) | 318 (37.1) | 2624 (33.0) | 2942 (33.4) |
| Never smoker (%) | 269 (31.4) | 3885 (48.8) | 4154 (47.1) |
| Cigarettes per day, mean (SD) | 19.6 (12.3) | 16.7 (11.7) | 17.0 (11.8) |
| Pack-years, mean (SD) | 34.5 (24.7) | 23.7 (20.6) | 25.1 (21.5) |
| Smoking duration (years), mean (SD) | 35.2 (13.4) | 28.0 (12.8) | 28.9 (13.1) |
| Spirometry (post-BD), mean (SD) | |||
| FVC (L) | 3.6 (1.1) | 3.7 (1.0) | 3.7 (1.0) |
| FVC (%) | 101.6 (17.8) | 101.5 (14.5) | 101.5 (14.8) |
| FEV1 (L) | 2.3 (0.8) | 2.9 (0.8) | 2.9 (0.8) |
| FEV1 (%) | 84.0 (17.1) | 103.5 (15.0) | 101.6 (16.2) |
| FEV1/FVC | 64.0 (6.2) | 80.2 (4.9) | 78.7 (7.0) |
| FVC predicted (%) | 2.7 (0.7) | 2.9 (0.7) | 2.8 (0.7) |
| FEV1 predicted (%) | 3.5 (0.9) | 3.6 (0.9) | 3.6 (0.9) |
| Quality of spirometry | |||
| GRADE A (%) | 667 (77.7) | 5862 (73.6) | 6259 (74.0) |
| GRADE B (%) | 167 (19.5) | 1891 (23.7) | 2058 (23.3) |
| GRADE C (%) | 24 (2.8) | 208 (2.6) | 232 (2.6) |
| GOLD | |||
| GOLD I (%) | 524 (61.1) | – | – |
| GOLD II (%) | 311 (36.2) | – | – |
| GOLD III/IV (%) | 23 (2.7) | – | – |
| Charlson comorbidity index | |||
| Absence of comorbidity (0–1p) (%) | 750 (87.5) | 7453 (93.6) | 8203 (93.0) |
| Low comorbidity (2–3p) (%) | 87 (10.1) | 419 (5.3) | 506 (5.7) |
| High comorbidity (>3p) (%) | 20 (2.3) | 88 (1.1) | 108 (1.2) |
| COTE index | |||
| Low risk (<4p) (%) | 735 (85.7) | 6837 (85.9) | 7572 (85.9) |
| High risk (≥4p) (%) | 123 (14.3) | 1123 (14.1) | 1246 (14.1) |
| Total | 858 (100) | 7961 (100) | 8819 (100) |
COPD: Chronic Obstructive Pulmonary Disease; FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; p: points; post-BD: post-bronchodilator; SD: standard deviation.
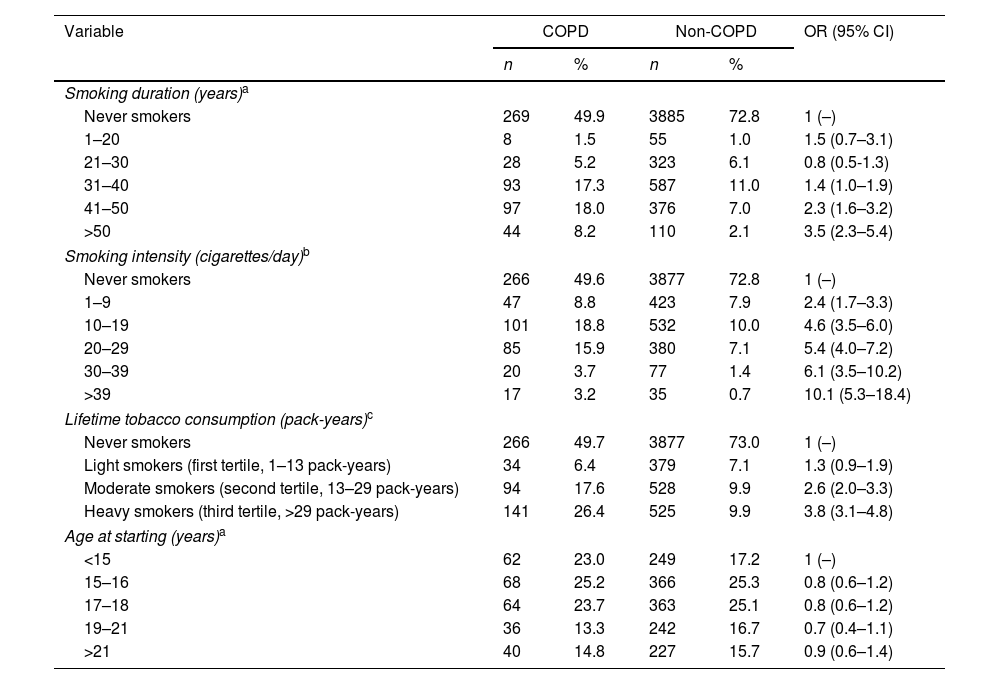
The parametric analysis by categories applied to analyze the risk of COPD in current smokers versus never smokers included 5877 individuals, of whom 540 were subjects with COPD and 5337 without COPD. The COPD risk increased with smoking duration up to the age of ≥50 years [OR=3.5 (95% CI: 2.3–5.4)]. This upward trend was also observed when smoking intensity increases. Thus, those people who smoked ≥40cig/day had a 10-fold increased risk of COPD compared to a never smoker [OR=10.1 (95% CI: 5.3–18.4)]. In relation to lifetime tobacco consumption, the highest risk was reached in the last tertile corresponding to heavy smokers [OR=3.8 (95% CI: 3.1–4.8)]. The COPD risk for those who started smoking at 22 or later was 0.9 (95% CI: 0.6–1.4) compared to those starting at the earliest age (Table 2).
