In the light of relationships reported between hypoxemia (tissue hypoxia) and cancer, Abrams et al. concluded in 2008 that sleep apnea-hypopnea syndrome (SAHS) and its main consequence, intermittent hypoxia, could be related with increased susceptibility to cancer or poorer prognosis of a pre-existing tumor. This pathophysiological association was confirmed in animal studies. Two large independent historical cohort studies subsequently found that the degree of nocturnal hypoxia in patients with SAHS was associated with higher cancer incidence and mortality. This finding has been confirmed in almost all subsequent studies, although the retrospective nature of some requires that they be considered as hypothesis-generating only. The relationship between sleep apnea and cancer, and the pathophysiological mechanisms governing it, could be clarified in the near future in a currently on-going study in a large group of melanoma patients.
En 2008, Abrams et al. publicaron que, habida cuenta de las anteriores relaciones encontradas entre la hipoxemia (hipoxia tisular) y el cáncer, el síndrome de apneas e hipopneas del sueño (SAHS) y su principal consecuencia, la hipoxia intermitente, podrían relacionarse con una mayor propensión a padecer cáncer o a un peor pronóstico de un tumor preexistente. Con esta base fisiopatológica y tras algunos estudios en animales que confirmaron esta asociación, 2 grupos independientes de investigación observaron en sendos estudios clínicos amplios de cohortes históricas que el grado de hipoxia nocturna aparecida en pacientes con SAHS se asociaba a una mayor incidencia y mortalidad por cáncer. Este dato ha sido confirmado por casi todos los estudios posteriores, si bien el carácter retrospectivo de todos ellos obliga a considerarlos tan solo como trabajos generadores de hipótesis. Un estudio puesto en marcha actualmente sobre un amplio grupo de pacientes con melanoma posiblemente arroje más luz en un futuro cercano sobre la existencia o no de esta relación y de los mecanismos fisiopatológicos que la gobiernan.
The relationship between obstructive sleep apnea-hypopnea syndrome (SAHS) and cancer is set to become an exciting area of research in the near future, although at the moment the clinical basis is far from solid. Nevertheless, both physiopathological and animal studies conducted to date appear to bestow credibility on this relationship. From a clinical point of view, several studies have shown an association between SAHS (particularly when evaluated by hypoxemia) and cancer incidence and mortality, although their retrospective nature means that more in-depth studies will be required before a more definitive conclusion can be reached. Nevertheless, everything indicates that if this association is proven, it will be one of the most significant discoveries of the last 100 years, since both SAHS and cancer share common epidemiological characteristics: they are both highly prevalent, cause a significant social and healthcare burden, and are potentially treatable. The aim of this review is to present the findings reported to date on the relationship between SAHS and cancer, and the mechanisms supporting and clarifying the physiopathological hypothesis.
