Pseudomonas aeruginosa (PA) is a notorious contributor to chronic lung diseases like bronchiectasis, historically associated with frequent exacerbations, faster lung function decline, reduced quality of life (QoL), and higher mortality.1,2 International guidelines advocate inhaled antibiotics for bronchiectasis patients with PA infection experiencing three or more exacerbations annually, or eradication therapy upon first isolation.3,4 However, the assumption that PA always drives severe outcomes warrants closer examination. Does PA always mean more hospitalization, or does it harm patients in subtler ways? A new study by Fernández-Barat and colleagues, published in this issue, challenges conventional views, revealing a complex relationship between chronic and intermittent PA infections, exacerbations, QoL, and lung microbiome dynamics.5
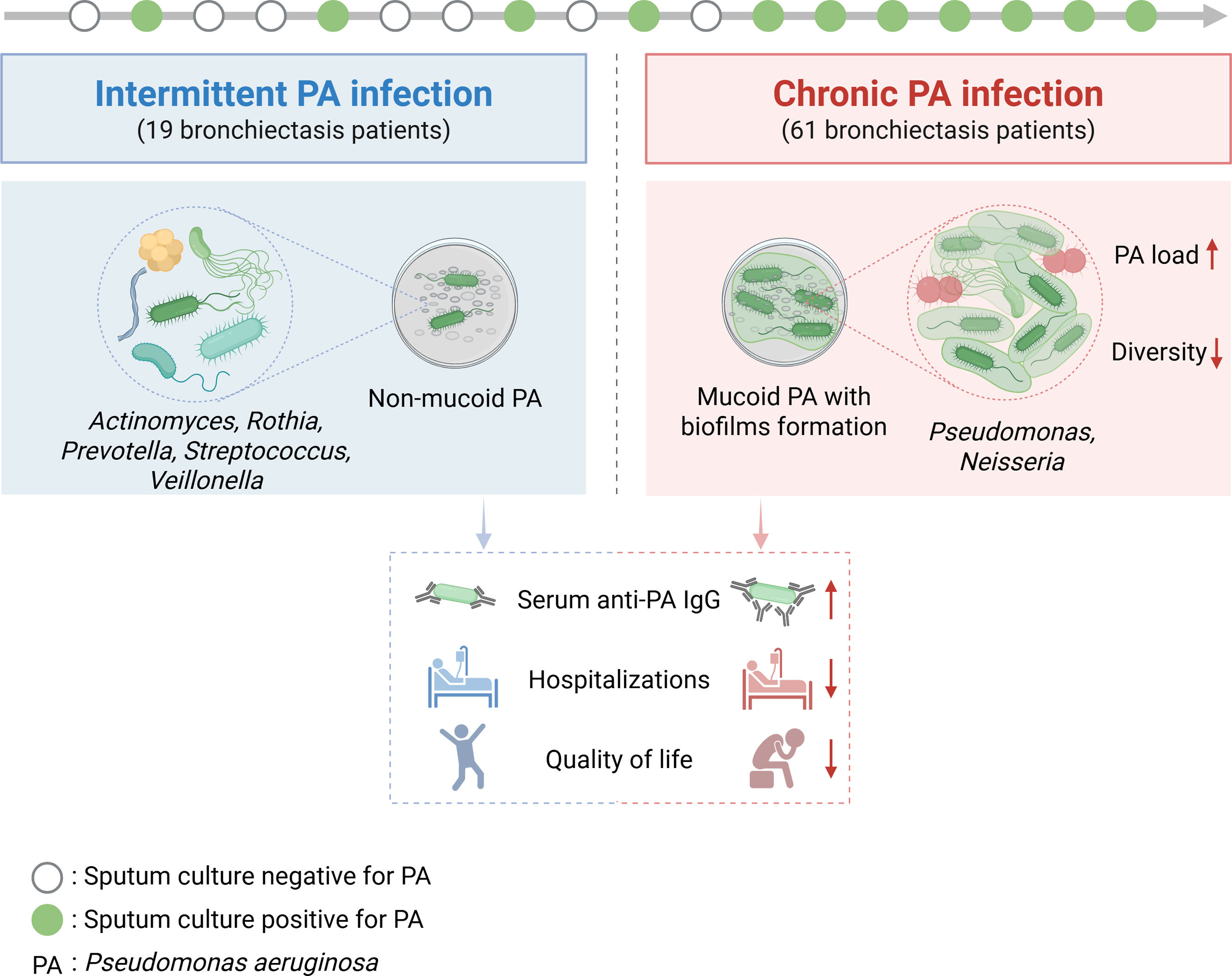
The study followed 80 bronchiectasis patients over one year—61 with chronic PA infection and 19 with intermittent infection—analyzing 235 sputum and 262 blood samples. Surprisingly, chronic PA infection, marked by mucoid phenotypes and biofilm formation, led to fewer severe exacerbations requiring hospitalization than intermittent infection. This was linked to elevated anti-PA IgG antibodies, suggesting an immune response that may reduce severe exacerbations. However, this “protective” effect came at a cost: chronic infection correlated with significant worsening emotional and social QoL domains, alongside reduced lung microbiome diversity dominated by Proteobacteria. In contrast, intermittent PA infection maintained a more diverse microbiome (e.g., enriched with Prevotella and Streptococcus) and better QoL over time, despite more hospitalizations (Fig. 1). This contrast challenges the old belief that PA always causes nonstop trouble, showing that long-term infection might impose a quieter, persistent burden on well-being.
