Obstructive sleep apnea (OSA) is a chronic condition characterized by intermittent hypoxia (IH) and sleep fragmentation (SF). OSA can induce excessive daytime sleepiness (EDS) and is associated with impaired cognition and anxiety. Solriamfetol (SOL) and modafinil (MOD) are widely used wake-promoting agents in OSA patients with EDS.
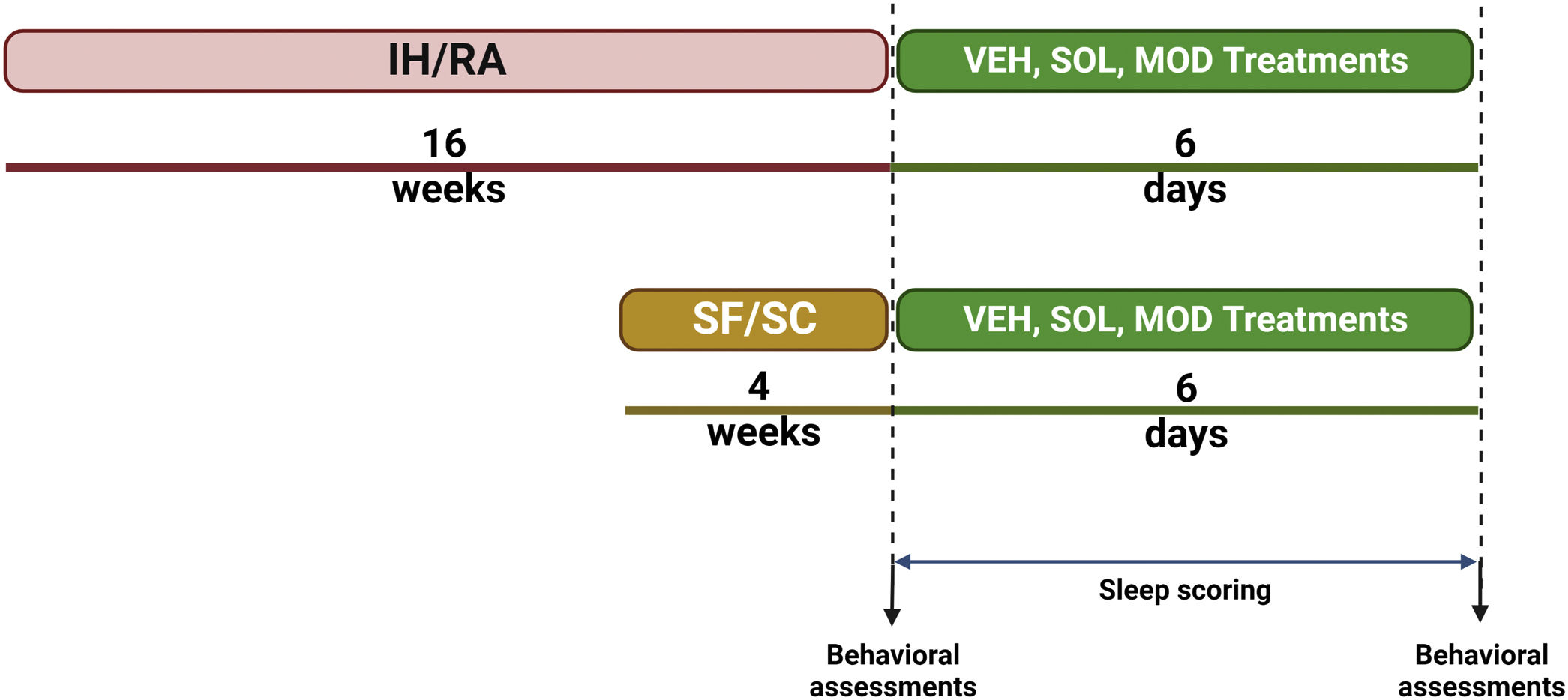
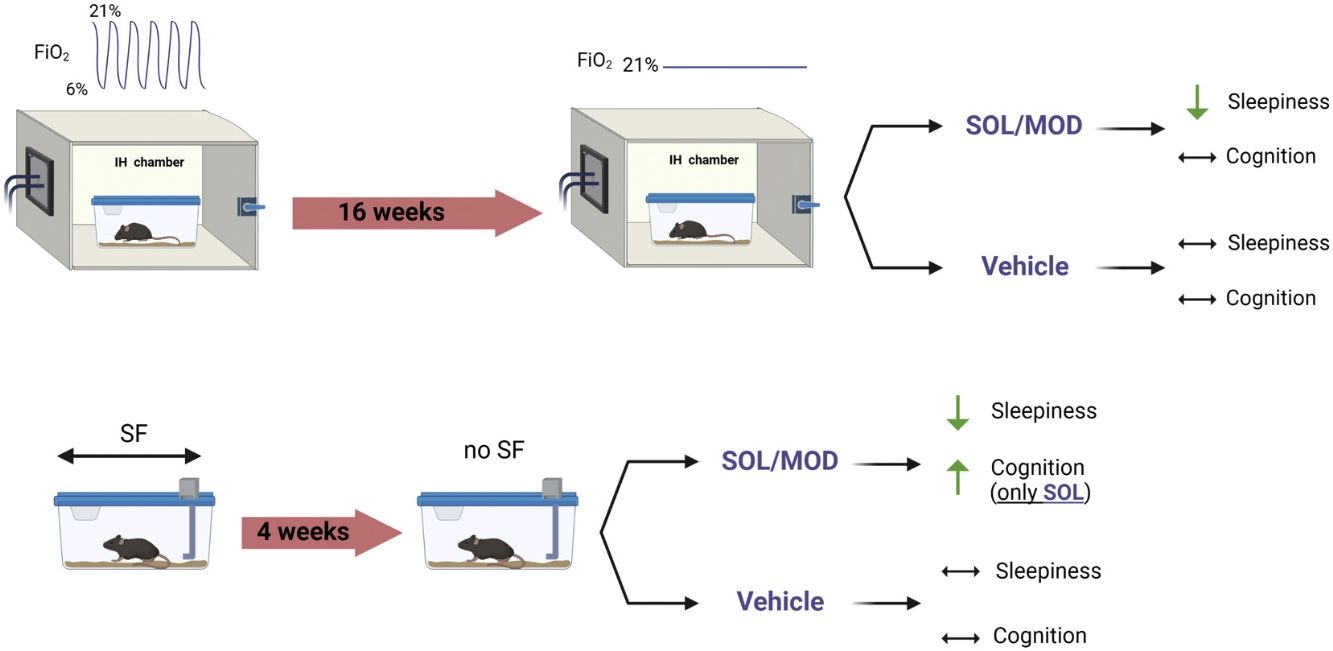
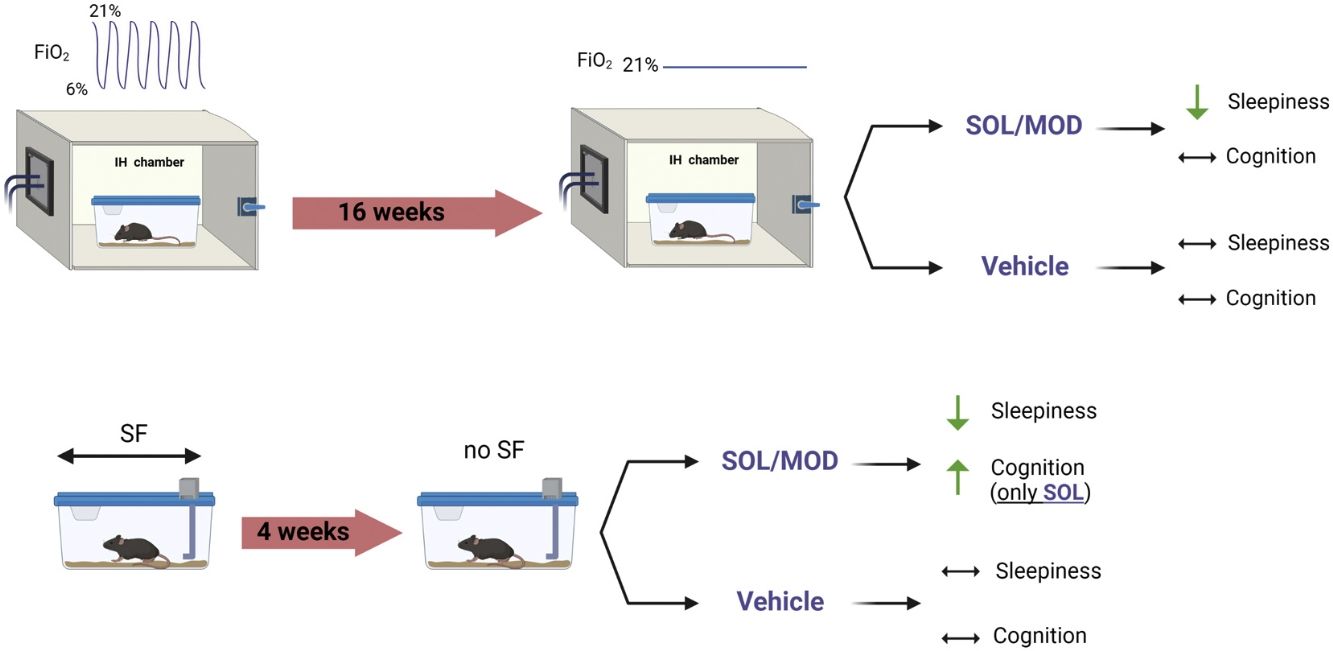
MethodsMale C57Bl/6J mice were exposed to SF along with sleep controls (SC) or to IH and room air (RA) controls during the light (inactive) phase for 4 and 16 weeks, respectively. Both IH and SF exposures were then discontinued to mimic “ideal” continuous positive airway pressure (CPAP) adherence. All groups were then randomly assigned to receive once daily intraperitoneal injections of SOL, MOD, or vehicle (VEH) for 6 days. Sleep/wake activity was assessed along with tests of explicit memory, anxiety and depression were performed before and after treatments.
ResultsIH and SF exposures increased sleep percentage in the dark phase and reduced wake bouts lengths (i.e., EDS), and induced cognitive deficits and impulsivity in mice. Both SOL and MOD treatments effectively mitigated EDS when combined with recovery, while recovery alone did not improve EDS over the 6-day period. Furthermore, improvements explicit memory emerged only after SOL.
ConclusionChronic IH and SF induce EDS in young adult mice that is not ameliorated by recovery except when combined with either SOL or MOD. SOL, but not MOD, significantly improves IH-induced cognitive deficits. Thus, SOL emerges as a viable adjuvant medication for residual EDS in OSA along with its positive impact on cognition.
Obstructive sleep apnea (OSA) is a common and long-lasting condition nearly affecting 1billion people worldwide.1 OSA is characterized by the increased upper airway collapsibility during sleep that leads to recurring episodes of obstructive respiratory events. These episodes induce characteristic perturbations such as intermittent hypoxia (IH) and sleep fragmentation (SF).2 Extensive research has established a link between OSA and a large spectrum of comorbidities, including cognitive and neurobehavioral impairments.3 OSA patients commonly experience mood swings, anxiety, memory problems, and emotional disturbances, which can be attributed, at least in part, to excessive daytime