This research investigates quadriceps muscle fatigability (MF) in chronic obstructive pulmonary disease (COPD) patients with chronic respiratory failure (CRF) at different levels of lung obstruction [severe obstruction (SO)=FEV1 <50% and >30% versus very severe obstruction (VSO)=FEV1 ≤30%]. It explores the relationships between quadriceps MF and lung function, respiratory muscles, and oxygenation status.
MethodsA post hoc cross-over analysis in 45 COPD patients (20 SO and 25 VSO) undergoing long-term oxygen therapy was performed. Delta change in quadriceps maximum voluntary contraction (MVC) (absolute value and percentage) before and after a constant workload was calculated. Associations between quadriceps MF and lung function, respiratory muscles, and gas exchange were examined using Pearson's correlation and multivariate linear regression analysis.
ResultsSO patients experience a more substantial reduction in MVC compared to VSO (−15.15±9.13% vs −9.29±8.90%, p=0.0357), despite comparable resting MVC. Dyspnea is more pronounced in VSO at the beginning and end of the exercise. Correlations were found between MF and maximal inspiratory pressure (MIP) (r=−0.4412, p=0.0056), maximal expiratory pressure (MEP) (r=−0.3561, p=0.0282), and a tendency for FEV1% (r=−0.2931, p=0.0507). The regression model (R2=0.4719) indicates that lower MIP and FEV1 and high total lung capacity are significant factors in reducing quadriceps muscle fatigability after a fatiguing task.
ConclusionCOPD patients with more severe pulmonary obstruction and hyperinflation and lower respiratory muscle strength have lower quadriceps MF but higher dyspnea both at rest and during exercise.
Muscle fatigability (MF) is an exercise-induced reduction in maximal voluntary muscle force, that depends on several mechanisms involving the central nervous system and peripheral muscle components.1 In clinical and rehabilitation settings, it can be assessed by the reduction in maximal voluntary contraction (MVC) i.e. the maximum force-generating capacity of the quadriceps after a fatiguing task: this parameter provides general but immediate information on the reduction in muscle performance.2 In healthy subjects, the reduction in MVC after a generic fatiguing task (i.e. cycling) has been found to average 15%, which is considered to be a commonly used threshold in the literature.3
The measure of muscle fatigability may serve as a primary limitation to peripheral muscle performance; however, after adequate recovery, it emerges as an essential prerequisite for improved muscle performance following exercise training.4
In addition, by measuring muscle fatigability in patients with COPD, healthcare providers can formulate individualized treatment strategies that incorporate tailored exercise regimens into pulmonary rehabilitation programs. This approach aims to improve both muscle function and overall health.5,6 However, it is known that not all patients with chronic obstructive pulmonary disease (COPD) are able to develop MF during different exercise tasks.7 Burtin et al.8 reported that 40% of patients with COPD were unable to reach a certain level of MF during a cycling task due to an early effort interruption, limiting potential adaptation to training.
In light of muscular fatigability, Mador et al.9 investigated this phenomenon using a mono-muscle fatigue protocol with minimal cardiorespiratory engagement, and found that people with severe COPD had heightened MF compared to both healthy counterparts and those with moderate COPD.9 These authors also evaluated MF during cycling by comparing subjects with moderate COPD and healthy subjects, at moderate intensity and iso-oxygen consumption, with similar results.10
However, in people with severe COPD during high-intensity “endurance” exercise, such as walking and cycling, an abnormal increase in ‘central’ cardio-respiratory involvement (e.g., higher dynamic hyperinflation, mechanical lung constraint, and respiratory muscle fatigability) can lead to an early interruption of the effort due to dyspnea and progressive deconditioning.11,12 These patients may experience susceptibility to locomotor muscle fatigability partly due to insufficient transport of O22 as a consequence of exaggerated arterial hypoxemia11,12 and supplemental oxygen administration can improve exercise tolerance in patients with COPD by reducing ventilatory requirements for the same workload and producing lower levels of lactate.11,12
Few studies have investigated MF in patients with COPD and chronic respiratory failure (COPD-CRF) who have already developed chronic hypoxia. Paneroni et al.13 recently reported that COPD-CRF patients had lower workability but similar endurance and quadriceps MF to COPD patients without CRF during high-intensity exercise under long-term oxygen therapy (LTOT). The authors speculated that these results might be related to the oxygen supplementation given to CRF patients.
No studies have investigated the impact of lung function parameters as well as respiratory muscle strength and oxygenation status on MF following a high-intensity “endurance” exercise task in COPD-CRF. The aim of this study was to investigate the influence of these parameters on MF as a decrease in quadriceps maximal voluntary contraction (MVC), in COPD-CRF subjects following an endurance exercise task.
We hypothesized that COPD-CRF patients on oxygen therapy with blood concentration within the appropriate range (PaO2>60mmHg) would have varying degrees of quadriceps MF based on the severity of lung obstruction, hyperinflation, respiratory muscle strength, and/or oxygenation status (SpO2/FiO2).
