Pulmonary arterial hypertension (PAH) is a rare and severe disease that causes a progressive remodelling of the pulmonary vessels, increasing the right ventricle (RV) afterload, and leading to death if untreated.1 This risk of death is influenced by multiple variables. European and American Guidelines for PAH management recommend the use of multiparametric models for risk stratification and treatment decisions.2,3 Pulmonary veno-occlusive disease (PVOD) is a particularly infrequent form of PAH. Both its heritable form caused by homozygous or compound heterozygous variants in the gene EIF2AK4,4 and sporadic cases have an especially low survival.5 Nevertheless, there is no strong evidence confirming the applicability of any risk score used for PAH specifically in PVOD. Our aim in this study was to describe the applicability of risk scores in patients with PVOD.
We included PVOD patients from the Spanish registry of PAH (REHAP) between 2011 and 2022. The diagnosis of heritable PVOD required a homozygous or a biallelic compound heterozygous variant in EIF2AK4. Patients with sporadic PVOD had a definitive pathological diagnosis, or a low diffusing capacity for carbon monoxide, as well as the presence of three radiological signs of PVOD (septal lines, ground glass opacities, and mediastinal lymphadenopathy).6 All cases required a diagnostic right heart catheterization (RHC). Both the 3-strata model proposed by the European Society of Cardiology (ESC)/European Respiratory Society (ERS) Guidelines,2 and the REVEAL Lite 23 were applied in this population at baseline. Goodness-of-fit and calibration of the model in this population were evaluated. All participants signed informed consent before entering the REHAP registry. The study was conducted following the Declaration of Helsinki and after approval by the local Ethics Committee.
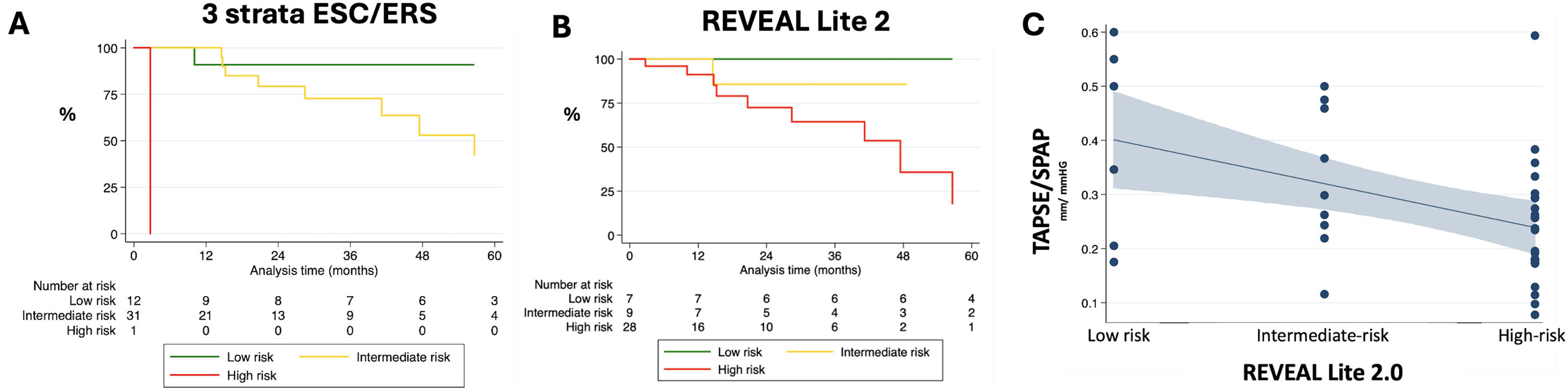
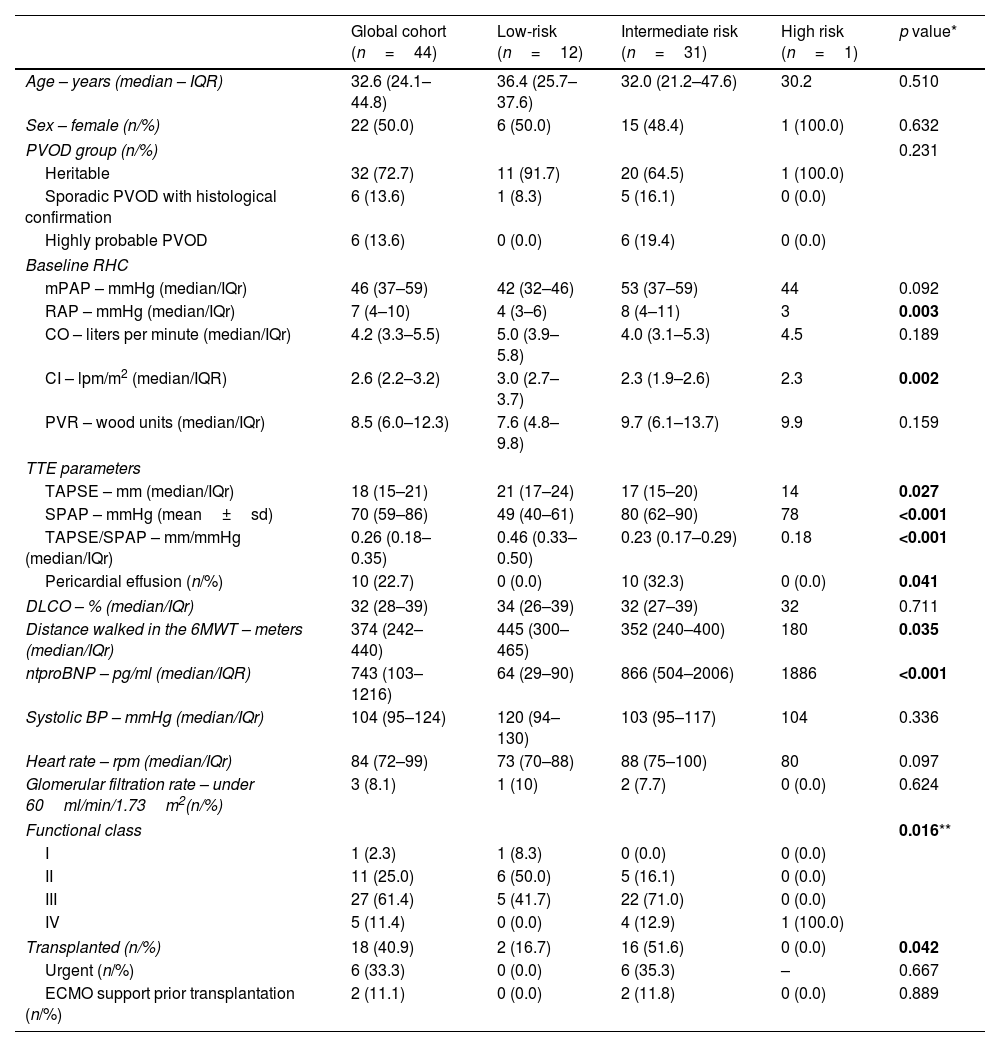