sleepiness (EDS).4 EDS significantly impacts daytime functioning, attention, cognition, psychomotor tasks, work productivity, risk of accidents, and overall quality of life.5 Although continuous positive airway pressure (CPAP) therapy has proven beneficial in treating EDS, a significant proportion of CPAP-treated OSA patients still experience residual EDS, with an estimated prevalence of 9–22%.6 Consequently, wake-promoting medications are commonly prescribed to manage OSA-induced EDS.
Over the years, various wake-promoting agents, such as psychostimulants have been prescribed to manage residual EDS in OSA patients. However, due to their undesirable side effects and the risk of rebound hypersomnolence, their usage has gradually declined.7 Consequently, more effective, and selective wake-promoting agents have been developed specifically targeting residual EDS in both treated and untreated OSA patients. One of the main agents commonly used for hypersomnolence disorders is the dopamine reuptake inhibitor modafinil (MOD) and its R-enantiomer armodafinil.8,9 Clinical and experimental studies have shown the beneficial effects of MOD in improving wakefulness, daytime performance, cognition, and depression.10,11 Despite the improvements experienced by OSA patients using MOD, the repeated dosing required to maintain its wake-promoting effects throughout the day has limited its use.12 Furthermore, MOD has been associated with increased cardiovascular and neuropsychiatric problems.13 In contrast, a more recently developed wake-promoting agent, solriamfetol (SOL), a norepinephrine/dopamine reuptake inhibitor with lower affinity to dopamine and norepinephrine transporters and lacking monoamine-releasing effects, has been approved by the FDA for treating EDS associated with conditions such as narcolepsy and OSA.14 Clinical trials have revealed robust SOL wake-promoting effects in a dose-dependent manner, regardless of concurrent OSA therapy adherence,15 making it a promising candidate as a first-line treatment for OSA-induced EDS.
We previously performed a head-to-head comparison between SOL and MOD in murine models of OSA, including IH and SF, on sleep and behavior.16,17 In the present study, we examine the effects of both SOL and MOD during the period after cessation of exposures (i.e., an attempt to mimic “ideal” CPAP adherence) on sleep propensity as well as cognition, depression, and anxiety.