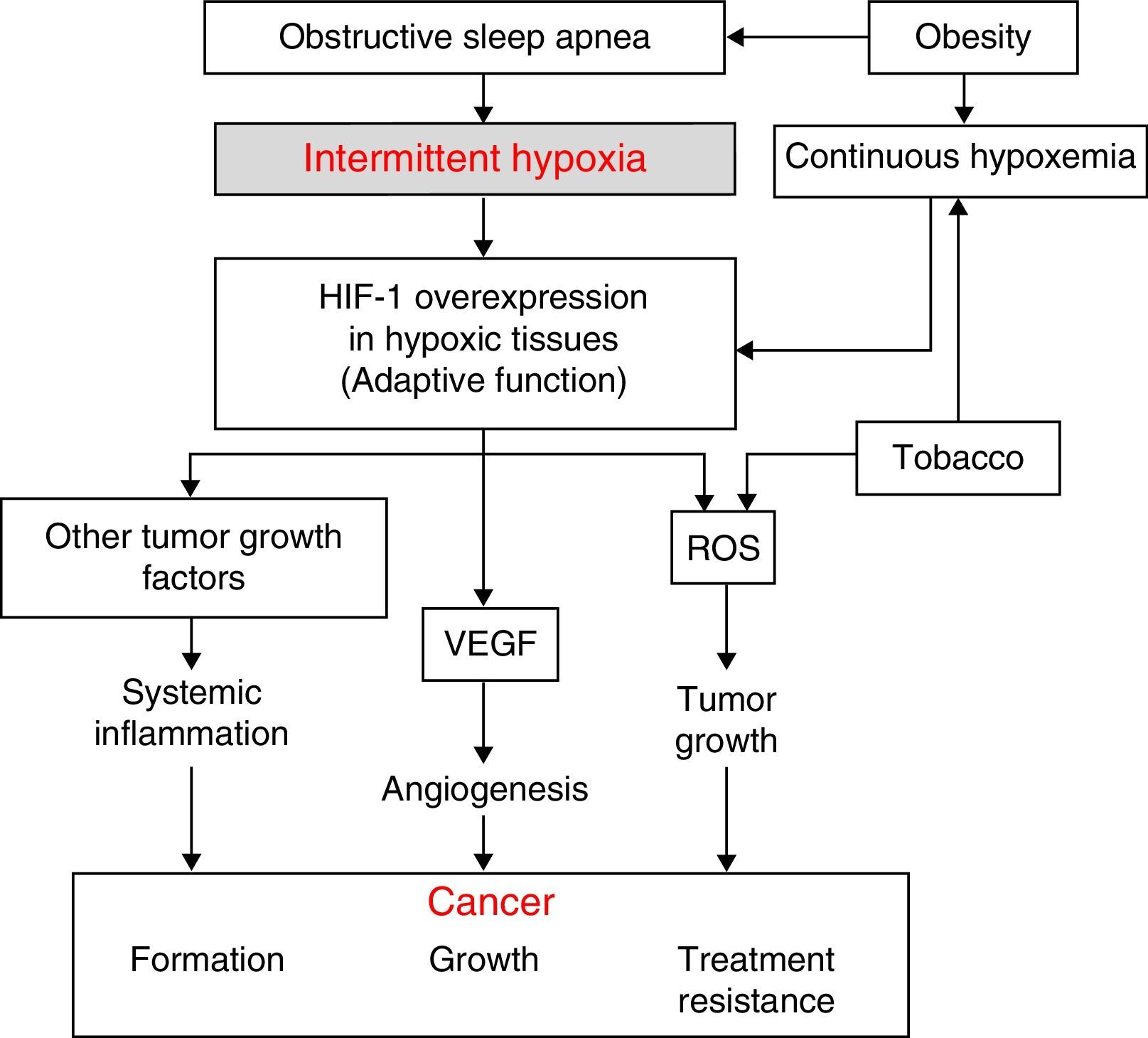
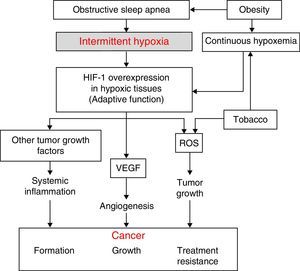
Physiopathological AspectsSeveral physiopathological mechanisms have been uncovered suggesting that a relationship between SAHS and the transformation of healthy to malignant cells or the spread and growth of tumors may be biologically plausible. The 3 most significant mechanisms are oxidative stress,1,2 a higher degree of systemic inflammation3,4 (both usually compounded in these patients by concomitant obesity), and, possibly most importantly, the presence of intermittent hypoxemia (IH)5 (and, in particular, the intermittent tissue hypoxia resulting from IH). The latter is probably the underlying cause of all these mechanisms (Fig. 1).