MethodsThis is a cross-sectional study, being a post hoc analysis performed on partial data obtained between 2019 and 2022 in Istituti Clinici Scientifici Maugeri Lumezzane (BS) relative to a prospective study in COPD-CRF (Maugeri Ethics Committee, CE 2288; 14/05/2019). Participants gave their specific informed written consent and all experimental procedures were performed in accordance with the Declaration of Helsinki.
Patients and EligibilityInclusion criteria for this study were all patients with COPD and CRF according to spirometry, COPD diagnosis14 and blood gas analysis, and who met the following conditions: (a) LTOT for at least 3 months according to guidelines15 and declaration of compliance; (b) CRF determined with a partial pressure of O2 <60mmHg by breathing ambient air at rest; (c) the presence of PaO2 >60mmHg with the oxygen level prescribed by pulmonologist at rest and during exercise; (d) forced expiratory volume at 1s (FEV1) <50% prd; (e) stable clinical condition; (f) absence of orthopedic, neurological, cardiac, and cognitive impairments; (g) admission to the hospital for rehabilitation program. All patients breathed oxygen through special nasal probes, and the oxygen flow during effort was set at the level prescribed by the pulmonologist. Individuals with contraindications to exercise due to cardiovascular, orthopedic, or oncological comorbidities, diabetes, clinical instability within the previous 4 weeks, and cognitive deficits were excluded from the study.
MeasuresBaseline demographics, anthropometrics, spirometry values (FEV1% prd, FVC % prd, FEV1/FVC, residual volume – RV % prd), blood gases analysis (PaO2/FiO2), and comorbidities [using the cumulative illness rating scale (CIRS)16] were recorded while breathlessness on daily activities was assessed by Medical Research Council (MRC) Dyspnea Scale.17 Patients with COPD-CRF were classified according to the GOLD spirometry classification [severe obstruction (SO)=FEV1 between 30% and 50% and very severe obstruction (VSO)=FEV1 below/equal to 30% of predicted].
Exercise capacity was assessed by the 6-minute walk test (6MWT). Patients were instructed to walk as far as possible within the 6-minute time limit along a dedicated 30-m corridor. A physiotherapist recorded the distance walked (m) and administered the Borg scale RPE of dyspnea and leg fatigability,18 before and after the test, and measured the heart rate and oxygen saturation with a pulse oximeter (8500A, Nonin). Patients could stop during the test for dyspnea or leg discomfort and restart as soon as possible, without stopping the timer.19
Patients performed an incremental test (10W/min, 60rpm) using an electromagnetically braked cycle ergometer (Ergoline 800; Sensor Medics, Anaheim, CA, USA) to determine their peak power output (PPO).20 The constant work rate cycling test (CWRCT) was performed at 80% of PPO. Patients were instructed to exercise for as long as possible, with continuous verbal encouragement. Exercise was stopped as dyspnea became intolerable, or if they were unable to pedal at the required rate (60/min) for more than 10s. Dyspnea and leg RPE were recorded every 60s using the modified Borg scale18 which ranges from 0 to 10 points. Heart rate was measured using the R–R interval from a 12-lead online electrocardiogram (Marquette Max) and oxygen saturation using a pulse oximeter (Nonin), while blood pressure was measured every minute using a manual sphygmomanometer.
Maximal voluntary contraction of quadriceps was performed in the dominant limb using a customized setup. Subjects were seated in an upright position with a backrest and the knee and hip were flexed at 90°. The ankle was attached to the force transducer (DBBSE-100 kg, A2829; Applied Measurements Ltd, Aldermaston, Berkshire, UK) via a strap and a rigid steel bar. The force transducer's output was amplified using INT2-L (London Electronics Ltd, Sandy, Bedfordshire, UK) and recorded using a PowerLab-16/35 data acquisition system (Powerlab, AD Instruments Inc., USA). Patients performed a focused warm-up, consisting of eight short contractions at increasing but not fatiguing intensity. The MVC was repeated three times with 30s of rest between each repetition. One minute elapsed between the MCV assessment and the CWRCT. Quadriceps MF was calculated as the percentage reduction in MVC (ΔMVC) after the CWRCT. The calculated value has a negative sign, indicating that a lower ΔMVC corresponds to a higher MF. Conversely, a ΔMVC approaching zero indicates the absence of muscular fatiguability.
All tests were performed with the usual patient's oxygen dosage, prescribed by the pulmonologist.
Statistical AnalysesDescriptive statistics were reported as mean±standard deviation for continuous variables and as a percentage (%) for discrete variables. The patients were grouped into severe obstruction (SO) and very severe obstruction (VSO) based on their FEV1 being above or below/equal to 30% of predicted.14 Comparisons between groups for continuous variables were performed using the unpaired t-test and the chi-square test was applied for categorical variables. The associations between quadriceps MF (percentage of delta change on the baseline) and baseline lung function and gas exchange variables [FEV1, forced vital capacity (FVC), residual volume (RV), total lung capacity (TLC), maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP), PaO2/FiO2 ratio] were assessed by Pearson's correlation.