MethodsAll experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Missouri (protocol:). One hundred and eighty male C57BL/6J mice (8-week-old) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and randomized into 4 different groups subjected to sleep fragmentation (SF) or sleep control (SC) for 4 weeks, or intermittent hypoxia (IH) for or room air (RA) for 16 weeks. Mice were then randomly assigned to receive SOL, MOD, or vehicle (VEH) at the beginning of the dark phase for 6 days following the cessation of all exposures (Fig. 1). Non-invasive sleep recordings were taken before and during the treatments and recovery, while behavioral tests we performed twice, once before IH and SF cessation and once at the end of treatments with recovery. All material and Methods are extensively described in the Supplementary material.
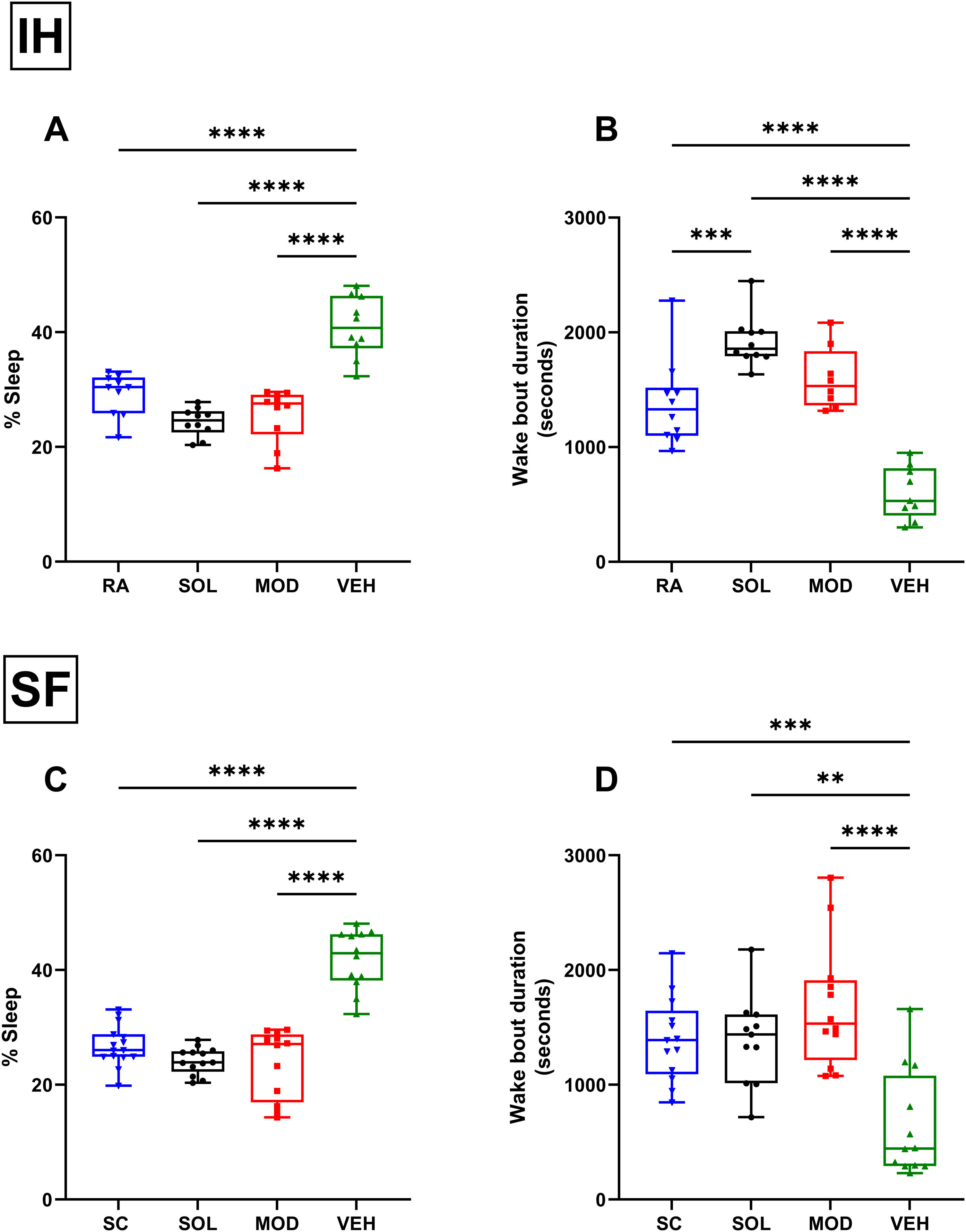
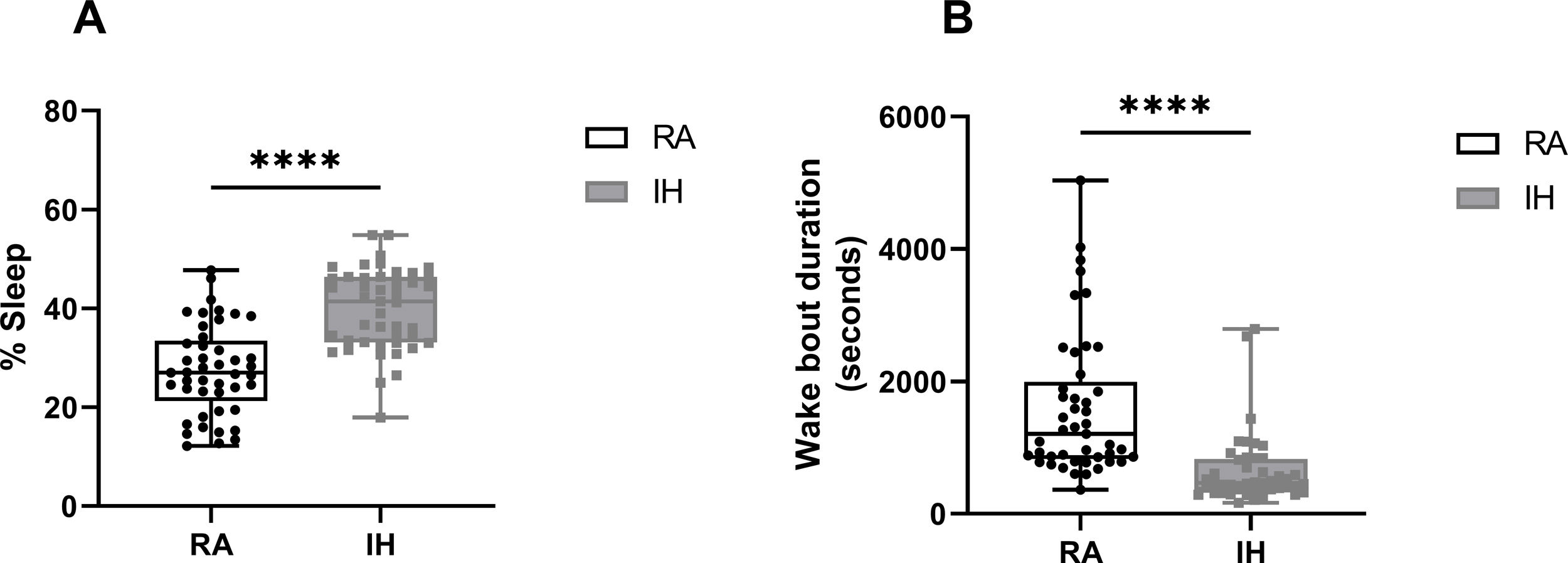
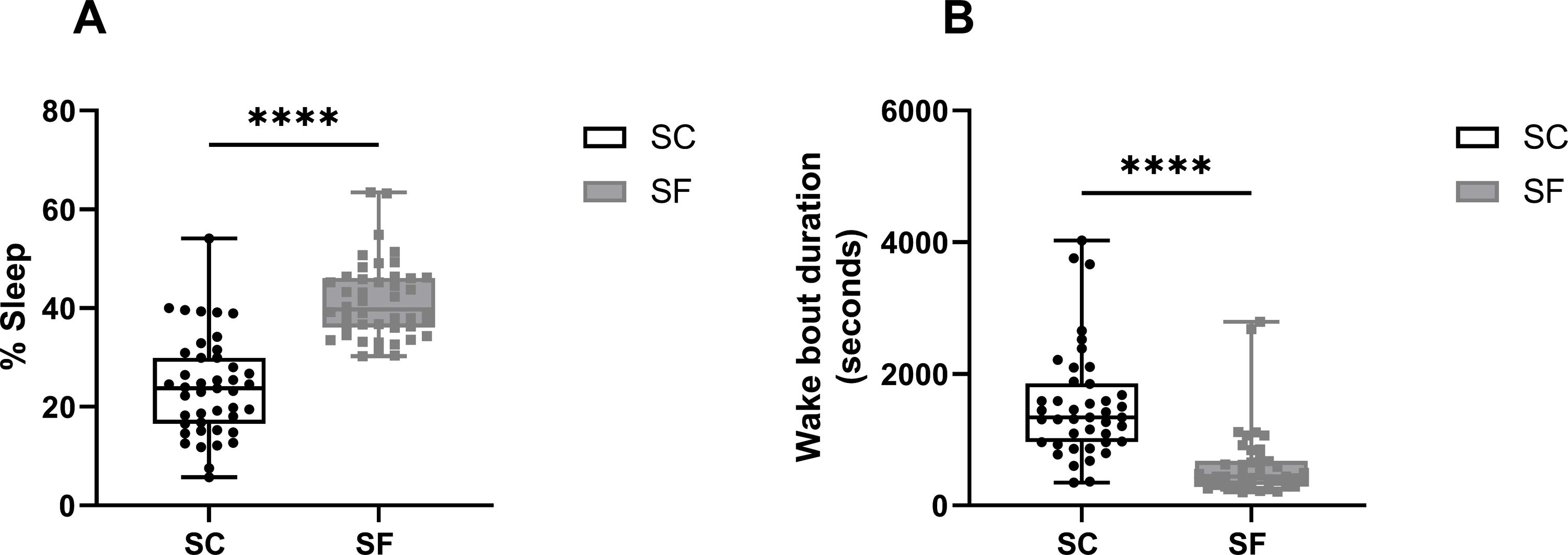
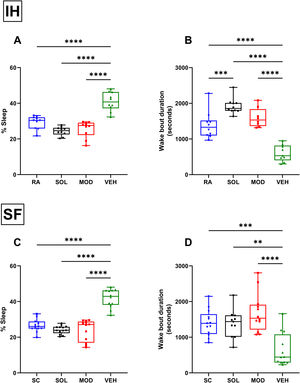
ResultsMice exposed to 16 weeks of IH exhibited excessive sleepiness during the active phase described as increased sleeping time and reduced wake bout durations during the dark phase (Supplementary Fig. 1) before being randomly assigned to receive treatments consisting of normoxic recovery. Average percentage of sleep in the dark phase during the 6 days of normoxic treatment was significantly higher in VEH-treated mice (41±5%) when compared to VEH-treated RA controls (29±4%, p<0.0001) despite the complete cessation of IH exposures (Fig. 2A). Treatments with both SOL (24±3%) and MOD (26±5%) combined with normoxic recovery significantly reduced the percentage of time spent in sleep in IH-exposed animals when compared to VEH treated mice (41±5%, p<0.0001). Additionally, VEH-treated mice exposed to IH with normoxic recovery exhibited significantly reduced average wake bout durations (600±229s) when compared to RA VEH-treated group (1382±381s, p<0.0001). Both SOL (1921±221s) and MOD (1597±271s) markedly increased average wake bout durations when compared to VEH-treated mice (600±229s, p<0.0001) (Fig. 2B).
