Severe asthma (SA) prevalence ranges from 3.9% to 9.7% in Spain.1,2 As asthma is still an incurable disease, the goal of treatment is control. However, control remains poor in most patients,3 especially those with SA,4,5 and is not achieved even by a significant percentage of patients with SA treated with biologics.6 Since poor control could be due to the wide biological heterogeneity of asthma, a therapeutic strategy based on personalized therapeutic goals and treatable traits in each patient has been proposed.7,8 Taking another approach, a group of asthma experts has put forward “clinical remission” as a feasible therapeutic goal, characterized by a high level of disease control, including the absence of symptoms and exacerbations, and normalization or optimization of lung function with or without ongoing treatment.9
The aims of this study are to identify therapeutic goals (TG) and treatable traits (TT) in a cohort of SA patients, to assess the extent to which these objectives have been met in the long term, and to evaluate whether treatable traits have been effectively treated. TGs evaluated were: maintenance oral corticosteroid (OCS) use, asthma exacerbations (AE), asthma control (asthma control test: ACT) and lung function (FEV1). TT evaluated were: blood eosinophils, adherence to inhaled treatment, and fractional exhaled nitric oxide (FeNO).
Secondary objectives were to evaluate whether any of the analyzed variables were associated with “clinical remission” (composite of no exacerbations, no bronchial obstruction, no use of OCS and ACT≥20)10 and to assess whether historical values of blood eosinophils are better predictors of AE and asthma control than current values in patients with SA.
This was a prospective longitudinal cohort observational study. The cohort comprises 179 SA patients5 who were recruited in 23 asthma units from October 1st, 2017 to April 30th, 2018 (V1) and followed up (all patients) until June 30th, 2021 (V2). At the initial visit (V1), informed consent, sociodemographic data, clinical characteristics, ACT, Test of Adherence to Inhalers (TAI), AE during the previous 12 months, OCS burden during the previous 12 months, spirometry, FeNO, skin prick test, IgE, and blood eosinophils (the three latest determinations in stable condition before V1 and current value at V1) were collected. At V2, informed consent, changes in medication, ACT, TAI, AE during the preceding 12 months, OCS burden, spirometry, bronchodilator test and blood eosinophils were collected.
A total of 179 patients participated in V1, but only 128 patients completed V2. These dropouts were due to patients’ unwillingness to continue participating in the study during the coronavirus pandemic. We found no demographic, clinical or treatment differences between patients lost to follow-up and those who continued.
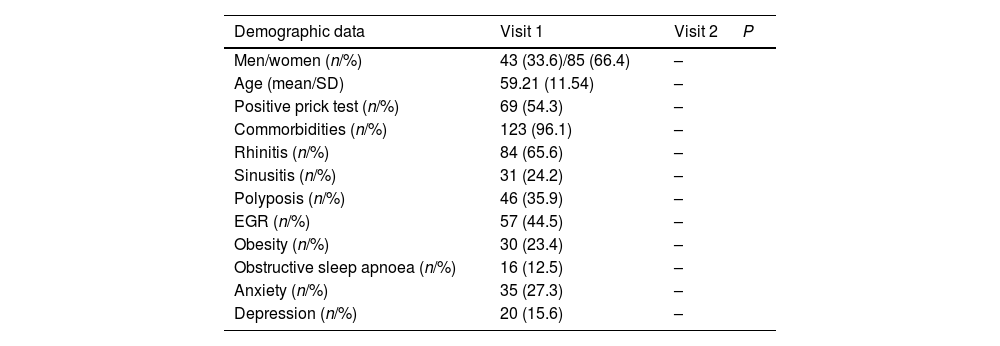
Patients had a mean age (SD) of 59.2 (11.5) years and were predominantly female (85, 66.4%). Sixty-nine (54.3%) had a positive prick test and 123 (96.1%) had at least one asthma-related comorbidity. Changes in asthma control, adherence to treatment, AE, OCS, pulmonary function tests, blood eosinophils and treatment between V1 and V2 are shown in Table 1. Remarkably, all patients were receiving high-dose inhaled corticosteroids at V1, and 47 (36.7%) and 64 (50%) were receiving a biologic at V1 and V2 respectively. Fifteen patients switched the biologic during follow-up.