The desaturation-reoxygenation pattern that defines IH is typical of SAHS. By increasing production of oxygen species (ROS), it constitutes an important stimulus in the activation of the oxidative stress system.1,2 This leads to an imbalance between the production and degradation of certain oxidant/antioxidant products. This situation has been related with an increase in both acute and chronic mutagenesis, changes in cell function and structure, DNA damage, genome instability that may cause greater cell proliferation and neoplastic transformation. Oxidative stress has also been related with some transcription factors such as activator protein-1 (AP-1) and nuclear factor-kB (NF-kB) that have been associated with a greater propensity to develop cancer.6
Hypoxia-Induced FactorAll cells in the body have several mechanisms to compensate for situations of both continuous and intermittent hypoxia (such as seen in SAHS). One of the most powerful is the increased production of a key molecule, known as hypoxia-induced factor (HIF-1). HIF-1 orchestrates the regulation of genes that code for mediators that enable cells to adapt to situations of tissue hypoxia. It is composed of 2 subunits: HIF-1α and HIF-1ß. Of these, the α form plays a greater role in tissue hypoxia regulation.7 It compensates hypoxia by triggering a series of mechanisms that activate the production of angiogenic molecules. The most important of these is vascular endothelial growth factor (VEGF), which regulates the formation of new collateral blood vessels for providing more oxygen to the hypoxic area or for avoiding areas of vascular obstruction.8–14 While this is an important compensatory mechanism in cardiovascular diseases that involve hypoxic areas, it seems to have a deleterious effect in cancer patients. Tumors contain large hypoxic areas (with pO2 <10mmHg) that act as potent activators of HIF-1-mediated compensatory systems. These systems generate tumor neovascularization that provides cancer cells with an excellent mechanism for propagation and generation of distant metastases. Moreover, this neovascularization is produced in a scenario of abnormal, fragile vessels, so reoxygenation does not occur in the best of conditions.