Recovery from IH and SF does not improve wakefulness unless it is combined with SOL and MOD. (A) Sleep percentages and (B) wake bouts duration during the dark phase in mice after 16 weeks of IH exposure and treated with either SOL, MOD or VEH for 6 days with recovery. (C) Sleep percentages and (D) wake bouts duration during the dark phase in mice after 4 weeks of SF and treated with either SOL, MOD or VEH for 6 days with recovery. Data presented as box plots with whiskers with min to max points (n=8–15/group). **p<0.01, ***p<0.001, ****p<0.0001. IH: intermittent hypoxia, RA: room air, SF: sleep fragmentation, SC: sleep control, MOD: modafinil, SOL: solriamfetol, VEH: vehicle.
Similar to IH, SF for 4 weeks induced increased sleep propensity before randomization to pharmacological treatments (Supplementary Fig. 2). Average percentage of sleep during the dark phase was significantly higher in VEH-treated mice with recovery after SF exposure (43±5%) when compared to VEH-treated SC controls (27±3%, p<0.0001). Treatments with both SOL (23±4%) and MOD (25±6%) combined with recovery significantly reduced the percentage of time spent in sleep in SF-exposed animals when compared to SC VEH treated mice (43±5%, p<0.0001) (Fig. 2C). Average wake bouts durations in VEH-treated mice after SF exposure with recovery decreased significantly (643±464s) when compared to SC VEH-treated group (1382±381s, p<0.0001). Both SOL (1386±387s) and MOD (1682±545s) significantly increased average wake bout durations when compared to VEH-treated mice (643±464s, p<0.0001) (Fig. 2D). All treatments in RA and SC controls did not induce any significant changes in wake bout durations.