Risk of Chronic Obstructive Pulmonary Disease (COPD) related to smoking duration, smoking intensity (cigarettes/day), lifetime tobacco consumption (pack-years) and age of starting consumption in current smokers vs. never smokers. Former smokers excluded.
| Variable | COPD | Non-COPD | OR (95% CI) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Smoking duration (years)a | |||||
| Never smokers | 269 | 49.9 | 3885 | 72.8 | 1 (–) |
| 1–20 | 8 | 1.5 | 55 | 1.0 | 1.5 (0.7–3.1) |
| 21–30 | 28 | 5.2 | 323 | 6.1 | 0.8 (0.5-1.3) |
| 31–40 | 93 | 17.3 | 587 | 11.0 | 1.4 (1.0–1.9) |
| 41–50 | 97 | 18.0 | 376 | 7.0 | 2.3 (1.6–3.2) |
| >50 | 44 | 8.2 | 110 | 2.1 | 3.5 (2.3–5.4) |
| Smoking intensity (cigarettes/day)b | |||||
| Never smokers | 266 | 49.6 | 3877 | 72.8 | 1 (–) |
| 1–9 | 47 | 8.8 | 423 | 7.9 | 2.4 (1.7–3.3) |
| 10–19 | 101 | 18.8 | 532 | 10.0 | 4.6 (3.5–6.0) |
| 20–29 | 85 | 15.9 | 380 | 7.1 | 5.4 (4.0–7.2) |
| 30–39 | 20 | 3.7 | 77 | 1.4 | 6.1 (3.5–10.2) |
| >39 | 17 | 3.2 | 35 | 0.7 | 10.1 (5.3–18.4) |
| Lifetime tobacco consumption (pack-years)c | |||||
| Never smokers | 266 | 49.7 | 3877 | 73.0 | 1 (–) |
| Light smokers (first tertile, 1–13 pack-years) | 34 | 6.4 | 379 | 7.1 | 1.3 (0.9–1.9) |
| Moderate smokers (second tertile, 13–29 pack-years) | 94 | 17.6 | 528 | 9.9 | 2.6 (2.0–3.3) |
| Heavy smokers (third tertile, >29 pack-years) | 141 | 26.4 | 525 | 9.9 | 3.8 (3.1–4.8) |
| Age at starting (years)a | |||||
| <15 | 62 | 23.0 | 249 | 17.2 | 1 (–) |
| 15–16 | 68 | 25.2 | 366 | 25.3 | 0.8 (0.6–1.2) |
| 17–18 | 64 | 23.7 | 363 | 25.1 | 0.8 (0.6–1.2) |
| 19–21 | 36 | 13.3 | 242 | 16.7 | 0.7 (0.4–1.1) |
| >21 | 40 | 14.8 | 227 | 15.7 | 0.9 (0.6–1.4) |
Note:
In the stratified analysis of smoking duration and intensity, COPD risk was found to increase with both duration and intensity. However, this increase seems to be more marked the greater the smoking intensity. Thus, a subject who smoked 1–15cig/day for more than 40 years had a COPD risk of 3.4 (95% CI: 2.6–4.5), but a person who smoked 31–40cig/day during the same time had a COPD risk of 4.8 (95% CI: 1.9–10.8) (Table 1 of the supplementary material).