Systemic InflammationIn SAHS, inflammation is increased both locally and systemically.15,16 The role of SAHS as a generator of systemic inflammation goes some way to explaining the possible association between SAHS and cancer. Oxidant/antioxidant imbalance and increased ROS have been related with a systemic increase in levels of proinflammatory substances such as tumor necrosis factor (TNF-α), interleukin (IL)-6, and IL-8, caused, as mentioned above, by the activation of transcription factors NF-kB and AP-1. The NF-kB factor is thought to be essential in the transcription of multiple genes associated with inflammation, arteriosclerosis, and cancer. IH appears to be the most important factor in the activation of this inflammatory element in SAHS patients.15,16
Obesity is very common among SAHS patients,17 and has been related per se with several tumor types.18–21 This means that obesity is a significant confounding factor in the analysis of the relationship between SAHS and cancer. Obesity-related inflammation, which may be described as chronic low grade inflammation generated by the fat cells themselves in response to excess calories and nutrients, appears to be the most important element in this relationship, and may be unrelated to the existence of SAHS.
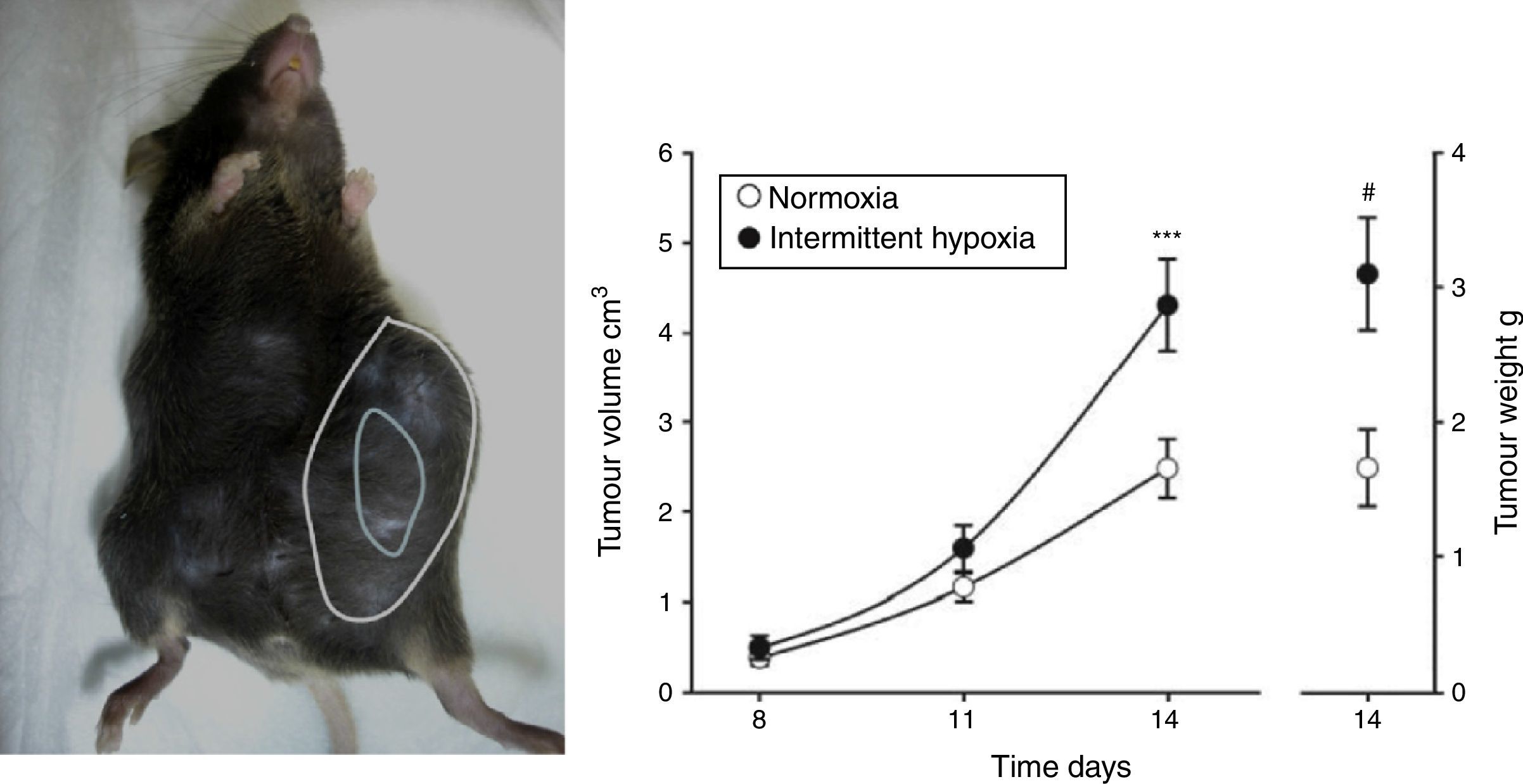
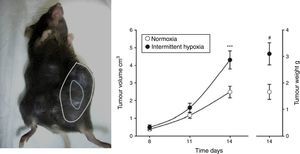
Evidence From SAHS in Animal ModelsEffects of Intermittent Hypoxia in CancerIn an initial study,22 IH mimicking the sequence occurring in SAHS was seen to double the rate of tumor growth in a melanoma model (Fig. 2). These results were confirmed in a second study,23 which addressed the question of whether obesity in SAHS could mask to a certain extent the effects of IH in tumor outcome. In the absence of IH, the authors observed greater tumor growth in obese animals than in controls, as described previously.18,24,25 However, exposure to IH in obese rats did not produce greater tumor growth than that already caused by the obesity itself. Plasma VEGF levels increased in all obese animals, whether exposed or not exposed to IH, and only in the slim animals exposed to IH. Moreover, circulating VEGF levels correlate closely with tumor size. This signaling protein, which has been proposed in various oncology studies as a possible marker for cancer prognosis,26–28 may be of relevance in SAHS, since SAHS patients have higher circulating VEGF levels.29–31
Animal model based on subcutaneous injection of 106 melanoma cells in mice (left). Tumor growth and weight at 14 days are significantly greater in mice exposed to intermittent hypoxemia (simulating SAHS) compared to the control group exposed to normoxia (right).