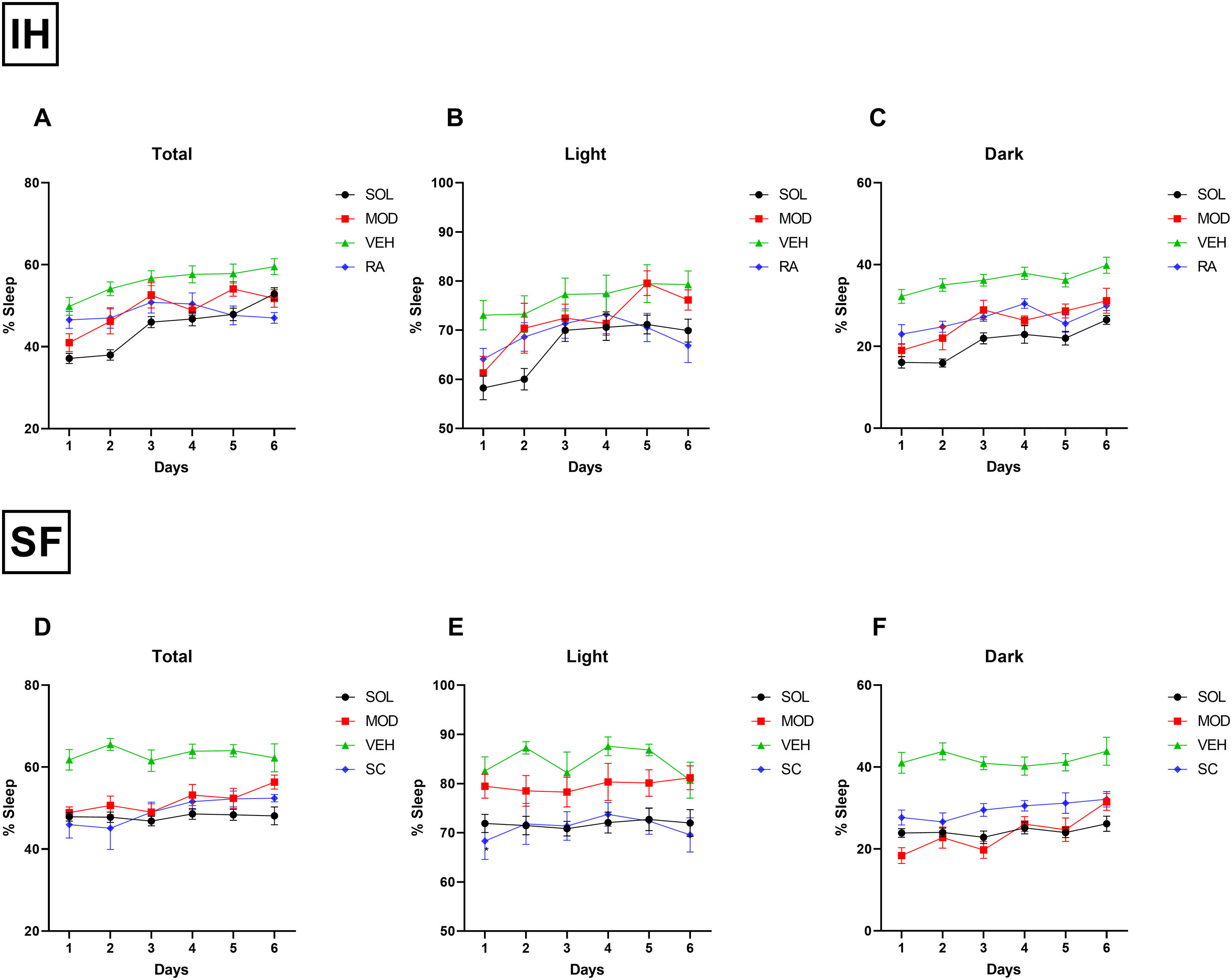
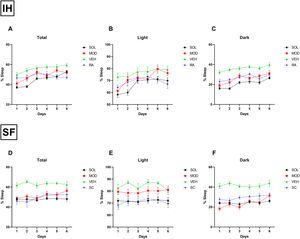
Daily total sleep percentage after IH cessation with SOL and MOD treatments for 6 days was nearly normalized to RA control levels when compared to recovery alone, with more pronounced reduction in total sleep time in mice treated with SOL (Fig. 3A). During the light phase, SOL and MOD treatments exhibited similar efficacies except on days 5 and 6 of treatments where MOD lost its efficacy (Fig. 3B). As described previously, both treatments significantly reduced time spent sleeping in the dark phase (Fig. 3C). After SF termination, daily total sleep percentage after SOL and MOD treatments with recovery returned to normal control levels when compared to recovery alone (Fig. 3D). However, only SOL exhibited enhanced efficacy in normalizing sleeping patterns during the light phase (Fig. 3E). Both drugs improved wakefulness during the dark phase when compared to recovery alone with VEH (Fig. 3F).
SOL or MOD combined with recovery improve sleep patterns in mice exposed to chronic IH and SF. (A) Total, (B) light phase, and (C) dark phase sleep percentages in mice after 16 weeks of IH exposure and treated with either SOL, MOD or VEH for 6 days with recovery. (D) Total, (E) light phase, and (F) dark phase sleep percentages in mice after 4 weeks of SF and treated with either SOL, MOD or VEH for 6 days with recovery. Data presented as mean±SEM. (n=8–15/group). IH: intermittent hypoxia, RA: room air, SF: sleep fragmentation, SC: sleep control, MOD: modafinil, SOL: solriamfetol, VEH: vehicle.