Taking never smokers as a reference, the risk of COPD in former smokers decreased consistently with increasing years of smoking cessation. Thus, the lowest risk was found after 15 years of smoking abstinence [OR=1.2 (95% CI: 0.9–1.5)]. When current smokers were taken as the reference category, a decreasing trend in COPD risk was observed as the years of abstinence increase. Again, the lowest risk was observed after 15 years of abstinence [OR=0.3 (95% CI: 0.3–0.4)] (Table 3). This implies that, compared to a current smoker, a former smoker with more than 15 years of abstinence has reduced his/her COPD risk by two thirds.
Risk of Chronic Obstructive Pulmonary Disease (COPD) related to years of abstinence from tobacco consumption in former smokers compared to never smokers and current smokers.
| Variable | COPD | Non-COPD | OR (95% CI)a | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Abstinence (years since smoking cessation) | |||||
| Never smokers | 266 | 45.7 | 3877 | 59.7 | 1 (–) |
| 1–2 | 26 | 4.5 | 158 | 2.4 | 3.1 (1.9–5.0) |
| 3–5 | 27 | 4.6 | 227 | 3.5 | 1.9 (1.2–3.0) |
| 6–10 | 46 | 7.9 | 354 | 5.5 | 2.0 (1.3–2.9) |
| 11–15 | 40 | 6.9 | 427 | 6.6 | 1.3 (0.9–1.9) |
| >15 | 177 | 30.4 | 1451 | 22.3 | 1.2 (0.9–1.5) |
| Abstinence (years since smoking cessation) | |||||
| Current smokers | 270 | 46.1 | 1447 | 35.6 | 1 (–) |
| 1–2 | 26 | 4.4 | 158 | 3.9 | 0.9 (0.5–1.3) |
| 3–5 | 27 | 4.6 | 227 | 5.6 | 0.5 (0.3–0.8) |
| 6–10 | 46 | 7.8 | 354 | 8.7 | 0.5 (0.4–0.7) |
| 11–15 | 40 | 6.8 | 427 | 10.5 | 0.4 (0.2–0.5) |
| >15 | 177 | 30.2 | 1450 | 35.7 | 0.3 (0.3–0.4) |
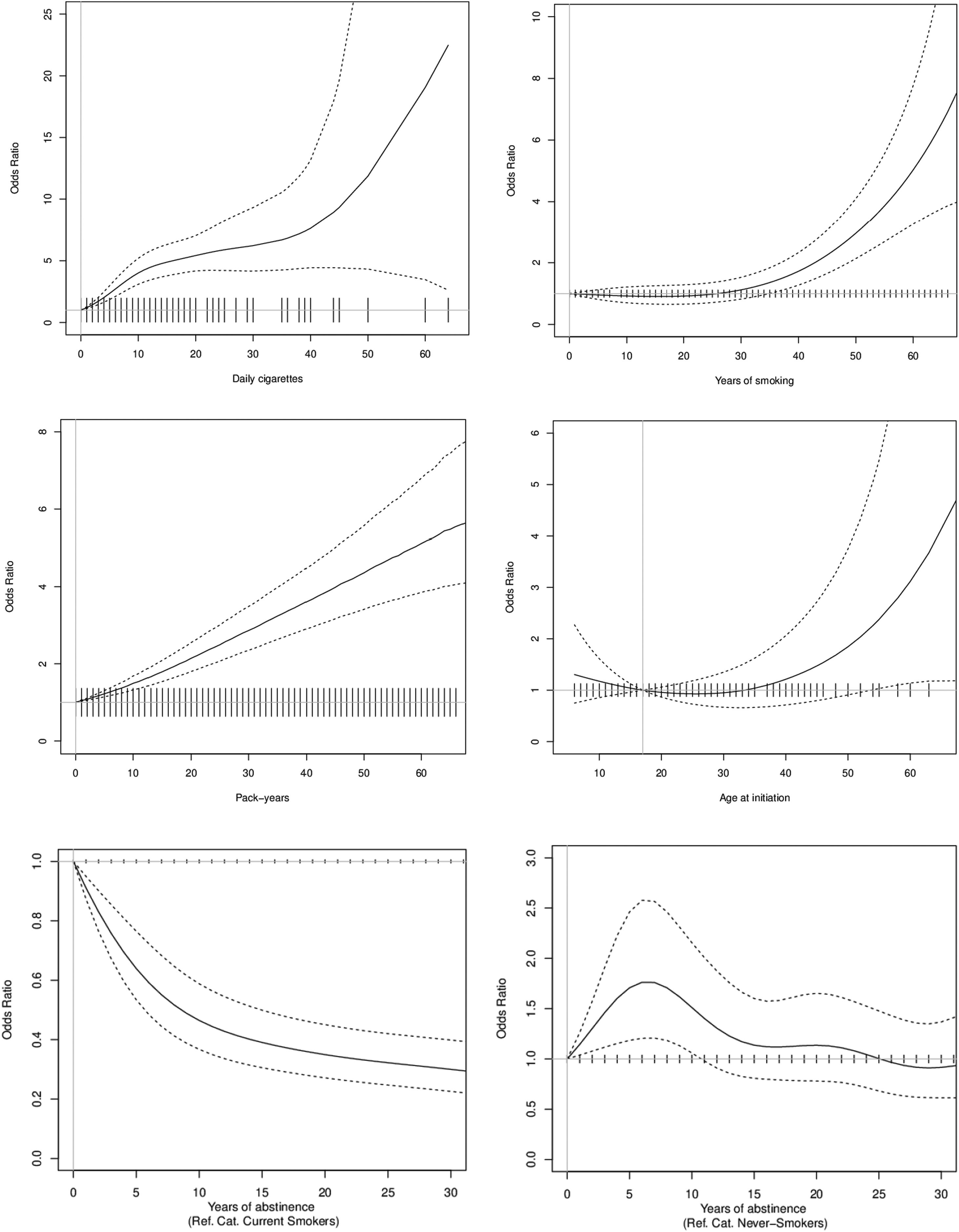
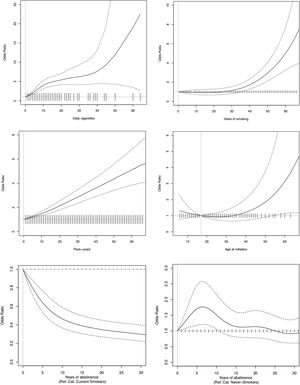