Reproduced from Almendros et al.22
A subsequent study was conducted to evaluate the metastatic capacity of melanoma in response to IH in two experimental metastasis models, one induced and one spontaneous.32 In both models, IH was found to induce an increase in both number and size of metastatic melanoma. Following this same line of research in a similar IH model, Eubank et al.18 found more melanoma cells in blood (intravasation) and in lung samples from animals exposed to IH. The authors suggested that the metastatic capacity observed in IH was a result of increased HIF-1α activity (regulating extracellular matrix metalloproteinases [MMP]-7) and reduced HIF-2α (MMP-9 and 12). Moreover, previous exposure of tumor cells to cyclic hypoxia similar to that occurring in SAHS appears to increase their metastatic capacity in vivo.12,13
A very recent study explored whether changes in malignancy associated with IH were modulated in part by the immune system.33 A very large amount of data is now available on the mediating role of tumor-associated macrophages (TAM); for example, a greater presence of TAMs has been associated with poorer cancer prognosis.34 Indeed, TAMs are capable of modulating tumor growth and invasive properties by regulating a series of growth factors, cytokines and proteases.35 Thus, TAMs can be “re-educated” from an anti-tumor state (classic activation) to a pro-tumor state (alternative activation) in which the synthesis of tumor-promoting molecules and angiogenesis are promoted. To demonstrate the possible role of macrophages in tumor growth observed in animal models, melanoma cells were cultivated in isolation or in co-culture with macrophages under IH conditions in vitro.33 In both cases, they were exposed to IH or normoxia, and at 48h both cell populations were quantified to evaluate melanocyte proliferation. The results showed that melanocytes in isolation did not proliferate more rapidly in IH conditions, but in the presence of macrophages they increased by 30%. IH, then, causes macrophage phenotypes to switch to a more tumor-promoting or alternative activation state. The results from this study suggest that the effects of IH seen in in vivo tumors may be regulated more by the immunological response of the host than by changes occurring directly in the tumor cells.
Effects of Fragmented Sleep on CancerAltered sleep patterns are very common and may be related with cancer growth. This relationship has been explored over the last 10 years or so, and an association has been established between malignancy of different types of cancer and insomnia or lack of sleep.36–38
Until now, very little work has been done on the effects of sleep on cancer, due to difficulties in developing experimental models that simulated the physiopathological conditions of the disease. Indeed, early studies were based on the effects of sleep deprivation in both mice and rats,39,40 finding evidence that lack of sleep acts negatively on tumorigenesis, tumor growth and mortality. Nevertheless, sleep fragmentation characteristic of obstructive sleep apnea (OSA) was not reproduced, thus limiting the interpretation of the results.
In a recent study, Hakim et al.41 managed to reproduce OSA sleep-disordered breathing in a newly developed mouse model. The authors reported that fragmented sleep doubled tumor size and increased tumor capacity for invading adjacent tissues. This study suggests that TAMs play an active role in the increased malignancy observed in response to sleep fragmentation, but the mechanisms that might be involved have not yet been described in detail. Nonetheless, there is evidence that sleep fragmentation may cause macrophages to move toward the aorta wall,42 producing metabolic changes43,44 that may serve as a basis for the mechanisms involved in increased malignancy.