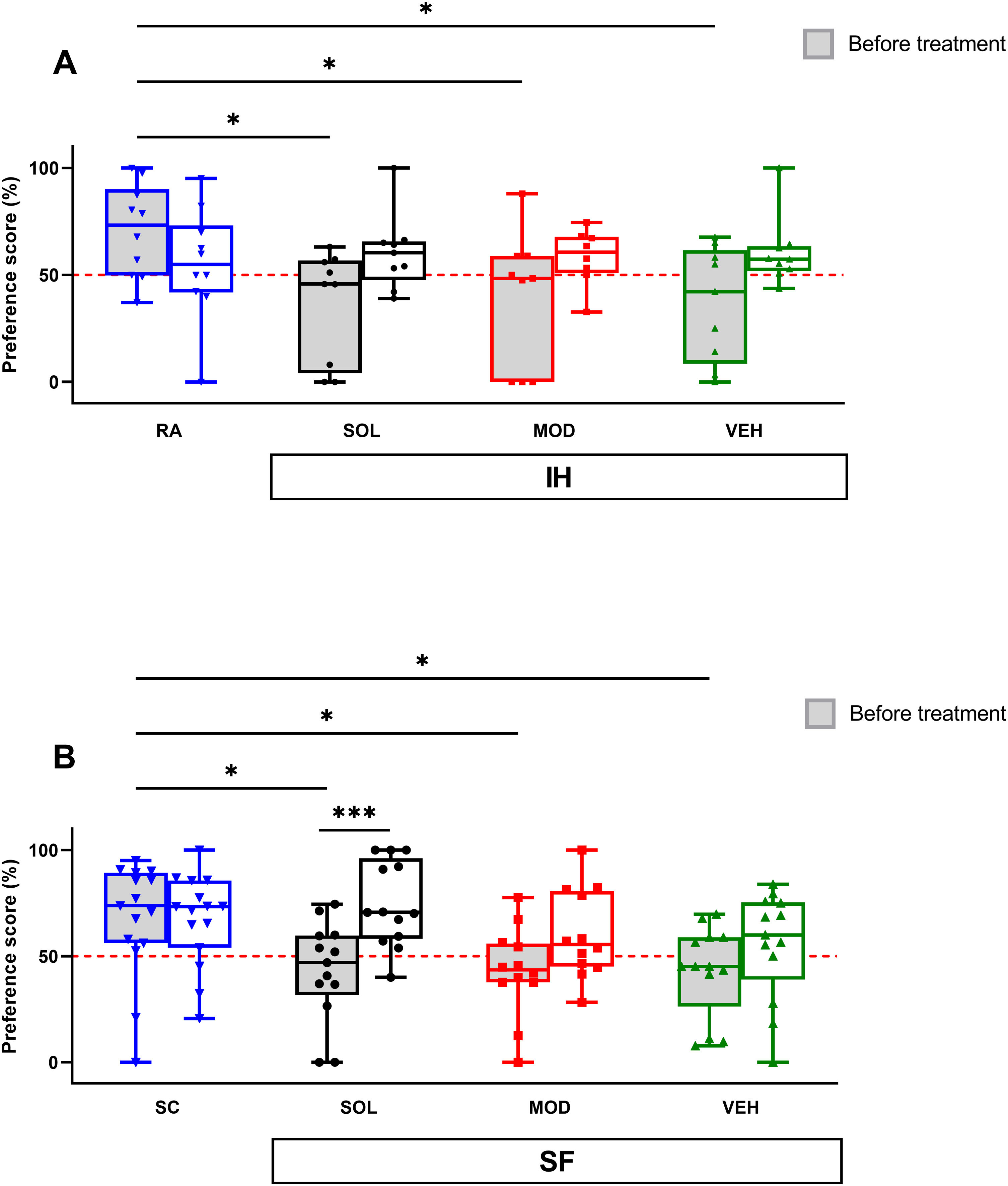
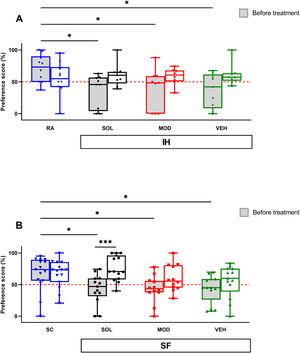
Before treatments, exposures to IH for 16 weeks significantly impaired the novel object recognition (NOR) preference scores when compared to RA mice (71±22% vs. VEH: 37±28, p<0.05; vs. MOD: 39±31%, p<0.05; vs. SOL: 36±25%, p<0.05) as previously documented.16,18 After IH cessation, the preference scores were increased, although not significantly, in all groups regardless of treatment (Fig. 4A). Similarly, before treatment, the NOR preference scores for all groups subjected to 4 weeks SF were significantly lower when compared to SC (67±27% vs. VEH: 43±21, p<0.05; vs. MOD: 43±21%, p<0.05; vs. SOL: 43±23% p<0.05) as previously shown.17,18 All preference scores were improved after the cessation of the SF exposures but only the SOL-treated mice with recovery had a significantly higher preference scores (74±19%) when compared to the same animal group before treatment (43±23%, p<0.001, Fig. 4B). All treatments in the SC or RA condition induced no modifications in the preference scores (data not shown).
SOL, but not MOD combined with recovery or recovery alone, improve NOR preference only in mice exposed to chronic SF. (A) Preference scores in NOR test in mice before and after 16 weeks of IH and treated with SOL, MOD, or VEH with recovery for 6 days. (B) Preference scores in mice before and after 4 weeks of SF and treated with SOL, MOD, or VEH with recovery for 6 days. Data presented as box plots with whiskers with min to max points (n=8–15/group). *p<0.05, ***p<0.001. IH: intermittent hypoxia, RA: room air, SF: sleep fragmentation, SC: sleep control, MOD: modafinil, SOL: solriamfetol, VEH: vehicle.