Demographic data and outcome over 5 years of follow-up.
| Demographic data | Visit 1 | Visit 2 | P |
|---|---|---|---|
| Men/women (n/%) | 43 (33.6)/85 (66.4) | – | |
| Age (mean/SD) | 59.21 (11.54) | – | |
| Positive prick test (n/%) | 69 (54.3) | – | |
| Commorbidities (n/%) | 123 (96.1) | – | |
| Rhinitis (n/%) | 84 (65.6) | – | |
| Sinusitis (n/%) | 31 (24.2) | – | |
| Polyposis (n/%) | 46 (35.9) | – | |
| EGR (n/%) | 57 (44.5) | – | |
| Obesity (n/%) | 30 (23.4) | – | |
| Obstructive sleep apnoea (n/%) | 16 (12.5) | – | |
| Anxiety (n/%) | 35 (27.3) | – | |
| Depression (n/%) | 20 (15.6) | – |
| Outcomes | Visit 1 | Visit 2 | |
|---|---|---|---|
| Asthma control | |||
| Suboptimal control (n/%) | 90 (70.3) | 46 (35.9%) | 0.002 |
| Controlled (n/%) | 38 (29.7) | 82 (64.1) | |
| ACT (mean/SD) | 16.20 (5.8) | 19.75 (5.2) | <0.001 |
| TAI (mean/SD) | 48.79 (3.98) | 51.05 (9.54) | 0.014 |
| AE (mean/SD) | 2.55 (2.3) | 0.88 (1.8) | <0.001 |
| Moderate AE (mean/SD) | 1.54 (1.72) | 0.57 (1.41) | <0.001 |
| Severe AE (mean/SD) | 1.01 (1.43) | 0.30 (0.98) | <0.001 |
| FEV1 (mL) (mean/SD) | 1959.56 (706.9) | 2076.28 (720.6) | 0.008 |
| FEV1 (%) (mean/SD) | 73.0 (22.1) | 78.3 (20.2) | 0.001 |
| FEV1/FVC (mean/SD) | 64.95 (12.6) | 75.7 (18.7) | <0.001 |
| FeNO (ppb) | 43.9 (45.1) | 33.0 (28.6) | 0.009 |
| Blood eosinophils (n/mL) (mean/SD) | 338.87 (297.5) | 221.35 (264.2) | 0.001 |
| Treatment | |||
| ICS+LABA+LAMA (n/%) | 51 (39.8) | 90 (70.3) | 0.102 |
| OCS maintenance treatment (n/%) | 39 (30.7) | 16 (12.6) | 0.018 |
| Monoclonal antibodies (n/%) | 47 (36.7) | 64 (50) | <0.001 |
| Omalizumab | 31 (24.2) | 19 (14.8) | <0.001 |
| Mepolizumab | 15 (11.7) | 22 (17.2) | <0.001 |
| Reslizumab | 1 (0.8) | 7 (5.5) | 0.809 |
| Benralizumab | 0 (0) | 16 (12.5) |
%: percentage. ACT: asthma control test. AE: asthma exacerbations. FEV1: forced expiratory volume in one second. ICS: inhaled corticosteroids. LABA: long-acting beta agonist. LAMA: long-acting muscarinic antagonist. N: number of patients. OCS: oral corticosteroids. SD: standard deviation. TAI: test of Adherence to Inhalers. V1: initial visit. V2: final study visit.