In the analysis of current smokers versus never smokers, for smoking duration, the COPD risk began to increase at around 30 years of smoking duration. In terms of smoking intensity, the COPD risk increased rapidly from the first cigarette and then stabilized slightly upwards between 10 and 40cig/day. Regarding lifetime tobacco consumption, the COPD risk increased almost linearly up to 60 pack-years. In the nonparametric model for age of onset, a slight decreasing trend was observed that was not significant. Regarding years of abstinence, there was a clear reduction on COPD risk compared to current smokers. When comparing with never smokers, COPD risk seems to equal that of never smoker around 25 years since quitting (Fig. 1).
Dose–response relationships between smoking and Chronic Obstructive Pulmonary Disease as related to smoking duration (years of smoking), smoking intensity (daily cigarettes), lifetime tobacco consumption (pack-years), age at smoking initiation, and years of abstinence. Note: The reference category for smoking duration is 0 years, for smoking intensity is 0cig/day, for lifetime tobacco consumption is 0 pack-years, for age of onset is 17 years, and for years of abstinence is never smokers and current smokers.
To our knowledge, this is the first study to analyze the relationship between COPD and five tobacco consumption characteristics using categorical and nonparametric models. The COPD risk increases with smoking duration, smoking intensity and lifetime tobacco consumption. Age at smoking initiation shows no effect. It seems that smoking intensity presents a higher COPD risk than the smoking duration. COPD risk decreases with years since quitting and seems to equal to that of a never smoker after 15–25 years of abstinence. Perhaps this is the most relevant result, there is an immediate benefit for quitting during the first 1–5 years of abstinence. Nonparametric models show a latency period for COPD of about 30 years from the onset of smoking and display similar results to the conventional models.
