To describe an evidence- and experience-based expert consensus on the most relevant issues of patients with COPD exacerbations.
MethodsThe Delphi technique was used. Evidence was reviewed by a scientific committee and 60 experts. A questionnaire was prepared containing 3 sections: diagnosis of the exacerbator; treatment, and healthcare processes. The survey was answered in 2 rounds by 60 pneumologists on an online platform. Statements were scored on a Likert scale from 1 (total disagreement) to 9 (total agreement). Agreement and disagreement were defined as a score of 7–9 or 1–3, respectively, given by more than two thirds of the participants.
ResultsA total of 48 statements were included, one of which was added in the second round. Consensus was reached in 37 items (78.7%) after the first round (agreement), and in 43 (89.5%) after the second round (42 agreement, 1 disagreement). The statements with the highest proportion of experts agreeing were as follows: in exacerbators, chronic bronchial infection favors lung function decline (93.1%); long-acting bronchodilators should not be withdrawn (93.1%); treatment must be personalized if new exacerbations occur despite optimal bronchodilator treatment (96.6%); management must be coordinated between primary care and the respiratory medicine department (93.1%), and patients must be followed up in specific integrated multicomponent programs (94.8%).
ConclusionsThe findings of this study could assist in the diagnosis and treatment of COPD exacerbators in our area.
Describir un acuerdo entre expertos basado en la evidencia y la experiencia sobre los aspectos más relevantes del paciente exacerbador con EPOC.
MétodosSe siguió la metodología Delphi. Tras revisar la evidencia por un comité científico y 60 expertos, se elaboró un cuestionario con 3 apartados: diagnóstico del paciente exacerbador; tratamiento, y proceso asistencial. La encuesta fue respondida online en 2 rondas por 60 neumólogos. El grado de acuerdo siguió la escala Likert de 1 (total desacuerdo) a 9 (total acuerdo), definiéndose acuerdo y desacuerdo como una puntuación de 7-9 o 1-3, respectivamente, otorgada por más de dos tercios de los participantes.
ResultadosSe incluyeron un total de 48 aseveraciones, una añadida en la segunda ronda. Hubo consenso en 37 (78,7%) tras la primera ronda (acuerdo), y en 43 (89,5%) tras la segunda (42 acuerdo y 1 desacuerdo). Las afirmaciones con mayor proporción de expertos en el rango de acuerdo se refirieron a que, en el paciente exacerbador, la infección bronquial crónica favorece el deterioro de la función pulmonar (93,1%), a que no se deben retirar los broncodilatadores de larga duración (93,1%), a la conveniencia de personalizar el tratamiento si se dan nuevas exacerbaciones pese a un tratamiento broncodilatador óptimo (96,6%), o al cuidado y manejo de este paciente, que debe ser coordinado entre atención primaria y neumología (93,1%) y controlado en programas integrados específicos multicomponente (94,8%).
ConclusionesLa información proporcionada por este consenso puede facilitar el diagnóstico y tratamiento del paciente exacerbador con EPOC en nuestro ámbito.
Chronic obstructive pulmonary disease (COPD) is a highly prevalent pathology that places a heavy burden on both the patient and the health and social welfare system.1–3 In Spain, a prevalence of 10.2% has been reported in the population aged 40–80 years.4,5 Patients frequently suffer exacerbations during the clinical course of the disease. These episodes are characterized by an acute worsening of symptoms, and contribute decisively to a deterioration of health status, affect disease progression and control. They increase the risk of death and generate a strong demand for care, and their socio-economic impact is considerable.6–10 In a cost-effectiveness study conducted in Belgium and the Netherlands, an average cost of €4007, €579 and €86 was calculated for severe, moderate, and mild exacerbations, respectively.10
In clinical trials, it is common for patients to have between 1 and 3 exacerbations per year.5 However, the frequency of exacerbations varies dramatically from 1 patient to another: while some individuals scarcely experience any exacerbations, others present them frequently. Patients with frequent exacerbations, commonly known as “exacerbators”, are generally associated with worse health-related quality of life, greater lung function decline, worse prognosis, and greater consumption of resources.7,9,11,12 The total costs of COPD were more than double in patients with at least 3 exacerbations compared with none, while direct costs multiplied 7-fold.12
Despite the remarkable importance of this special subgroup of patients, some confusion surrounds the concept of COPD exacerbator. The Spanish COPD Guidelines (GesEPOC)13 were one of the first clinical practice guidelines (CPG) to recognize the importance of this group of patients and to propose the term “exacerbator phenotype” for patients who have had 2 or more exacerbations of at least moderate severity in the last year. However, this definition, based on previous proposals in the literature,14,15 contains some ambiguity regarding the number of events required, the intensity of the episodes, and even the concept of exacerbation. The same could be said of some controversies surrounding possible exacerbator subtypes, how to define the type of exacerbation, how to monitor these patients, the specific treatments to be used, or how to organize the care process. The main CPGs barely touch on these aspects or only do so tangentially,16–18 not least because the available evidence in this respect is scarce and inaccurate. In this setting, the consensus opinion of a group of experts can be helpful to promote healthcare changes which should subsequently be endorsed by scientific evidence.