In the 2011–2022 period up to 32 cases of heritable PVOD in 23 unrelated families were collected (Supplementary material). Six cases of sporadic PVOD were confirmed histologically after transplantation, and the other 6 cases were diagnosed as probable PVOD by means of the radiological findings. Multiple variables including cardiac biomarkers, TAPSE/sPAP ratio, pericardial effusion, six-minute walk distance, or functional class were differently distributed between risk groups (Table 1). After a median follow-up of 22.1 months, up to 18 patients with PVOD needed lung transplantation (40.9%), and 12 patients died (27.3%). The observed rate of death at 1, 3, and 5 years in PVOD was 5.3%, 22.9%, and 50.5%, respectively. The majority of the included population had an intermediate risk of mortality after the application of the European model (70.5%, Fig. 1A), a different distribution of that observed after applying the REVEAL Lite 2 score (63.6% at high risk, Fig. 1B). Using the European score in our PVOD population, those patients at intermediate risk had a hazard ratio of 9.53 (2.15–42.17, p=0.003) when compared with patients at low risk, and patients at high-risk had a hazard ratio of 9.49 (0.86–104.69, p=0.066) when compared with patients at intermediate risk (Fig. 1A). This model demonstrated a good global prognostic capacity (C Index of 0.707), with a modest calibration of the model (Supplementary material). These data were similar when compared with those reflected by the REVEAL Lite 2.0 model (C Index of 0.694, modest calibration; Supplementary material). Compared with patients at low risk, patients at intermediate and high risk together had a risk of death of 4.13 (1.42–12.00, p=0.009) applying the ESC/ERS score, and 5.60 (0.75–47.80, p=0.091) applying the REVEAL Lite 2. The rate of lung transplantation or death at one year was 10.0%, 25.7%, and 100% in the low-, intermediate-, and high-risk groups defined by the ESC/ERS, respectively. These rates were 0.0%, 11.1%, and 28.6% in the low-, intermediate-, and high-risk groups defined by the REVEAL Lite 2 (Supplementary material).