Furthermore, we also performed a multivariate linear regression analysis with quadriceps MF (expressed as % delta change in MVC after CWRCT) as the dependent variable and FEV1, FVC, RV, TLC, MIP, MEP, and PaO2/FiO2 ratio as independent variables to elucidate the primary pulmonary factors influencing MF expression. The method used was backward stepwise. The best model, highlighted in terms of R2, was presented. Only variables with a significance level (p-value) below 0.10, as determined by the stepwise method of variable selection, were included in the model. Statistical significance was set at p<0.05.
The minimum sample size was calculated on NMF considering the change in the percentage of MVC after the fatiguing task (primary outcome) according to Paneroni et al.13 who found a reduction in MVC% in CRF patients of 9.6±9.0. According to the difference between moderate and severe COPD reported by Mador et al.,9 we estimated an additional reduction in MVC of approximately 10% between SO and VSO groups (delta MVC%: −19.6±9). With an alpha significance level of 0.05 (2-sided) and a beta of 90%, a minimum total sample size of 38 participants (19 for each group) was considered sufficient to detect significant differences between the SO and the VSO groups.
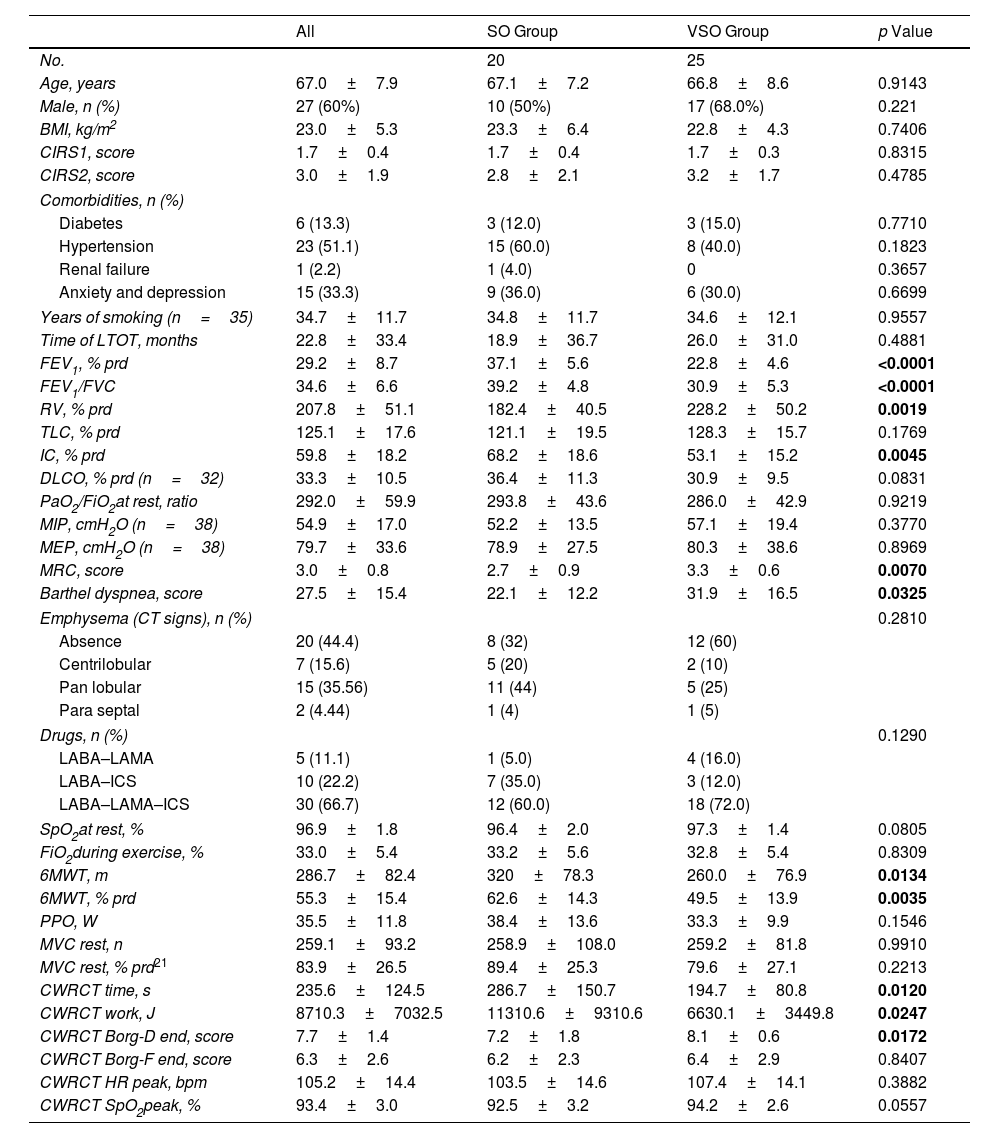
ResultsWe included 45 patients [20 with severe obstruction (SO) and 25 with very severe obstruction (VSO)] (Table 1). The whole group was predominantly males and had a high rate of comorbidities. As expected, patients with VSO had worse lung obstruction, degree of hyperinflation, dyspnea during ADL, effort tolerance at 6MWT distance, and endurance time than patients with SO.