Sleep Apnea and Cancer. Current Evidence in HumansIndirect DataUnfortunately, very few clinical studies are currently available on the possible association between SAHS and cancer in humans (Table 1), although some indirect data have been published. In mortality studies in SAHS patients, cancer is often the second cause of death after cardiovascular diseases.45–47 Some of these studies have even reported an association between SAHS and cancer death.48 However, a recent study assessing the merits of symptoms as SAHS markers did not find that snoring, breathing pauses or excessive daytime sleepiness were associated with a higher incidence of cancer.49
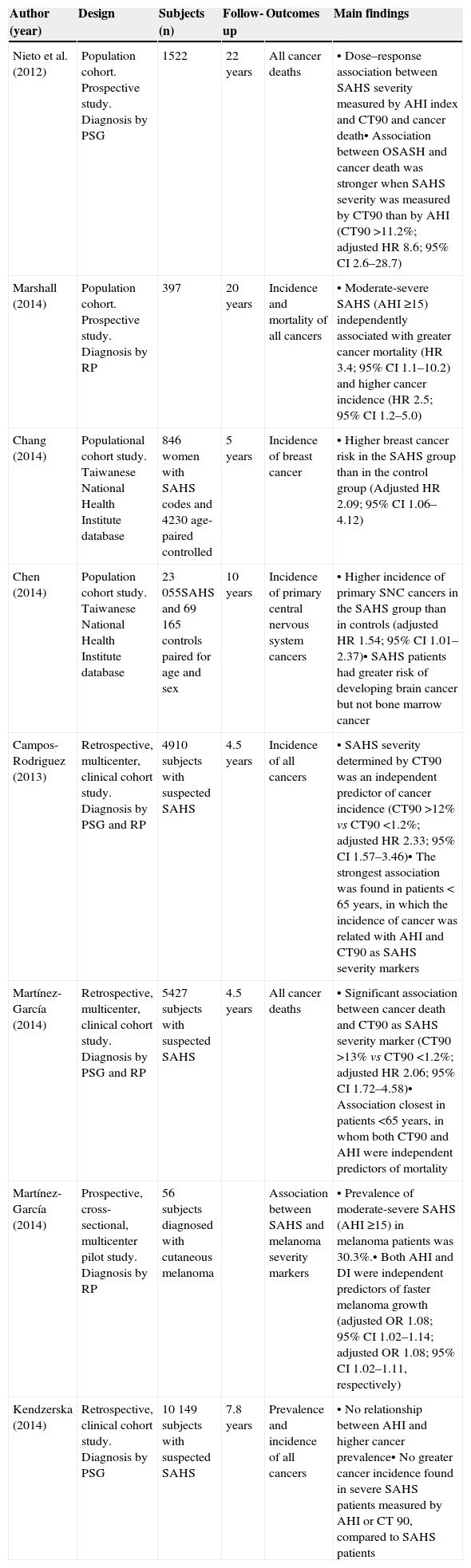
Studies Analyzing the Association Between SAHS and Cancer in Humans.
| Author (year) | Design | Subjects (n) | Follow-up | Outcomes | Main findings |
|---|---|---|---|---|---|
| Nieto et al. (2012) | Population cohort. Prospective study. Diagnosis by PSG | 1522 | 22 years | All cancer deaths | • Dose–response association between SAHS severity measured by AHI index and CT90 and cancer death• Association between OSASH and cancer death was stronger when SAHS severity was measured by CT90 than by AHI (CT90 >11.2%; adjusted HR 8.6; 95% CI 2.6–28.7) |
| Marshall (2014) | Population cohort. Prospective study. Diagnosis by RP | 397 | 20 years | Incidence and mortality of all cancers | • Moderate-severe SAHS (AHI ≥15) independently associated with greater cancer mortality (HR 3.4; 95% CI 1.1–10.2) and higher cancer incidence (HR 2.5; 95% CI 1.2–5.0) |
| Chang (2014) | Populational cohort study. Taiwanese National Health Institute database | 846 women with SAHS codes and 4230 age-paired controlled | 5 years | Incidence of breast cancer | • Higher breast cancer risk in the SAHS group than in the control group (Adjusted HR 2.09; 95% CI 1.06–4.12) |
| Chen (2014) | Population cohort study. Taiwanese National Health Institute database | 23055SAHS and 69165 controls paired for age and sex | 10 years | Incidence of primary central nervous system cancers | • Higher incidence of primary SNC cancers in the SAHS group than in controls (adjusted HR 1.54; 95% CI 1.01–2.37)• SAHS patients had greater risk of developing brain cancer but not bone marrow cancer |
| Campos-Rodriguez (2013) | Retrospective, multicenter, clinical cohort study. Diagnosis by PSG and RP | 4910 subjects with suspected SAHS | 4.5 years | Incidence of all cancers | • SAHS severity determined by CT90 was an independent predictor of cancer incidence (CT90 >12% vs CT90 <1.2%; adjusted HR 2.33; 95% CI 1.57–3.46)• The strongest association was found in patients < 65 years, in which the incidence of cancer was related with AHI and CT90 as SAHS severity markers |
| Martínez-García (2014) | Retrospective, multicenter, clinical cohort study. Diagnosis by PSG and RP | 5427 subjects with suspected SAHS | 4.5 years | All cancer deaths | • Significant association between cancer death and CT90 as SAHS severity marker (CT90 >13% vs CT90 <1.2%; adjusted HR 2.06; 95% CI 1.72–4.58)• Association closest in patients <65 years, in whom both CT90 and AHI were independent predictors of mortality |