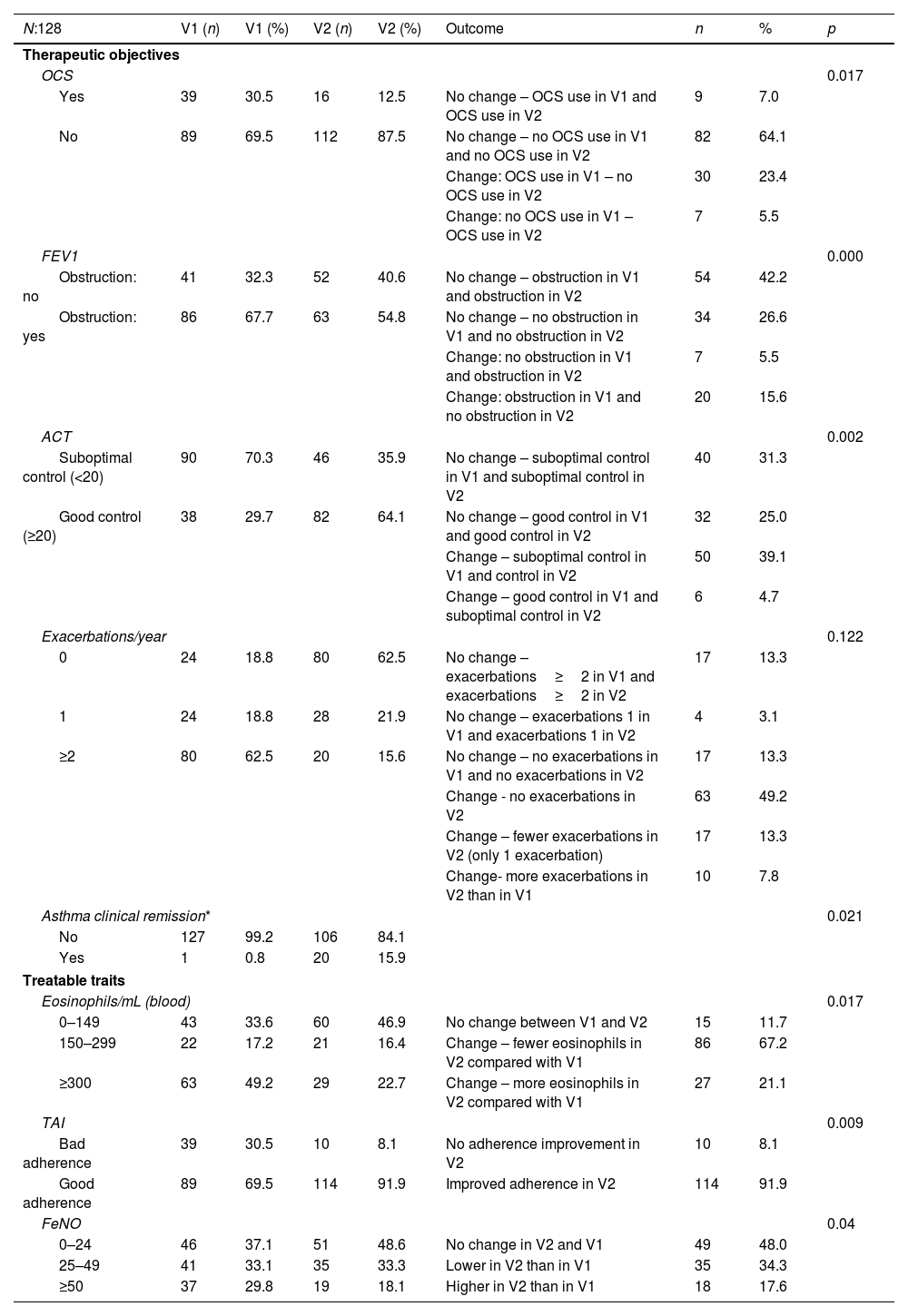
All TG (OCS, AE, FEV1 and ACT) significantly improved from V1 to V2 (Table 2). In V1 only 1 patient (0.8%) achieved “clinical remission” vs. 20 patients (15.9%) in V2. Mean AE significantly decreased from the 12 months prior to V1 to the 12 months prior to V2: 2.55 (SD 2.3) vs 0.88 (SD 1.8); p<0.001. The rate of patients receiving OCS significantly decreased from the 12 months prior to V1 to the 12 months prior to V2 (30.7% vs 12.6%; p=0.01). Mean ACT scores significantly increased from V1 to V2: 16.2 (SD 5.8) vs 19.7 (SD 5.2); p<0.001. Mean FEV1 significantly improved from V1 to V2: 1959mL (SD 706) vs 2076mL (SD 720); p<0.001. This is of note, since patients with SA would be expected to lose lung function within 5 years. All TT recorded in the study also improved. There was a significant reduction in blood eosinophil counts (but just in biologic-treated patients), FeNO levels and rate of non-adherence to inhalers (both in non-biologic and biologic-treated patients). A detailed description is provided in Table 2.