Baseline characteristics of patients with heritable or sporadic PVOD included in the Spanish REHAP cohort between 2011 and 2022 for each ESC/ERS risk stratum.
| Global cohort (n=44) | Low-risk (n=12) | Intermediate risk (n=31) | High risk (n=1) | p value* | |
|---|---|---|---|---|---|
| Age – years (median – IQR) | 32.6 (24.1–44.8) | 36.4 (25.7–37.6) | 32.0 (21.2–47.6) | 30.2 | 0.510 |
| Sex – female (n/%) | 22 (50.0) | 6 (50.0) | 15 (48.4) | 1 (100.0) | 0.632 |
| PVOD group (n/%) | 0.231 | ||||
| Heritable | 32 (72.7) | 11 (91.7) | 20 (64.5) | 1 (100.0) | |
| Sporadic PVOD with histological confirmation | 6 (13.6) | 1 (8.3) | 5 (16.1) | 0 (0.0) | |
| Highly probable PVOD | 6 (13.6) | 0 (0.0) | 6 (19.4) | 0 (0.0) | |
| Baseline RHC | |||||
| mPAP – mmHg (median/IQr) | 46 (37–59) | 42 (32–46) | 53 (37–59) | 44 | 0.092 |
| RAP – mmHg (median/IQr) | 7 (4–10) | 4 (3–6) | 8 (4–11) | 3 | 0.003 |
| CO – liters per minute (median/IQr) | 4.2 (3.3–5.5) | 5.0 (3.9–5.8) | 4.0 (3.1–5.3) | 4.5 | 0.189 |
| CI – lpm/m2 (median/IQR) | 2.6 (2.2–3.2) | 3.0 (2.7–3.7) | 2.3 (1.9–2.6) | 2.3 | 0.002 |
| PVR – wood units (median/IQr) | 8.5 (6.0–12.3) | 7.6 (4.8–9.8) | 9.7 (6.1–13.7) | 9.9 | 0.159 |
| TTE parameters | |||||
| TAPSE – mm (median/IQr) | 18 (15–21) | 21 (17–24) | 17 (15–20) | 14 | 0.027 |
| SPAP – mmHg (mean±sd) | 70 (59–86) | 49 (40–61) | 80 (62–90) | 78 | <0.001 |
| TAPSE/SPAP – mm/mmHg (median/IQr) | 0.26 (0.18–0.35) | 0.46 (0.33–0.50) | 0.23 (0.17–0.29) | 0.18 | <0.001 |
| Pericardial effusion (n/%) | 10 (22.7) | 0 (0.0) | 10 (32.3) | 0 (0.0) | 0.041 |
| DLCO – % (median/IQr) | 32 (28–39) | 34 (26–39) | 32 (27–39) | 32 | 0.711 |
| Distance walked in the 6MWT – meters (median/IQr) | 374 (242–440) | 445 (300–465) | 352 (240–400) | 180 | 0.035 |
| ntproBNP – pg/ml (median/IQR) | 743 (103–1216) | 64 (29–90) | 866 (504–2006) | 1886 | <0.001 |
| Systolic BP – mmHg (median/IQr) | 104 (95–124) | 120 (94–130) | 103 (95–117) | 104 | 0.336 |
| Heart rate – rpm (median/IQr) | 84 (72–99) | 73 (70–88) | 88 (75–100) | 80 | 0.097 |
| Glomerular filtration rate – under 60ml/min/1.73m2(n/%) | 3 (8.1) | 1 (10) | 2 (7.7) | 0 (0.0) | 0.624 |
| Functional class | 0.016** | ||||
| I | 1 (2.3) | 1 (8.3) | 0 (0.0) | 0 (0.0) | |
| II | 11 (25.0) | 6 (50.0) | 5 (16.1) | 0 (0.0) | |
| III | 27 (61.4) | 5 (41.7) | 22 (71.0) | 0 (0.0) | |
| IV | 5 (11.4) | 0 (0.0) | 4 (12.9) | 1 (100.0) | |
| Transplanted (n/%) | 18 (40.9) | 2 (16.7) | 16 (51.6) | 0 (0.0) | 0.042 |
| Urgent (n/%) | 6 (33.3) | 0 (0.0) | 6 (35.3) | – | 0.667 |
| ECMO support prior transplantation (n/%) | 2 (11.1) | 0 (0.0) | 2 (11.8) | 0 (0.0) | 0.889 |
BP (blood pressure); CO (cardiac output); DLCO (diffusing capacity of the lung for carbon monoxide); IQR (interquartile range); ntproBNP (N-terminal pro b-type natriuretic peptide); PVOD (pulmonary venoocclusive disease); PVR (pulmonary vascular resistance); RAP (right atrial pressure); RHC (right heart catheterization); RPM (rates per minute); SPAP (systolic pulmonary artery pressure); TAPSE (Tricuspid annular plane systolic excursion).