Comparison of Patients with Severe Obstruction (SO) (FEV1 <50% and >30%) and Very Severe Obstruction (VSO) (FEV1 ≤30%).
| All | SO Group | VSO Group | p Value | |
|---|---|---|---|---|
| No. | 20 | 25 | ||
| Age, years | 67.0±7.9 | 67.1±7.2 | 66.8±8.6 | 0.9143 |
| Male, n (%) | 27 (60%) | 10 (50%) | 17 (68.0%) | 0.221 |
| BMI, kg/m2 | 23.0±5.3 | 23.3±6.4 | 22.8±4.3 | 0.7406 |
| CIRS1, score | 1.7±0.4 | 1.7±0.4 | 1.7±0.3 | 0.8315 |
| CIRS2, score | 3.0±1.9 | 2.8±2.1 | 3.2±1.7 | 0.4785 |
| Comorbidities, n (%) | ||||
| Diabetes | 6 (13.3) | 3 (12.0) | 3 (15.0) | 0.7710 |
| Hypertension | 23 (51.1) | 15 (60.0) | 8 (40.0) | 0.1823 |
| Renal failure | 1 (2.2) | 1 (4.0) | 0 | 0.3657 |
| Anxiety and depression | 15 (33.3) | 9 (36.0) | 6 (30.0) | 0.6699 |
| Years of smoking (n=35) | 34.7±11.7 | 34.8±11.7 | 34.6±12.1 | 0.9557 |
| Time of LTOT, months | 22.8±33.4 | 18.9±36.7 | 26.0±31.0 | 0.4881 |
| FEV1, % prd | 29.2±8.7 | 37.1±5.6 | 22.8±4.6 | <0.0001 |
| FEV1/FVC | 34.6±6.6 | 39.2±4.8 | 30.9±5.3 | <0.0001 |
| RV, % prd | 207.8±51.1 | 182.4±40.5 | 228.2±50.2 | 0.0019 |
| TLC, % prd | 125.1±17.6 | 121.1±19.5 | 128.3±15.7 | 0.1769 |
| IC, % prd | 59.8±18.2 | 68.2±18.6 | 53.1±15.2 | 0.0045 |
| DLCO, % prd (n=32) | 33.3±10.5 | 36.4±11.3 | 30.9±9.5 | 0.0831 |
| PaO2/FiO2at rest, ratio | 292.0±59.9 | 293.8±43.6 | 286.0±42.9 | 0.9219 |
| MIP, cmH2O (n=38) | 54.9±17.0 | 52.2±13.5 | 57.1±19.4 | 0.3770 |
| MEP, cmH2O (n=38) | 79.7±33.6 | 78.9±27.5 | 80.3±38.6 | 0.8969 |
| MRC, score | 3.0±0.8 | 2.7±0.9 | 3.3±0.6 | 0.0070 |
| Barthel dyspnea, score | 27.5±15.4 | 22.1±12.2 | 31.9±16.5 | 0.0325 |
| Emphysema (CT signs), n (%) | 0.2810 | |||
| Absence | 20 (44.4) | 8 (32) | 12 (60) | |
| Centrilobular | 7 (15.6) | 5 (20) | 2 (10) | |
| Pan lobular | 15 (35.56) | 11 (44) | 5 (25) | |
| Para septal | 2 (4.44) | 1 (4) | 1 (5) | |
| Drugs, n (%) | 0.1290 | |||
| LABA–LAMA | 5 (11.1) | 1 (5.0) | 4 (16.0) | |
| LABA–ICS | 10 (22.2) | 7 (35.0) | 3 (12.0) | |
| LABA–LAMA–ICS | 30 (66.7) | 12 (60.0) | 18 (72.0) | |
| SpO2at rest, % | 96.9±1.8 | 96.4±2.0 | 97.3±1.4 | 0.0805 |
| FiO2during exercise, % | 33.0±5.4 | 33.2±5.6 | 32.8±5.4 | 0.8309 |
| 6MWT, m | 286.7±82.4 | 320±78.3 | 260.0±76.9 | 0.0134 |
| 6MWT, % prd | 55.3±15.4 | 62.6±14.3 | 49.5±13.9 | 0.0035 |
| PPO, W | 35.5±11.8 | 38.4±13.6 | 33.3±9.9 | 0.1546 |
| MVC rest, n | 259.1±93.2 | 258.9±108.0 | 259.2±81.8 | 0.9910 |
| MVC rest, % prd21 | 83.9±26.5 | 89.4±25.3 | 79.6±27.1 | 0.2213 |
| CWRCT time, s | 235.6±124.5 | 286.7±150.7 | 194.7±80.8 | 0.0120 |
| CWRCT work, J | 8710.3±7032.5 | 11310.6±9310.6 | 6630.1±3449.8 | 0.0247 |
| CWRCT Borg-D end, score | 7.7±1.4 | 7.2±1.8 | 8.1±0.6 | 0.0172 |
| CWRCT Borg-F end, score | 6.3±2.6 | 6.2±2.3 | 6.4±2.9 | 0.8407 |
| CWRCT HR peak, bpm | 105.2±14.4 | 103.5±14.6 | 107.4±14.1 | 0.3882 |
| CWRCT SpO2peak, % | 93.4±3.0 | 92.5±3.2 | 94.2±2.6 | 0.0557 |
Data were expressed as mean±standard deviation. %: percentage; prd: predicted; BMI: body mass index; CIRS: cumulative illness rating scale; FEV1: forced expiratory volume at first second; FVC: forced vital capacity; RV: residual volume; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; MRC: Medical Research Council; CT: computed tomography; LABA: long-acting beta-agonists; TLC: Total Lung Capacity; IC: Inspiratory Capacity; DLCO: Diffusion Lung CO; PaO2: Partial pressure of oxygen; FiO2: Inspiratory Fraction of Oxygen; LAMA: long-acting muscarinic antagonists; ICS: inhaled corticosteroids; O2: oxygen; L: liters; SpO2: arterial oxygen saturation; 6MWT: 6-minute walking test; m: meters; PPO: peak power output at cyclo-ergometer incremental test; W: watts; MVC: maximal voluntary contraction; CWRCT: constant work rate cycling test; s: seconds; J: joules; Borg-F: Borg score muscle fatigability-related; Borg-D: Borg score dyspnea-related; HR: heart rate; bpm: beats per minute. p<0.05 are highlighted in bold.