The objective of this study was to develop a consensus document addressing ambiguities in the diagnosis, treatment, and care process of patients with exacerbations.
MethodsThis document was prepared using the Delphi method and nominal group methodology19 among a wide group of experts who regularly contribute to the EXPERT project. The initiative was sponsored by Boehringer Ingelheim Spain, which has been bringing together a group of Spanish experts in COPD every year since 2014 to discuss various aspects related to the disease. The project has a Scientific Committee formed of 7 pulmonologists (a national coordinator and 6 members) selected by the sponsor on the basis of their experience, scientific insight, and professional recognition.
ParticipantsA group of 60 leading Spanish pulmonologists specializing in COPD were invited by the scientific committee to participate in this study. All are members of the COPD working group of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR), and were initially proposed on the basis of their experience, publications in recent years, participation in research projects on the subject, and geographic diversity.
Experts were defined as professionals seeing more than 100 patients with COPD/year or who had published more than 1 article in the previous year or had made more than 2 communications to congresses on COPD in the previous year. The selection of participants was not random and was conducted mainly in response to criteria of availability and willingness to participate.
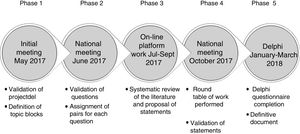
Stages in Consensus DevelopmentThe statements included in the Delphi questionnaire were derived from a series of questions that were investigated in a literature review. The results of the review were shared among the experts and debated. The steps followed are set out in detail below, in Fig. 1.
Phase 1. Initial Meeting of the Scientific CommitteeIn this meeting, the study objective, the characteristics of the participants, and the different topic areas of the document were defined. Each member of the Scientific Committee was assigned a topic block and developed a proposal for an initial series of questions that would be studied in subsequent phases (Table 1).
Questions on COPD Exacerbators That Were Analyzed in the Systematic Literature Review.
| Topic Block | Questions |
|---|---|
| What?(concept) | What is an exacerbator?What defines the severity of the exacerbator?What are the exacerbator phenotypes?What are the exacerbation patterns of each COPD phenotype?What is used to define the etiology of an exacerbation? |
| When?(time, progress) | When is worsening is not really an exacerbation?When is bronchodilator treatment sufficient?When should the treatment of the exacerbator be changed?When should an exacerbator be offered intervention?When should specific care programs be implemented? |
| Where?(department/treating physician) | Where should the exacerbator be monitored in a respiratory medicine department?Must an exacerbator attending the emergency department be admitted for a new exacerbation?Is a home care program (telemedicine) efficient for exacerbators?Is an integrated (coordinated) care program efficient for exacerbators?Where should exacerbators be followed up? |
| Why?(consequences) | Why is the risk of death higher in exacerbators?Why do exacerbators have such an impact on health resources?Why is lung function decline observed?Why do exacerbators show a deterioration in quality of life?Why does bronchodilator therapy impact on exacerbations? |
| With what?(treatment) | What criteria do we use to administer inhaled corticosteroids in the treatment of exacerbators?What criteria do we use to add oral drugs in the treatment of exacerbators?What criteria do we use to reduce treatment in exacerbators?What criteria do we use to administer dual bronchodilation to exacerbators?What criteria do we use to administer single-agent bronchodilators? |
| How?(management) | How can we promptly identify a potential exacerbator?How can we prescribe a respiratory rehabilitation program to an exacerbator?Do we always prevent exacerbations in the same way?How can we identify a non-infectious inflammatory exacerbation?How should the exacerbator be followed up? |
The 60 expert pulmonologists were divided in 6 groups corresponding to the topic areas (see Annex 1). Each group selected by vote the 5 questions they considered most relevant from the list of questions proposed by the Committee members. These were distributed among the experts, who were then asked to perform a literature review in order to draw conclusions which would serve as the basis for the Delphi questionnaire. The review was not systematic, but instead was performed at the discretion of each expert. Five questions were chosen, so that each question would be addressed simultaneously by 2 of the 10 expert members of each group.