Therapeutic objectives and treatable traits achieved at initial visit (V1) and final visit (V2).
| N:128 | V1 (n) | V1 (%) | V2 (n) | V2 (%) | Outcome | n | % | p |
|---|---|---|---|---|---|---|---|---|
| Therapeutic objectives | ||||||||
| OCS | 0.017 | |||||||
| Yes | 39 | 30.5 | 16 | 12.5 | No change – OCS use in V1 and OCS use in V2 | 9 | 7.0 | |
| No | 89 | 69.5 | 112 | 87.5 | No change – no OCS use in V1 and no OCS use in V2 | 82 | 64.1 | |
| Change: OCS use in V1 – no OCS use in V2 | 30 | 23.4 | ||||||
| Change: no OCS use in V1 – OCS use in V2 | 7 | 5.5 | ||||||
| FEV1 | 0.000 | |||||||
| Obstruction: no | 41 | 32.3 | 52 | 40.6 | No change – obstruction in V1 and obstruction in V2 | 54 | 42.2 | |
| Obstruction: yes | 86 | 67.7 | 63 | 54.8 | No change – no obstruction in V1 and no obstruction in V2 | 34 | 26.6 | |
| Change: no obstruction in V1 and obstruction in V2 | 7 | 5.5 | ||||||
| Change: obstruction in V1 and no obstruction in V2 | 20 | 15.6 | ||||||
| ACT | 0.002 | |||||||
| Suboptimal control (<20) | 90 | 70.3 | 46 | 35.9 | No change – suboptimal control in V1 and suboptimal control in V2 | 40 | 31.3 | |
| Good control (≥20) | 38 | 29.7 | 82 | 64.1 | No change – good control in V1 and good control in V2 | 32 | 25.0 | |
| Change – suboptimal control in V1 and control in V2 | 50 | 39.1 | ||||||
| Change – good control in V1 and suboptimal control in V2 | 6 | 4.7 | ||||||
| Exacerbations/year | 0.122 | |||||||
| 0 | 24 | 18.8 | 80 | 62.5 | No change – exacerbations≥2 in V1 and exacerbations≥2 in V2 | 17 | 13.3 | |
| 1 | 24 | 18.8 | 28 | 21.9 | No change – exacerbations 1 in V1 and exacerbations 1 in V2 | 4 | 3.1 | |
| ≥2 | 80 | 62.5 | 20 | 15.6 | No change – no exacerbations in V1 and no exacerbations in V2 | 17 | 13.3 | |
| Change - no exacerbations in V2 | 63 | 49.2 | ||||||
| Change – fewer exacerbations in V2 (only 1 exacerbation) | 17 | 13.3 | ||||||
| Change- more exacerbations in V2 than in V1 | 10 | 7.8 | ||||||
| Asthma clinical remission* | 0.021 | |||||||
| No | 127 | 99.2 | 106 | 84.1 | ||||
| Yes | 1 | 0.8 | 20 | 15.9 | ||||
| Treatable traits | ||||||||
| Eosinophils/mL (blood) | 0.017 | |||||||
| 0–149 | 43 | 33.6 | 60 | 46.9 | No change between V1 and V2 | 15 | 11.7 | |
| 150–299 | 22 | 17.2 | 21 | 16.4 | Change – fewer eosinophils in V2 compared with V1 | 86 | 67.2 | |
| ≥300 | 63 | 49.2 | 29 | 22.7 | Change – more eosinophils in V2 compared with V1 | 27 | 21.1 | |
| TAI | 0.009 | |||||||
| Bad adherence | 39 | 30.5 | 10 | 8.1 | No adherence improvement in V2 | 10 | 8.1 | |
| Good adherence | 89 | 69.5 | 114 | 91.9 | Improved adherence in V2 | 114 | 91.9 | |
| FeNO | 0.04 | |||||||
| 0–24 | 46 | 37.1 | 51 | 48.6 | No change in V2 and V1 | 49 | 48.0 | |
| 25–49 | 41 | 33.1 | 35 | 33.3 | Lower in V2 than in V1 | 35 | 34.3 | |
| ≥50 | 37 | 29.8 | 19 | 18.1 | Higher in V2 than in V1 | 18 | 17.6 | |
.