Smoking duration and intensity are the strongest predictors of the COPD risk. COPD risk increases linearly with smoking duration, though an induction period is needed. This may be because longer smoking duration is associated with several genetic changes19 produced by the accumulation of specific polycyclic aromatic hydrocarbons, aromatic amines and nitrosamines from tobacco smoke.20 Long periods of tobacco exposure can lead to alterations in the pulmonary microbiome and reduced microbial diversity that contribute to COPD progression.21,22 The results of this study show that the COPD risk increases with smoking intensity. Previous studies that assessed the lung cancer risk also observed an increase as the smoking intensity increased.7,9,23
It is important to discuss in-depth the lack of effect observed for smoking duration between 1 and 20 years of smoking duration. While this could be due to the need of an induction period where tobacco chemicals may produce a sustained inflammatory effect, we cannot disregard that these results may just be due to chance, because of the low number of participants having smoked less than 20 years. For smokers, it is uncommon to have smoking periods shorter than 20 years and, in this study, only 8 participants with COPD and 55 without COPD (all current smokers at the time of the interview) were included.
Stratified analysis between smoking duration and intensity suggests that intensity has a greater influence on the COPD risk. However, this result should be taken with caution because of the low sample size in some of the established categories. Previous studies have observed that smoking duration has the greatest influence on COPD risk and this also occurs in other diseases such as lung cancer8–10,14,15 and cardiovascular disease.24 Thus, people who smoke with a lower intensity for a longer period of time have a higher risk of lung cancer than those who smoke with a higher intensity in a shorter period.8 In relation to COPD, this aspect has not been as extensively studied as in lung cancer. Even so, previous studies point to smoking duration having a major impact on the development of COPD.14,15
Some authors point out that collecting smoking duration is a much more accurate measure than smoking intensity.9 Using daily cigarettes to assess smoking intensity may be an inaccurate measure as a smoker is unlikely to smoke the same number of cigarettes per day throughout his/her life. Therefore, smoking intensity does not take into account possible variations in tobacco consumption over different time periods.9–11 In addition, some studies indicate that measures of cig/day correlate poorly with chemical assessments of tobacco exposure.14,25 This lack of precision in smoking intensity could eventually influence the estimated risks for different lung diseases.
The use of pack-years to assess the risk of developing COPD or some other lung diseases has been criticized for being a measure based on the assumption that both smoking intensity and duration are of equal importance.11,16,26 However, some authors argue that it should continue to be used because of its simplicity and because it is a measure that provides information on the cumulative amount of tobacco consumption.9,16,27,28 In this study, COPD risk increases linearly with the increase in pack/years, an aspect that also occurs in the nonparametric model. These results are in agreement with a previous study that has analyzed this aspect in relation to lung cancer risk.7 Some authors suggest that the increase in smoking intensity is more harmful for light smokers than for heavy smokers9 and that many light smokers decrease their daily consumption because they believe it is safer, when this is not the case.16
Although the pack-years variable takes into account the intensity and duration of consumption together, it is important to assess the impact of these three variables independently. This is because by equalizing the impact of intensity and duration in the same variable (pack-years), the differences between these independently assessed variables can be compensated for.11 This can be observed in this study in the results obtained in the nonparametric models. Thus, when the duration of consumption is analyzed, a lack of effect is observed during the first years, while in the case of intensity, an effect is observed from practically the first daily cigarette consumed. These differences between the two variables translate into a linear increase in the COPD risk with pack-years from practically the beginning, so that the lack of effect observed in duration is compensated by the effect observed in intensity. This aspect was also observed in the study by Leffondré et al.11 in which it was indicated that, in current smokers, the use of pack-years underestimates the impact of intensity and overestimates that of duration. The different analysis of duration and cig/day provide also different biological hypotheses on COPD risk. A longer duration of smoking even at low cig/day may pose a sustained inflammatory activity finally causing COPD.