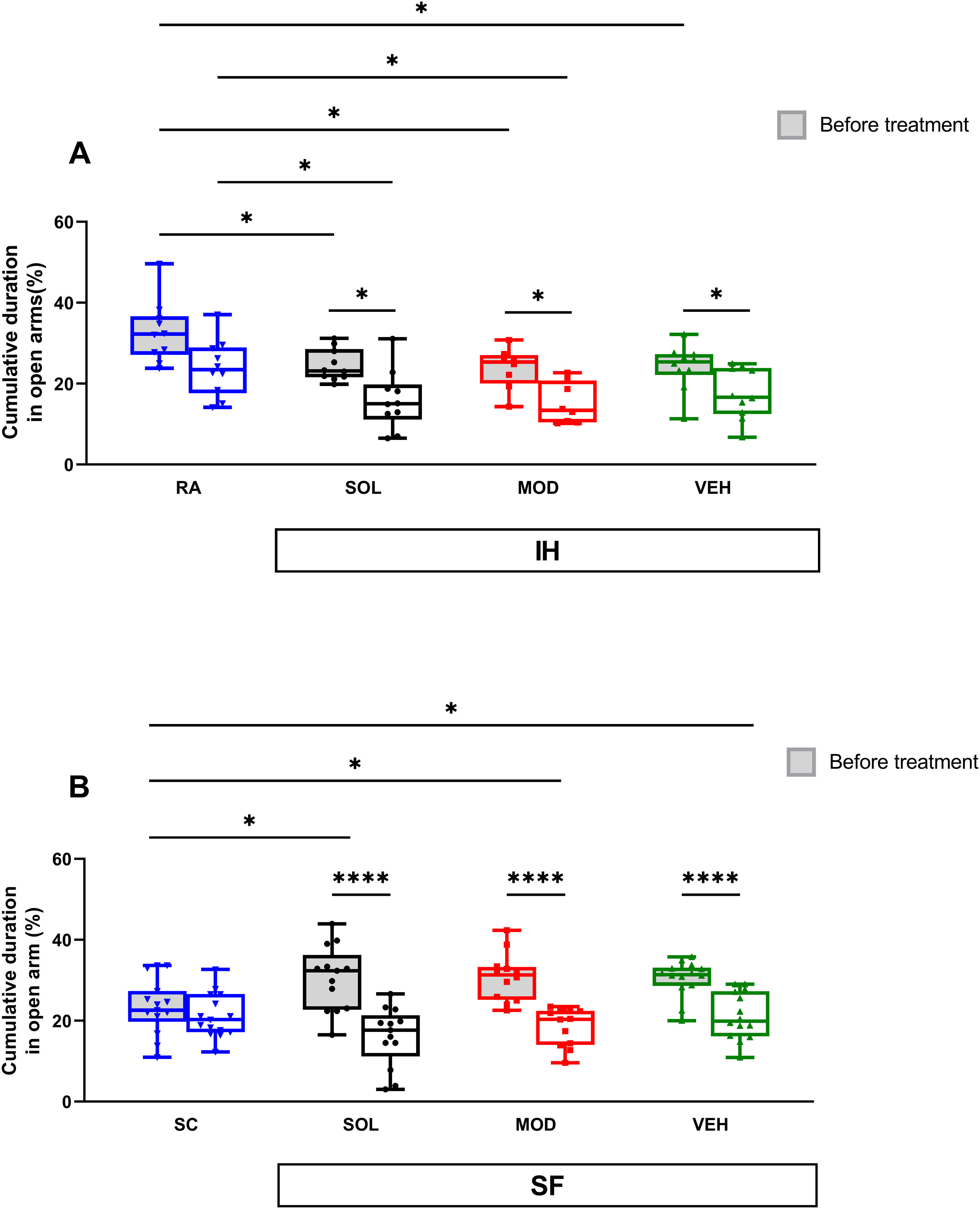
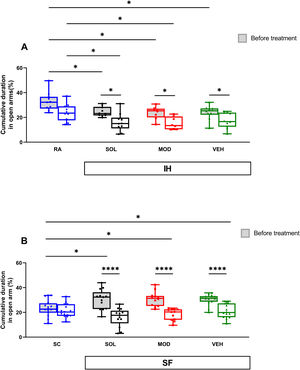
IH exposures for 16 weeks reduced time spent in open arms in the elevated plus maze test (EPMT) in all pretreatment groups when compared to RA controls (RA: 33±8% vs. VEH: 24±6%, p<0.05; MOD: 24±4, p<0.05; SOL: 25±4, p<0.05, Fig. 5A). After treatments with recovery, time spent in open arms significantly decreased in all IH exposed mice (p<0.05). The time spent in open arms was shorter than the RA group (24±7%) in both treatment SOL (16±7%, p<0.05) or MOD (15±5%, p<0.05, Fig. 5A). The time spent in open arms of the EPMT for all groups of mice exposed to SF was significantly higher when compared to SC (p<0.05, Fig. 5B). These results suggest an increase in the impulsivity of mice exposed to SF. After treatment and the cessation of the SF, all SF groups showed a significant decrease of the time spent in open arms (p<0.0001, Fig. 5B). All treatments in the SC or RA condition induced no modifications in the time spent in open arm (data not shown).
SOL and MOD combined with recovery or recovery alone promote anxiogenic effects in mice exposed to chronic IH and SF. (A) Time spent in open arms in mice before and after 16 weeks of IH and treated with SOL, MOD, or VEH with recovery for 6 days. (B) Time spent in open arm in mice before and after 4 weeks of SF and treated with SOL, MOD, or VEH with recovery for 6 days. Data presented as box plots with whiskers with min to max points (n=8–15/group). *p<0.05, ****p<0.0001. IH: intermittent hypoxia, RA: room air, SF: sleep fragmentation, SC: sleep control, MOD: modafinil, SOL: solriamfetol, VEH: vehicle.
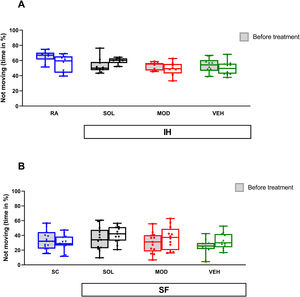
In the context of forced swim test (FST), IH exposure for 16 weeks was not associated with significant differences in the duration of immobility. No differences were detected in pre- and post-treatment responses (Fig. 6A). Similarly, neither SF exposures nor treatments with recovery were associated with significant differences in duration of immobility when compared to SC or to the pretreatment responses (Fig. 6B).
Neither IH nor SF induce depressive-like behavior in mice. (A) Time spent immobile in mice before and after 16 weeks of IH and treated with SOL, MOD, or VEH with recovery for 6 days. (B) Time spent immobile in mice before and after 4 weeks of SF and treated with SOL, MOD, or VEH with recovery for 6 days. Data presented as box plots with whiskers with min to max points (n=8–15/group). IH: intermittent hypoxia, RA: room air, SF: sleep fragmentation, SC: sleep control, MOD: modafinil, SOL: solriamfetol, VEH: vehicle.
This study illustrates a comprehensive set of experiments designed to explore residual EDS in murine models of OSA and to compare two commonly used FDA approved wake-promoting agents on sleep and behavioral functions in mice exposed to IH and SF,16,17 the two major hallmark manifestations of OSA, with cessation of IH and SF (mimicking “ideal” CPAP adherence), thus addressing a frequently encountered clinical situation. Firstly, the findings of this study corroborate the previous published findings showing that IH and SF can increase sleep propensity in the dark phase (EDS) and will generate impaired cognitive function while also modulating anxiety-like behaviors.16,17 Importantly, even cessation of sleep perturbations or of gas exchange abnormalities as achieved by discontinuation of the experimental exposures did not result in the disappearance of the excessive sleep propensity even after 6 days. Secondly, both treatments combined with recovery improved wakefulness in the dark phase after chronic SF and IH exposures, while recovery alone along with vehicle injections was not sufficient to improve wakefulness after either one of the two exposures. Moreover, SOL treatment was more efficacious in normalized sleeping patterns compared to MOD, especially in SF. Thirdly, improvements in cognition were only significantly apparent when SOL was combined with recovery in the SF group.