The study found statistically significant differences in the duration in CWRCT and total work between groups. Patients with greater lung obstruction had lower effort tolerance and stopped the test earlier. However, there were no differences in baseline MVC or reasons for stopping the test (45%, 15%, and 40% of patients stopped for dyspnea, perceived muscle fatigability, or both, respectively) between the two groups of patients.
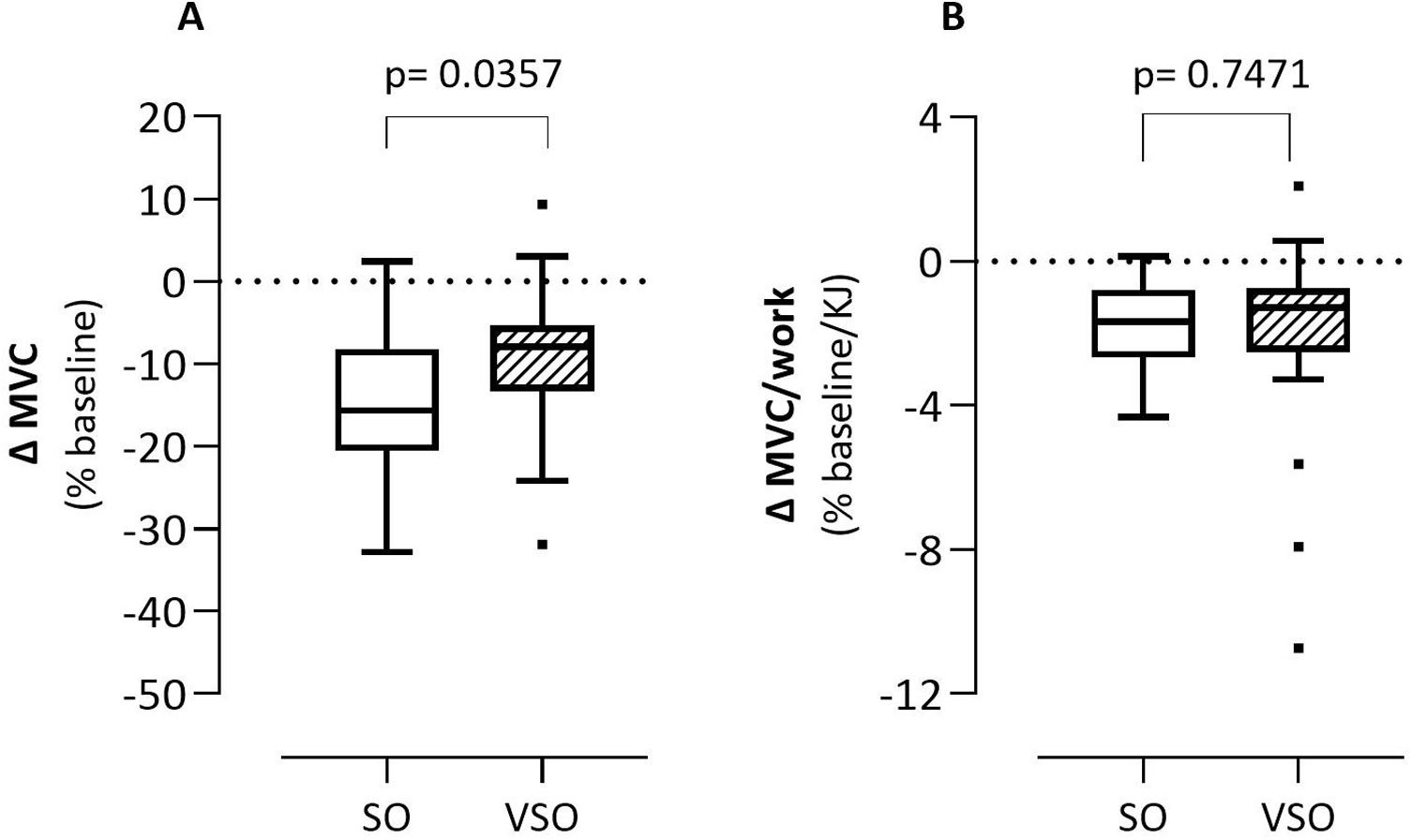
There were 10 patients (50%) in the SO group and 4 patients (16.0%) in the VSO group with a decrease in MVC of more than 15% (p=0.014). Fig. 1 shows the percentage of MVC change after CWRCT: the absolute values are defined in panel A, while the values normalized by the work performed are shown in panel B. The figure describes that, at exhaustion, SO patients had a greater and statistically significant reduction in MVC when compared to VSO patients (−15.15±9.13% vs −9.29±8.90%, p=0.0357) although with a high variability of the measurements. When MVC was normalized to CWRCT work, quadriceps MF was similar between groups (SO −1.7±1.2 vs VSO −2.0±2.7, p=0.7471).