Age is one of the risk factors related to the onset of COPD and its interaction with tobacco consumption may be determinant for this disease.14,17,29 In our study, taking as a reference the onset of smoking before the age of 15 years and adjusting for cig/day, the risk of COPD is lower in those individuals who start smoking after the age of 15 years. However, this aspect is not so evident in the nonparametric model. Safiti et al.17 also point to the existence of an increased COPD risk for smoking initiating before the age of 15 years. They also indicate that starting smoking before the age of 20 years causes the lungs to not develop properly in terms of capacity and function. This has also been observed in studies of lung cancer. Ruano-Ravina et al.7 note that the onset of smoking after the age of 25 years decreases the lung cancer risk compared to an earlier onset. This may be due to the fact that young smokers may be more susceptible to DNA adduct formation, and therefore accumulate more damaged DNA compared to subjects who start smoking later.20
Smoking cessation reduces the COPD risk. In this study, it was observed that after 15–25 years of abstinence the risk of COPD could be equal to that of a never smoker. In comparison with smokers, the decrease in COPD risk is still high during the first years of smoking cessation but always lower than the risk of a current smoker. This result coincides with previous studies of lung cancer.7,12 In one study, it was observed that the shorter the smoking cessation time, the greater the decrease in FEV1 compared to a never smoker. However, in smokers who had not smoked for more than 20 years, the decrease in FEV1 was equal to that of a never smoker.30 In addition, there are many studies that mention the beneficial health effects of smoking cessation in lung diseases15,31 or in lung function.32,33 Thus, in the Lung Health Study, it was observed that people who had not smoked for one year had an average increase in FEV1 of 47ml.32
This study has some limitations. The possibility of recall bias may be present in the collection of some variables such as the smoking intensity (cig/day) or the age of smoking onset. The data from this study are based on the EPISCAN-II study in which the diagnosis of COPD was made exclusively by spirometry, without taking into account other aspects such as exposure to risk factors or the presence of respiratory symptoms.18 Being a cross-sectional design also means that the number of participants with COPD and without COPD are not well balanced and this can affect the precision of estimations. The last disadvantage may be related with the fact of diagnosing people from general population with COPD. COPD is usually diagnosed following symptoms and many of the participants with COPD included did not have any symptoms, but they were underdiagnosed. We cannot exclude the possibility that the fact of presenting milder disease might mean that tobacco consumption is less intense compared to other COPD populations diagnosed following symptoms. Lastly, neither CO levels in expired air nor cotinine levels in blood were assessed in this study, which could have provided more clarity in the results obtained, particularly when defining a participant as a current smoker.
This study also has some advantages. It has a large sample size and it is representative of the Spanish population aged 40 years and older. The COPD diagnosis has been made using gold standard procedures and the possibility of misdiagnosing the disease is very low. A further advantage is that we are including only incident cases of COPD, since prevalent cases were disregarded to avoid any changes of tobacco consumption characteristics following COPD diagnosis.
In conclusion, the COPD risk increases with duration, intensity and lifetime tobacco consumption and decreases with years of abstinence. It seems to be confirmed that there is an induction period of about 30 years for the onset of COPD after smoking initiation. The COPD risk increases rapidly after the first cigarette is smoked. In smokers, smoking cessation continues to be one of the most important aspects in reducing the COPD risk incidence, since after 15–25 years of abstinence from smoking it seems that the risk could be equal to that of a never smoker.
FundingThe EPISCAN II study was funded and sponsored by GlaxoSmithKline, Spain through an unrestricted grant. The EPISCAN II study is registered in ClinicalTrial.gov under registry number: NCT01122758. Dr. Ahluwalia funded in part by P20GM130414, a NIH funded Center of Biomedical Research Excellence (COBRE). The sponsors were not involved in study design, data collection, analysis and interpretation, or original report writing.
Conflict of interestB.G. Cosío has received speaker or consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Sanofi and TEVA, and research grants from Menarini, AstraZeneca and Boehringer Ingelheim. C. Casanova has received speaker or consulting fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini and Novartis, and research grants from GlaxoSmithKline, Menarini and AstraZeneca. J.J. Soler-Cataluña has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GlaxoSmithKline, Menarini, Novartis and Teva, and consulting fees from AstraZeneca, Bial, Boehringer Ingelheim, GlaxoSmithKline, Ferrer and Novartis. F. García-Río has received speaker or consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Pfizer and Rovi, and research grants from Chiesi, Esteve, Gebro Pharma, GlaxoSmithKline, Menarini and TEVA. J. Ancochea has received speaker or consulting fees from Actelion, Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Carburos Médica, Chiesi, Faes Farma, Ferrer, GlaxoSmithKline, InterMune, Linde Healthcare, Menarini, MSD, Mundipharma, Novartis, Pfizer, Roche, Rovi, Sandoz, Takeda and Teva. M. Miravitlles has received speaker or consulting fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Laboratorios Esteve, Gebro Pharma, Kamada, GlaxoSmithKline, Grifols, Menarini, Mereo Biopharma, Novartis, pH Pharma, Palobiofarma SL, Rovi, TEVA, Spin Therapeutics, Verona Pharma and Zambon, and research grants from Grifols. Dr. Ahluwalia serves as a consultant and has equity in Qnovia, a start-up company developing a prescription nicotine replacement product for FDA approval. The rest of the authors declare no conflicts of interests.