EDS is a prominent and frequently reported symptom in individuals with OSA.19 EDS in OSA is associated with neurocognitive disturbances and has profound overall health implications, social functioning, and economic productivity. Numerous research studies have documented the detrimental effects of EDS on quality of life, work performance, and driving safety among OSA patients.20 By restoring normal breathing patterns, CPAP therapy significantly improves sleep quality.21 As a result, EDS, which is commonly associated with OSA, is alleviated in many patients. Despite its proven effectiveness, some patients may struggle with CPAP adherence due to discomfort, mask-related issues, or other factors.22 Furthermore, some patients continue to experience residual EDS despite adherent treatment. Consequently, there is a growing need for adjunct interventions that not only target EDS, but also improve cognitive function and daily performance. This necessity is further compounded by the rising prevalence of OSA, particularly among the elderly population at risk of cognitive decline.23 As a result, wake-promoting agents have emerged as essential adjuvant interventions aimed at alleviating OSA-related EDS.14,24
IH and SF are widely employed models recapitulating two of the most prominent characteristics of OSA.25 We have previously documented that both perturbations increase sleep propensity in mice,16–18 a finding that is in accordance with multiple published studies using a variety of exposure paradigms.26,27 For instance, research by Veasey et al. showed that IH exposures shortened sleep latencies and increased total sleep time in mice.27 Similarly, our studies replicated these findings with increased sleep percentages and shorter wake bout lengths following IH exposures. Building upon this evidence, our current findings indicate that both SOL and MOD effectively enhance wakefulness in animals exposed to chronic IH and SF when combined with recovery. However, we found that recovery alone did not alleviate the increased sleep propensity even after 6 successive days of recovery. These findings align with previous studies that revealed that IH exposures are associated with reduced wake times and shorter sleep latencies compared to controls, persisting even after 6 months of normoxic recovery.28 Similarly, acute SF (4 days) resulted in a sustained sleep debt.29 Studies conducted in humans have also shown that CPAP treatment is not entirely effective in addressing EDS.6,30 Notably, while both SOL and MOD demonstrated effectiveness in mitigating EDS when combined with recovery, the differential impact was more pronounced with SOL, especially following SF, suggesting its potential for greater improvement in wakefulness and reduction of sleepiness. This observation has been supported by systematic reviews of extant clinical studies.14,31 Recently, two meta-analyses systematically reviewed multiple clinical trials evaluating the effects of SOL and MOD in patients with OSA and EDS.24,32 The analysis confirmed the overall efficacy of both medications in reducing EDS symptoms. However, the meta-analyses also highlighted that SOL exhibited superior effectiveness compared to MOD in improving wakefulness and reducing sleepiness scores across various study populations.32 Collectively, these data support the rationale for combining wake-promoting agents with CPAP in OSA to maximize the extinction of EDS and ultimately enhance the quality of life for patients.
OSA is associated with an increased risk of cognitive impairments, including problems with memory, attention, executive function, and overall cognitive performance.23 With the world's population steadily aging, the prevalence of OSA is likely to increase, as is the susceptibility to cognitive decline.33 Both IH and SF can induce cognitive dysfunction but present some differences and such differences are recapitulated in the present study.16–18 Numerous publications have shown improvements in quality of life and attention in patients with OSA or those with residual EDS when treated with wake-promoting agents.14,34 To evaluate explicit memory, we selected the NOR test as previously published.35 IH for 16 weeks and SF for 4 weeks decreased preference scores as expected. After the cessation of the SF or IH exposures, all mice had an improvement in their preference scores whatever the pharmacological treatment used. However, only SOL treatment enhanced preference significantly with recovery from SF. This finding may indicate that SOL can facilitate the recovery to control conditions, i.e., may enhance restitution of functional circuits involved in declarative memory. This supposition is further reinforced by our previous studies showing that under SF or IH exposures, only SOL treatment was able to induce a recovery in terms of explicit memory.16,17
Anxiety and depression are highly prevalent in patients with OSA.36 In this study, we used the EPMT to appraise anxiety in mice. Fearful anxious mice would tend to spend less time in the open arms of EPMT, and in contrast, mice with high impulsivity and inattention would spend more time in the open areas.18 SF and IH have opposite effects, as previously described.18 IH exposure induces an increase in anxiety whereas SF leads to higher impulsivity. However, for all treatments and exposures, repetition of the test leads to a decrease in the time spent in the open arms of the EMPT. This result could be associated with the generation of familiarity with the test.17,18 No differences appeared between pharmacologic treatment with recovery when compared to recovery alone. SOL and MOD has heterogeneous effects on anxiety in humans and anxiety-like behaviors in animal models.34,37 In the context of depression, we used the FST based on the natural tendency of mice to try to escape from water. Neither SF exposures nor pharmacological treatments were associated with significant differences in either duration of immobility when compared to SC or VEH treatments with recovery. The absence of the effect of SF on the depressive like behavior is consistent with the literature and our previous fundings.16,18,38 However, we should also point out that these results differ from those reported in other studies, likely reflecting different methodologies and experimental models.25,39 After the IH exposure, the time spent immobile was decreased in comparison to RA animals, but no differences emerged with SOL, MOD, or VEH administration. A few studies have assessed the FST effects after treatment with MOD and IH exposure in mice, but the results are inconclusive.40
Several limitations are worthy of mention. Firstly, we performed one test for each behavioral functional assessment rather than conduct a more extensive battery of tests aimed at exploring multiple cognitive and behavioral functions. Expansion of the battery of tests in future studies should allow for more comprehensive evaluation of behavioral outcomes in the preclinical models of OSA. Secondly, treatments and recovery periods after IH and SF cessation were short and prolonging those periods may provide a better insight into the effects of “ideal” CPAP adherence and wake-promoting agents on OSA-induced EDS in the immediate, mid-term and long-term. Thirdly, the study exclusively used male mice. Thus, the effects of sex, age, and obesity on IH- or SF-induced EDS and the effects of SOL and MOD under such constraints remain to be explored.
ConclusionsChronic IH and SF, two of the major perturbations induced by OSA, induce EDS and cognitive deficits in young male mice. Short-term recovery mimicking “ideal” CPAP adherence in OSA patients is insufficient to promote normal wakefulness patterns or achieve improvements in cognition, unless combined with wake-promoting agents. However, SOL displays superior efficacy in mitigating sleepiness and enhancing performance in the NOR test when compared to MOD. Thus, adding SOL as an adjuvant therapy to CPAP in OSA patients may not just alleviate residual EDS, but also improve aspects of cognitive function and overall quality of life.
Author's contributionConceptualization, D.G.; IH and SF exposures, M.B., C.P.; Sleep recording, M.B., C.P.; Behavioral tests, C.P., M.B.B., A.R.; Treatments, M.B.; Data analysis, M.B., C.P.; Manuscript writing, M.B., C.P., D.G.; All authors read and approved the final version of the manuscript.
Data sharing statementThe datasets generated in this study are available from the corresponding author upon reasonable request.
FundingInvestigator-initiated grant from Jazz Pharmaceuticals, and NIH grant AG061824.
Conflict of interestsThe sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. The authors declare no competing interests.