Percentage of MVC change on baseline after CWRCT: absolute values (panel A) and values normalized by work performed (panel B). Δ: delta; MVC: maximal voluntary contraction; KJ: kilojoules; SO: patients with severe obstruction group; VSO: patients with very severe obstruction group. Boxplots (median and 25̊-75̊ percentile values).
Significant correlations were found between MF (% delta change of MVC after CWRCT) and MIP (r=−0.4412, p=0.0056) and MEP (r=−0.3561, p=0.0282), while there was a trend toward significance for FEV1% (r=−0.2931=0.0507), suggesting that patients with less pronounced pulmonary obstruction and higher respiratory muscle strength were more likely to develop quadriceps MF.
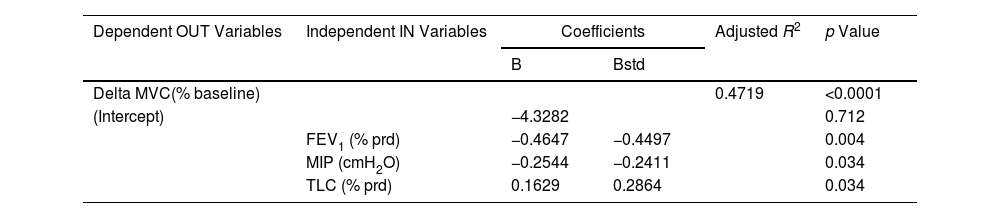
According to these results, Table 2 describes the best multivariate linear regression model to explain the quadriceps MF. Taking into account respiratory and oxygenation factors, the most important variables with a significant influence are FEV1, MIP and TLC. Patients with higher values of FEV1 and MIP and lower levels of TLC may develop higher quadriceps MF. These three factors contribute similarly to MF, but the ability to reduce MVC (i.e. the ability to achieve a higher MF) was only partially explained (coefficient of determination R2=47%).
Multivariate Linear Regression Model Explaining the Quadriceps MF, According to Lung Function and Oxygenation Status.
| Dependent OUT Variables | Independent IN Variables | Coefficients | Adjusted R2 | p Value | |
|---|---|---|---|---|---|
| B | Bstd | ||||
| Delta MVC(% baseline) | 0.4719 | <0.0001 | |||
| (Intercept) | −4.3282 | 0.712 | |||
| FEV1 (% prd) | −0.4647 | −0.4497 | 0.004 | ||
| MIP (cmH2O) | −0.2544 | −0.2411 | 0.034 | ||
| TLC (% prd) | 0.1629 | 0.2864 | 0.034 | ||
OUT: output; IN: input; B: beta coefficient; Bstd: standardized beta; R2: coefficient of determination; MVC: maximal voluntary contraction. Independent variables included in the model of the multivariate linear regression (backward stepwise approach) were FEV1 (% prd): forced expiratory volume at first second expressed in % of predicted value; FVC: forced vital capacity in % of predicted value; RV: residual volume; TLC: total lung capacity in % of predicted value; MIP: maximal inspiratory pressure (cmH2O); MEP: maximal expiratory pressure (cmH2O); PaO2: partial pressure of oxygen; FiO2: inspiratory fraction of oxygen.
Our study supports the hypothesis that among COPD-CRF patients, those with different degrees of lung obstruction have different responses to quadriceps muscle fatigability (MF).
Compared with COPD-CRF patients with severe lung obstruction (FEV1 between 50% and 30% predicted), our findings demonstrate that individuals with more severe lung obstruction (FEV1 below/equal to 30% predicted) have a shorter endurance time, a lower propensity to develop quadriceps MF and increased dyspnea both at rest and after a fatiguing cycling task. Better lung function expressed as FEV1% prd and TLC % prd and better respiratory muscle strength are associated with a higher likelihood of developing quadriceps muscle fatigability during exercise.