The collaboration of Monica Sarmiento and Neus Canal of IQVIA, and Carolina Peña and José Julio Jiménez from GSK is explicitly acknowledged. Principal Investigators, collaborators and participating centres: MADRID: H. La Princesa, Julio Ancochea Bermudez (IP)/Elena García Castillo/Claudia Valenzuela/Joan B Soriano. CASTILLA LEÓN: H. U. de Burgos, Ana Pueyo Bastida (IP)/Lourdes Lázaro Asegurado/Luis Rodríguez Pascual/Mª José Mora. ARAGÓN: H. Gral. San Jorge, Luis Borderias Clau (IP)/Lourdes Arizón Mendoza/Sandra García. EXTREMADURA: H. San Pedro de Alcántara, Juan Antonio Riesco Miranda (IP)/Julián Grande Gutiérrez/Jesús Agustín Manzano/Manuel Agustín Sojo González. CASTILLA LEÓN: H. Clínico U. de Salamanca, Miguel Barrueco Ferrero (IP)/Milagros Rosales. GALICIA: H. Álvaro Cunqueiro, José Alberto Fernández Villar (IP)/Cristina Represas/Ana Priegue/Isabel Portela Ferreño/Cecilia Mouronte Roibás/Sara Fernández García. I. BALEARES: H. Son Espases, Borja G Cosío (IP)/Rocío Cordova Díaz/Nuria Toledo Pons/Margalida Llabrés. ARAGÓN: H. U. Miguel Servet, José María Marín Trigo (IP)/Marta Forner/Begoña Gallego/Pablo Cubero/Elisabet Vera. C. VALENCIANA: H. Arnau de Vilanova (Valencia), Juan José Soler-Cataluña (IP)/Mª Begoña Picurelli Albero/Noelia González García. ANDALUCÍA: H. Virgen de la Macarena, Agustín Valido Morales (IP)/Carolina Panadero/Cristina Benito Bernáldez/Laura Martín-Bejarano y Maria Velar. MURCIA: H. Gral. U. Santa Lucía (Cartagena), Antonio Santa Cruz Siminiani (IP)/Carlos Castillo Quintanilla/Rocío Ibáñez Meléndez/José Javier Martínez Garcerán/Desirée Lozano Vicente/Pedro García Torres/Maria del Mar Valdivia. NAVARRA: Clínica Universidad de Navarra, Juan Pablo de Torres Tajes (IP)/Montserrat Cizur Girones/Carmen Labiano Turrillas. LA RIOJA: H. de San Pedro (Logroño), Carlos Ruiz Martínez (IP)/Elena Hernando/Elvira Alfaro/José Manuel García/Jorge Lázaro. PAÍS VASCO: H. Santiago Apóstol (H. Txagorritxu), David Bravo (IP)/Laura Hidalgo/Silvia Francisco Terreros/Iñaki Zorrilla/Ainara Alonso Colmenero. ASTURIAS: H. Central de Asturias, Cristina Martínez González (IP)/Susana Margon/Rosirys Guzman Taveras/Ramón Fernández/Alicia Álvarez. CANTABRIA: H. de Valdecilla, José Ramón Agüero Balbín (IP)/Juan Agüero Calvo. CATALUÑA: H. U. Vall d’Hebron, Jaume Ferrer Sancho (IP)/Esther Rodríguez González/Eduardo Loeb. CASTILLA LA MANCHA: H. U. de Guadalajara, José Luis Izquierdo Alonso (IP)/Mª Antonia Rodríguez García. I. CANARIAS: H. U. de Tenerife, Juan Abreu González (IP)/Candelaria Martín García/Rebeca Muñoz/Haydée Martín García. ASTURIAS: H. U. de San Agustín (Avilés), Miguel Angel Martínez Muñiz (IP)/Andrés Avelino Sánchez Antuña/Jesús Allende González/Jose Antonio Gullón Blanco/Fernando José Alvarez Navascues/Manuel Angel Villanueva Montes/María Rodríguez Pericacho/Concepción Rodríguez García/Juan Diego Alvarez Mavárez.