The microbiological and clinical disparities between bronchiectasis patients with intermittent and chronic Pseudomonas aeruginosa infections. This figure highlights the microbiological and clinical disparities between bronchiectasis patients with intermittent versus chronic Pseudomonas aeruginosa (PA) infections. The green spot represents the sputum culture positive for PA, and the hollow spot refers to the sputum culture negative for PA. Chronic PA infection was defined as two or more positive sputum cultures separated by 3 months within a year. Otherwise, patients were classified as exhibiting intermittent infection. Nineteen intermittent PA infection patients showed non-mucoid PA phenotype in sputum culture, rich microbial diversity, and detectable serum anti-PA IgG. Compared to those with intermittent PA infection, patients with chronic PA infection were characterized by mucoid PA phenotype with biofilm formation, elevated PA load and reduced microbial diversity, with a predominance of Pseudomonas and Neisseria. Additionally, patients with chronic PA infection demonstrated higher serum anti-PA IgG, fewer hospitalizations and worse quality of life compared to those with intermittent infection.
The study also spotlights a testing issue. Standard sputum culture techniques (SOCT) detected PA in only 44% of samples, whereas an optimized protocol—endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID),6 incorporating sonication and extended incubation—increased detection to 74%.5 This gap, especially for mucoid PA, underscores the need for better diagnostics to catch PA infections early, enabling interventions to prevent persistent colonization. The mucoid PA phenotype, tied to chronicity and biofilm resilience, suggests potential for targeted therapies like antibiofilm agents in combination with inhaled antibiotics.
Chronic PA infection in bronchiectasis shares features with cystic fibrosis (CF), such as biofilm formation, alginate production, and adaptive IgG responses.7–9 However, bronchiectasis patients with chronic PA often show no decline in forced expiratory volume in 1 second (FEV1),10,11 unlike CF, indicating unique disease mechanisms. This distinction cautions against directly applying CF treatment strategies to bronchiectasis, calling for tailored approaches that may include mucolytics or biofilm disruptors alongside bronchiectasis-specific outcomes.
The study offers practical guidance for clinicians. Distinguishing chronic from intermittent PA infection—achievable in routine practice—enables early eradication to prevent chronicity, preserving QoL and preventing irreversible lung damage.3,12,13 For chronic cases, biomarkers like mucoid PA prevalence or elevated anti-PA IgG could guide intensified strategies, such as biofilm-targeted therapies or immune modulation, to reduce long-term harm.14,15 These approaches aim to balance exacerbation control with QoL preservation.
While groundbreaking, the study's single-center design and small intermittent PA cohort (n=19) limit generalizability, necessitating validation across diverse populations. The reduced microbiome diversity in chronic cases, dominated by Proteobacteria, suggests a stable but risky ecological shift, previously linked to worse survival.16 Key research priorities include: (1) Clarifying the role of anti-PA IgG in reducing exacerbations; (2) Exploring ways to maintain microbiome diversity in chronic infection; and (3) Refining early eradication protocols to prevent chronicity.
Fernández-Barat et al. redefine PA infection in bronchiectasis as a spectrum, where chronicity reflects a unique state driven by immune–microbiome interactions, not just exacerbation severity. This shift urges clinicians to balance acute flare management with QoL preservation, using advanced diagnostics for precision care. As biofilm-targeted therapies evolve, this study lays a foundation for improving long-term outcomes in bronchiectasis patients.
Take-home message: Chronic Pseudomonas aeruginosa in bronchiectasis reduces severe exacerbations but harms quality of life and microbiome diversity. Early detection and eradication with advanced diagnostics and antibiofilm therapies are key to balancing exacerbation control and long-term well-being.
Author contributionsAll authors have contributed intellectually, comply with the conditions of authorship and have approved the final version to be published.
Declaration of generative AIThere was no involvement of AI in preparation of this manuscript.
FundingThis work was supported by Noncommunicable Chronic Diseases – National Science and Technology Major Project (No. 2024ZD0529700) and National Natural Science Foundation of China (No. 82270047).
Conflicts of interestThe authors have no conflicts of interest to disclose.