Application of the ESC/ERS and REVEAL Lite 2 scores at diagnosis in PVOD. (A) Kaplan–Meier survival curves for patients classified as low-, intermediate-, and high-risk strata using the ESC/ERS 3 strata score; (B) Kaplan–Meier survival curves for patients classified as low-, intermediate-, and high-risk strata using the REVEAL Lite 2 score; (C) Prediction by the mean interval between TAPSE/sPAP ratio and the REVEAL Lite 2.0 score. The result of the Kruskal–Wallis test (p=0.048) demonstrates differences of the TAPSE/sPAP ratio between risk groups. The result of the Jonckheere–Terpstra tendency test (p=0.012) confirms the trend for lower TAPSE/sPAP in higher risk groups.
Our work showed a good global prognostic capacity of both the ESC/ERS risk score and the REVEAL Lite 2 model in PVOD at diagnosis. The European Guidelines highlight the fact that those patients at high risk could have a mortality above 20% at 1 year, and between 10 and 20% in case of intermediate risk.2 The observed mortality in our PVOD population was higher than expected by the guidelines. More importantly, the need for lung transplantation during that first year of diagnosis was incredibly high in this young population in those cases at intermediate or high risk, independently of the two models evaluated in this work. These data reinforce the awareness that PVOD should always be referred for lung transplantation evaluation, and listed immediately if the baseline risk evaluation reveals an intermediate or high-risk status, whichever the score system is applied. A recent study was the first study showing that both the European risk model and the REVEAL scoring system could be very useful in PVOD, with also a similar global prognostic capacity for these two models. This last work showed likewise a bad prognosis in low-risk patients with PVOD.7 Nevertheless, the comparison of this work is difficult since there was a minority of cases of heritable PVOD (8% compared with 73% in our cohort), the median age was 65 years, and there was a predominance of men. Thus, as reflected in our data, a strategy based on the initiation of pulmonary vasodilators and early reevaluation, without the need for early listing for lung transplantation might be an option only for those patients with a baseline low risk in heritable PVOD forms, but always under close follow-up in a reference centre with the possibility for lung transplantation, bearing in mind the possible deleterious response to these drugs in this population.8
It is possible that the generalization of genetic testing and the better characterization of patients with PAH with a respiratory phenotype could unmask more PVOD cases in the future,9 making necessary the identification of proper scores for this specific condition. In our study, cardiac biomarkers and ventricular-arterial coupling evaluated by the TAPSE/sPAP ratio exhibited important differences between risk groups (Table 1, Fig. 1C), suggesting that these two variables could be rather important in this condition, in which the functional class, the ventilatory efficiency, or the hemodynamic impairment (indirectly evaluated by blood pressure or heart rate in the REVEAL Lite 2) were equally deteriorated in all risk groups. In line with our results in PVOD, some authors have suggested that the evaluation of the TAPSE/sPAP ratio in addition to ntproBNP, and functional class could be good enough for risk stratification in PAH.10
Although the study has limitations due to its small size and its retrospective nature, the work set the scene for future collaborative studies specifically designed for risk stratification in PVOD. The original REVEAL Lite 2 only included patients with at least 12 months of follow-up, excluding patients who received lung transplants within 1 year of enrollment. In our PVOD cohort, several patients received lung transplantation urgently or died during the first-year evaluation, limiting the extrapolation of the REVEAL Lite 2 in PVOD.
In conclusion, the applicability of the European 3 strata model or the REVEAL Lite 2 at baseline in PVOD is valid, showing an especially high risk of death in patients at intermediate- or high-risk. This work enhances the need for an immediate listing for lung transplantation in PVOD at intermediate or high risk. Ventricular–arterial uncoupling measured by the TAPSE/sPAP ratio could early identify those patients at higher risk of death.
FundingThis work was funded by the competitive projects “Bases Genético-Moleculares de la Medicina de Precisión en la Hipertensión Arterial Pulmonar” and “Moving toward to a -omic classification for pulmonary arterial hypertension”, Instituto de Salud Carlos III, Ministry of Science and Innovation, Spanish Government. Award numbers: PI18/01233, PI21-01593, and PI21-01690.
Alejandro Cruz-Utrilla holds a research contract “Juan Rodés” from the Instituto de Salud Carlos III, Ministry of Science and Innovation, Spanish Government (JR23/00071).
Conflict of interestsThe authors state that they have no conflict of interests.
We thank all the patients and professionals involved in the REHAP registry. We thank Natalia Gallego and Jair Antonio Tenorio, as well as other members of the INGEMM – Hospital Universitario La Paz for the genetic studies in PAH.