| Martínez-García (2014) | Prospective, cross-sectional, multicenter pilot study. Diagnosis by RP | 56 subjects diagnosed with cutaneous melanoma | Association between SAHS and melanoma severity markers | • Prevalence of moderate-severe SAHS (AHI ≥15) in melanoma patients was 30.3%.• Both AHI and DI were independent predictors of faster melanoma growth (adjusted OR 1.08; 95% CI 1.02–1.14; adjusted OR 1.08; 95% CI 1.02–1.11, respectively) | |
| Kendzerska (2014) | Retrospective, clinical cohort study. Diagnosis by PSG | 10149 subjects with suspected SAHS | 7.8 years | Prevalence and incidence of all cancers | • No relationship between AHI and higher cancer prevalence• No greater cancer incidence found in severe SAHS patients measured by AHI or CT 90, compared to SAHS patients |
CT90, nocturnal period of oxygen arterial concentration <90%; AHI, apnea-hypopnea index; RP, respiratory polygraphy; PSG, conventional polysomnography; SAHS, sleep apnea-hypopnea syndrome; CNS, central nervous system.
The first population study to analyze the association between SAHS and cancer was the Wisconsin cohort, comprising 1522 subjects followed up over 22 years.50 This study reported a dose-response association between SAHS severity measured by the apnea-hypopnea index (AHI) and all cancer deaths, an association that was maintained after adjusting for several confounding factors. Severe SAHS (AHI ≥30) was even found to be an independent predictor of cancer death. The association was even stronger when an oximetric index, i.e., percentage of the night with oxygen saturation <90% (CT90), was used instead of AHI. In this study, the association was not influenced by sex or age, although it was stronger in non-obese patients.
In the Busselton cohort, consisting of 400 subjects followed up over 20 years, an association was also found between moderate-to-severe SAHS (AHI ≥15) and higher mortality (HR 3.4; 95% CI 1.1–10.2) and incidence of cancer (HR 2.5; 95% CI 1.2–5.0).51
Finally, 2 large Taiwanese studies49,50 that used data from the National Health Institute reported that SAHS patients had a greater risk of breast cancer (HR 2.09; 95% CI 1.06–4.12)36 and primary central nervous system cancer (HR 1.54; 95% CI 1.01–2.37)52 compared to non-SAHS control groups. However, both studies had significant limitations, including lack of data on SAHS severity or adjustment for several basic confounding factors.
Clinical StudiesIn 2013, the Spanish Sleep Group (GES) published the first clinical study analyzing the association between SAHS and cancer. This was a retrospective, multicenter study with a mean follow-up of 4.5 years. In the first analysis, the association between SAHS severity and the incidence of any type of cancer was explored in 4910 patients with no previous history of cancer.53 After adjusting for several basic factors, a significant association was found between the incidence of cancer and SAHS severity when measured by CT90, but not by AHI. The strongest association was found for patients younger than 65 years: in this group, cancer incidence was related with both AHI and CT90 determinations. When mortality was analyzed in this series, the results were very similar to the incidence results, revealing a significant association between cancer mortality and CT90 as a marker for SAHS severity, particularly in men and in subjects <65 years of age.54 In a subanalysis including only patients with cancer (n=527), CT90 was found to be an independent predictive factor for mortality.