The literature on post-exercise MF in COPD remains inconclusive and somewhat controversial. Mador et al.9 conducted a study comparing severe COPD (FEV1%=26%), mild to moderate COPD (FEV1%=53%), and healthy controls using a quadriceps fatiguing protocol with little cardiopulmonary system involvement. The results showed that patients with severe COPD, without chronic respiratory failure (CRF), experienced more MF during exercise compared to those with milder disease and healthy controls.9
In patients with moderate to severe COPD (FEV1%=41%) without CRF, Burtin et al.8 demonstrated that only 63% of the general group reached a threshold for quadriceps MF after a cycling task. This assessment was based on a reduction in the potentiated resting quadriceps twitch (Qtw, pot), with no significant differences in clinical and functional characteristics observed between patients with and without contractile fatigability.
The development of MF in COPD patients has been suggested to depend on muscle characteristics such as fiber types, enzymes, capillarization, and lactate accumulation, as well as the nature of the exercise task. For example, walking may induce less peripheral MF than cycling,7 and short-term oxygen supplementation in COPD without CRF can reduce MF development about a third.12
In patients with severe COPD-CRF (FEV1%=25%) on oxygen therapy, perceived muscle fatigue and dyspnea were reported to be higher after high-intensity cycling exercise compared with less severe COPD without CRF (FEV1%=38%).13 However, the extent of quadriceps MF (defined as a decrease in MVC and Qtw, pot) was found to be similar in both groups.13 The authors speculated that the similar MF was due to comparable muscle conditions and characteristics between the groups, possibly due to the protective effect of oxygen consumption during exercise in CRF patients.13
Our study represents a pioneering investigation exclusively focused on chronic obstructive pulmonary disease with chronic respiratory failure (COPD-CRF) patients undergoing LTOT. This study has shown that the response of the MF varies between individuals with different degrees of lung obstruction. Our findings suggest that as lung obstruction progresses, there is a concomitant decrease in effort tolerance, which is accompanied by a decreased development of quadriceps MF observed at the peak of a high-intensity constant load test. In this regard, our dataset shows that only 15% of COPD-CRF patients with a FEV1 less than 30% of predicted reached a predefined threshold level of quadriceps MF (maximal voluntary contraction reduction <15%). These data support the notion that the decline in effort tolerance is associated with a reduced development of quadriceps MF, which is particularly pronounced in patients with severe lung obstruction.
Notably, the difference in quadriceps fatigability between patients with significant obstruction (SO) and very severe obstruction (VSO) is attenuated when the change in maximal voluntary contraction (MVC) is normalized to the total work performed. This normalization highlights that the observed differences in MF response may be influenced not only by the severity of lung obstruction but also by the overall workload endured during the test. Moreover, the fact that both groups of patients show the same normalized fatigability suggests that they have a common impairment of their peripheral muscles; the lower absolute fatigability in VSO could therefore be explained by the greater ventilatory limitation shown by this group.
Our data are consistent with the study by Butcher et al.22 which showed an inverse relationship between the magnitudes of ventilatory constraints due to dynamic hyperinflation and skeletal muscle contractile capacity after high-intensity exercise cycling in room air in patients with COPD. Their patients were more likely to increase exercise tolerance with heliox, resulting in greater leg MF. Unfortunately, we lack data describing dynamic volumes during effort, but these results are consistent with the notion that the worse the respiratory mechanics, the lower the potential for peripheral muscles to reach a given fatigue threshold. In this direction, our data showed that total lung capacity (TLC) is a component able to explain the quadriceps MF. Indeed, TLC increases during the course of the disease due to loss of lung recoil and is recognized as one of the markers of lung hyperinflation (LH). LH is partially independent of the degree of airflow limitation and is strongly associated with dyspnea, exercise limitation, reduced activities of daily living and mortality. A direct link between LH and peripheral muscle dysfunction has not been fully established. Peripheral muscle dysfunction is common in COPD, probably mainly driven by muscle deconditioning from disuse of the locomotor muscles.23
Disuse results from exertional dyspnea, dynamic hyperinflation and redistribution of blood flow during exercise from the musculoskeletal system to respiratory muscles.24 However, also LH may be indirectly related to peripheral muscle dysfunction.25
The reasons for the divergent MF development between SO and VSO patients at exhaustion may be primarily related to the early engagement of the respiratory system during exercise in patients with very severe obstruction (VSO). The early cessation of exertion may consequently lead to the development of less fatigability. In this context, the results of correlation and regression analyses show that patients with better lung function and inspiratory muscle strength were able to achieve higher levels of peripheral MF. Nevertheless, our results describe how the two patient groups show a “theoretical” similarity in intrinsic muscular fatigability when normalized for the actual work performed.