%: percentage. ACT: asthma control test. FeNO: fractional exhaled nitric oxide. FEV1: forced expiratory volume in 1s. N: number of patients. OCS: oral corticosteroids. TAI: test of adherence to inhalers. V1: initial visit. V2: final study visit.
A logistic regression analysis was performed to identify factors associated with failure to achieve clinical remission at V2. The model with the highest predictive performance included current blood eosinophils at V2 [OR (95%CI); p] [0.998 (0.996;1.000); p=0.038] together with age [1.038 (0.998;1.080); p=0.06] and male/female gender [0.474 (0.186;1.211); p=0.118], providing a moderate predictive model with an AUC of 0.684 (95% CI: 0.576;0.791). Replacing the current eosinophil value with the highest historical value gives worse results (p=0.170) with an AUC of 0.579 (0.473;0.686). The multivariate model maintained the significance of current eosinophils (p=0.031) with an AUC of 0.651 (0.540;0.763).
In this prospective study, with a long follow-up in a cohort of SA patients, we found that individual TGs can be achieved in a significant percentage of patients (no AE: 62.5%; symptom control: 64.1%; and no OCS use: 87.5%). However, the percentage of patients achieving “clinical remission” was low (15.9%), in line with the results from previous studies in biologic-treated patients.11 Possible explanations for this include inadequate therapeutic planning, misidentification of the inflammatory endotype or the existence of a maximal attainable response (“ceiling effect”) for biologics.
We found that the TTs assessed in this study (eosinophils, FeNO and adherence to therapy) improved over the course of the study, presumably due to the increase in the prescription of biologics during follow-up. In fact, our results support this explanation, as only patients treated with biologics had significantly reduced eosinophil numbers, and this variable was predictive of worse outcome. Although we cannot prove that one is a consequence of the other, it seems reasonable to think that improving TTs leads to the attainment of TGs.
The main strength of this study is its very long follow-up in a homogeneous group of SA patients treated in expert units. This study has limitations that should be acknowledged, such as a small sample size, the considerable number of patients lost to follow-up, and significant changes in maintenance treatment that make it difficult to draw conclusions about the effect of clinical and biological features on outcomes. Moreover, as this is a real-world study, we cannot know whether all patients received the most appropriate treatment for their clinical situation and phenotype (e.g., biologic drugs). The fact that patients were recruited in asthma units means that the sample is not representative of the situation of all subjects with SA in this country. Furthermore, we have to recognize that the full list of TTs includes items not considered in this study because at the time our study was designed, a clear description of TTs in asthma was not yet available. It is also possible that respiratory isolation measures carried out during the COVID-19 pandemic may have influenced the study results by reducing the number of exacerbations.
We conclude that the care of patients in expert asthma units leads to the achievement of TGs by addressing TTs in a high percentage of patients. However only a small percentage of SA patients achieved disease remission, a problem that requires further investigation.
Authors’ contributionsAll authors were involved in the conception and design of the work; acquisition, analysis, interpretation of data, drafting the work, revising it critically for important intellectual content.
Conflict of interestsCAS has received fees in the last 3 years for giving lectures, scientific advice, participation in clinical studies, or writing publications for (in alphabetical order): ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer. CAS declares never receiving, directly or indirectly, funding from the tobacco industry or its affiliates.
This research was supported by AstraZeneca Farmacéutica Spain, S.A. The final and published content is the authors’ sole work.