These results, however, were not reproduced in a very recent study that analyzed 10149 patients with suspected SAHS between 1994 and 2010 in Toronto, with a follow-up of 7.8 years.55 After adjusting for several factors, no greater incidence of cancer was found in patients with severe SAHS measured by AHI or CT90, compared to patients without SAHS (AHI >30 vs <5; adjusted HR 1.02; 95% 0.80–1.31; CT90: adjusted HR 1.00; 95% CI 0.99–1.02). These results were not affected when the authors used the same cut-off points for AHI and CT90 as used in the GES study. When the series was broken down into subgroups, some smoking-related tumors were seen to be associated with some SAHS severity parameters.
While an association between SAHS severity (especially when measured by oximetric markers) and cancer incidence and mortality has been found in 5 of the 6 available studies,36,50–54 their limitations must prompt researchers to approach their conclusions with caution and to continue investigating this association. None of the published series was originally designed to investigate the association between SAHS and cancer; most of them did not study a certain tumor type, histology or site (different cells may be more or less sensitive to the effects of hypoxia); no valid marker for intermittent hypoxemia was used50,51,53–55; and finally, due to the low number of events, some associations in certain subgroups, such as non-obese subjects, those <65 years or male sex, have very little statistical value.
Ongoing StudiesOnly 1 prospective study performed under the auspices of the GES, analyzing the association between SAHS and a specific tumor type, cutaneous melanoma, has been published. The aim of this study was to examine if SAHS severity measured by different markers is associated with more aggressive tumor disease. A pilot study was initially conducted in 56 patients with a diagnosis of melanoma who underwent respiratory polygraph.56 Prevalence of SAHS in this series was high; 60.7% of the patients had AHI ≥5 and 14.3% had AHI ≥30. Moreover, SAHS severity measured by AHI and desaturation index (DI) were correlated with greater tumor skin depth, and increased tumor growth rate. After adjusting for confounding factors, both AHI and DI were found to be independent predictors of faster melanoma growth rate. These findings led to the implementation of a larger multicenter study designed to confirm these preliminary results in the near future.
ConclusionsDespite the growing interest in the possible association between SAHS and cancer, the small amount of data available to date and the inherent limitations in study design require that the information is considered as hypothesis-generating only. More questions have been raised than have been answered, and new, better designed studies are needed to clarify this possible relationship (Table 2).57–59
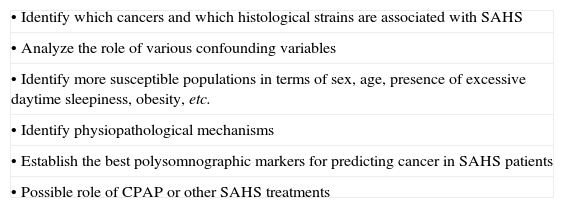
Future Research Goals for Associating of SAHS With Cancer.
| • Identify which cancers and which histological strains are associated with SAHS |
| • Analyze the role of various confounding variables |
| • Identify more susceptible populations in terms of sex, age, presence of excessive daytime sleepiness, obesity, etc. |
| • Identify physiopathological mechanisms |
| • Establish the best polysomnographic markers for predicting cancer in SAHS patients |
| • Possible role of CPAP or other SAHS treatments |
Healthcare Research Fund (FIS) PI12/01363, SEPAR Grant 058/2011 and the Beatriu de Pinos Program of the Government of Catalonia (2010 BP_00238).
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Martínez-García MÁ, Campos-Rodríguez F, Almendros I, Farré R. Relación entre apnea del sueño y cáncer. Arch Bronconeumol. 2015;51:456–461.