Structural alterations and dysfunction in limb muscles of patients with COPD have been studied with subsequent clinical implications such as exercise intolerance and premature mortality.26
Susceptibility to quadriceps contractile fatigability during exercise is more common in patients with higher glycolytic enzyme activity, lower muscle capillarization, and early lactate accumulation.9
In addition, several factors may influence the likelihood of developing peripheral fatigability. One such factor is the “metaboreflex phenomenon”27–29 induced by respiratory muscle fatigability, which increases sympathetic vasoconstrictor outflow, resulting in reduced blood flow to the locomotor muscles and subsequent fatigability of these muscles.30 It is plausible that more severe patients, in terms of lung obstruction and inspiratory muscle strength, may have had a relatively limited involvement of this mechanism, potentially hindering adequate quadriceps muscle stimulation. This limitation is suggested by their greater and earlier impairment of the central ventilatory component, as evidenced by greater dyspnea at rest and during exertion.
Further investigations are needed to elucidate the relationship between metaboreflex induced by respiratory muscle fatigability and quadriceps fatigability in the COPD-CRF population.
The utility of measuring muscle fatigability lies in the fact that it is an essential prerequisite for improved muscle performance following exercise training.4
Confirming this fact, Burtin et al.8 showed that COPD patients who were more likely to develop muscle fatigability had greater improvement in the 6-minute walk test (6MWT) after a training program.
Unfortunately, significant ventilatory limitations may prevent severe COPD patients with CRF from reaching a training intensity sufficient to induce a similar overload: this condition seems to be the most likely to occur in VSO patients.
To overcome this problem, several strategies have been proposed to reduce dyspnea and dynamic lung limitation during exercise allowing peripheral muscles to reach a sufficient training stress threshold, such as interval training,31 small muscle mass training,32 the use of external respiratory devices (e.g., mechanical ventilation, high-flow oxygen therapy)33 and magnetic versus electrical muscle stimulation.34
LimitationsOur study is a “real-life” investigation, which has certain limitations. First, we did not directly assess ventilatory and peripheral muscle oxygenation parameters during exercise, which limits our ability to analyze dynamic hyperinflation based solely on the trend of dyspnea and metabolic muscle activity. Second, we did not use specific electrical or magnetic muscle or nerve stimulation techniques to assess central and peripheral fatigability.35 Third, we calculated neuromuscular fatigability at the exhaustion of a CWRCT, and the result at iso-work was indirectly evaluated by normalization to the total work performed. It cannot be excluded that our results would have been different if patients had undergone an iso-time (and iso-workload) test.
In addition, the type of exercise may also have played a role; if less muscle mass is involved, and consequently, the lung mechanics are less stressed, the muscles may be more susceptible to fatigue.36 We cannot exclude the possibility that different results would be obtained if patients were exposed to a different type of exercise. As fatigability appears to be a task-dependent phenomenon,4 caution should be exercised in generalizing our results to everyday life. Finally, the study is a post hoc investigation and this aspect could have led to several problems: (a) some relevant measures for interpreting the study were not collected (e.g., Fat-Free Mass Index); (b) due to the small sample size, the risk of Type-II error could be relevant for some of the comparisons where no significant differences were found, although the sample was calculated as adequate for the evaluation of the primary outcome.
Practical ImplicationsThe novelty of our report is the demonstration that greater obstruction, greater hyperinflation, and lower respiratory muscle strength reduce the potential for peripheral muscles to reach a certain fatigue threshold during high-intensity endurance tasks in patients, probably due to the influence of the respiratory system in the early cessation of effort. This aspect should be considered when devising patient training regimens, as it can contribute to explain the presence of muscle deconditioning in severe populations.
Our evaluations provide a range of physiological information that can help to tailor the rehabilitation program for people with COPD/CRF. Rehabilitation staff should better characterize the patient's phenotype in terms of fatigability, as previous studies have shown that patients with higher fatigability on admission to pulmonary rehabilitation have a greater improvement in effort tolerance following a training program.
ConclusionIn patients with COPD and chronic respiratory failure, those with higher levels of airway obstruction, higher levels of hyperinflation and lower strength in inspiratory muscle experience greater exercise intolerance and reduced peripheral muscle fatigability at the peak of exercise due to increased dyspnea. This difference appears to be reduced by normalizing fatigability for the work performed. Further studies should compare both groups (VO and VSO) with mild COPD patients and healthy people, and describe the changes in MF after a structured training program.
Authors’ ContributionsThe contributions of the author were as follows: conceptualization and methodology, MP, MVi, MVe; validation, MP; formal analysis, AC; investigation, AC, LB, BS; data curation, AC, BS; writing original draft preparation, AC, MP, MVi; writing-review and editing, AC, MP, MVi, MVe, BS, LB; supervision, MVi; project administration, AC, MP, MVi.
FundingThis work refers to a post hoc analysis of data coming from a study conducted in our Institute related to different training modalities in COPD and CRF patients entitled: “Allenamento allo sforzo nei pazienti fragili e affetti da severa patologia respiratoria: confronto di tre modalità di allenamento”. The study was funded by a 5×1000 grant – year 2016 from the Italian Ministry of Health. The funder had no role in study design, data collection and analysis, and any interpretation of data; writing of the paper, or submission.
Conflict of InterestThe author reports no conflicts of interest in this work.
This work was supported by the “Ricerca Corrente” funding scheme of the Ministry of Health, Italy. The authors thank Laura Comini and Adriana Olivares for technical support.