Phase 3. Online Platform for Content DevelopmentThe experts uploaded a summary of the scientific review to an online platform, in which they included the original articles, the main clinical practice guidelines and available consensus documents. They used their conclusions to draw up a series of statements that would form the basis for the subsequent Phase 4 discussion.
Phase 4. National Meeting to Share the Results of the Review and Agree Upon the Final StatementsAt this meeting, which was attended by all project participants, conclusions on the review of the evidence were shared and the final wording of the statements was agreed. This consensus was originally reached at a group level (with members of each group) and subsequently discussed in a plenary meeting of all 60 experts.
Phase 5. Delphi SurveyThe scientific committee worked with an external methodological consultant to develop the Delphi questionnaire. The list of items agreed upon by the group underwent a process of selection, review and, where appropriate, adaptation, to achieve a version that was satisfactory to all members. The statements were distributed in 3 blocks: (1) diagnosis of the exacerbator; (2) treatment of the exacerbator, and (3) the care process.
The questionnaire was completed anonymously on the platform during the first quarter of 2018. The questions for which consensus was not reached in the first round of voting underwent a second round. Respondents had to score their degree of agreement with the statement on a 1–9 Likert scale, in such a way that 1 represented the greatest disagreement with the wording, moving progressively to 9, which represented the greatest agreement. Agreement on the question was consensual when the score of the responses was 7–9, and disagreement was consensual when it was 1–3. The remaining votes were classified as “indeterminate”. Consensus was reached when at least two thirds of the responses were in the median range.
Statistical AnalysisMedian values and the first and third percentiles for each of the questionnaire items were calculated as measures of central tendency and dispersion.
The internal consistency of the questionnaire was determined with the Cronbach's alpha statistic, values above 0.75 being considered acceptable. The correlation coefficient (ri) was calculated as a measure of reliability, and was considered good when values were between 0.4 and 0.75, and excellent above 0.75. Both values were calculated for the total questionnaire and for each of the blocks (Annex 2).
The correlation between rounds was measured using the Spearman correlation coefficient, in blocks and for the overall questionnaire (Annex 3). Similarly, concordance between rounds for each question was analyzed qualitatively using the kappa index, pooling the scores in 3 groups.1–9 Concordance was considered to be moderate when the score was between 0.41 and 0.60, good between 0.61 and 0.80, and very good for values above 0.80. The coefficient of variation (CV) was calculated for each questionnaire and in each round. The criterion for not needing consecutive rounds was that the relative increase from the previous round [(current CVround−previous CVround)/previous CVround] did not exceed 10%.
The level of statistical significance for all the estimators was established at P<0.05. Analyses were performed using SPSS software v24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.)
ResultsAll 60 experts were involved in both question rounds. The survey population was composed of 62% men, with an average age of 48±9 years and 21±9 years of experience in COPD, who saw an average of 30±15 COPD patients a month.
The internal consistency of the questionnaire was high in each of the blocks and very high overall (Annex 2). Spearman correlation coefficient values were high in all cases. Kappa index values indicated a high qualitative concordance in all cases (Annex 3). Finally, the delta CV of both rounds was determined. This was 0.23±0.10±0.07 and 0.25 in the first and second rounds, respectively, which meant a gross increase of 2% and relative increase of 8.7%. As this was less than 10%, it was established that there was no significant variability between the two rounds, so a third round was not necessary.
Table 2 shows the analysis of the responses after two rounds. In the first, the experts responded to 47 statements (23 in the first block, 13 in the second, and 11 in the third). The response rate was 100%, and 96.7% valid answers were received. There was a consensus agreement in 37 statements. Ten questions for which disagreement or indetermination had been detected passed into the second round, one of which was divided into 2 questions, so that the final questionnaire consisted of 48 questions. In this second round there was consensus regarding 6 statements (5 in agreement and 1 in disagreement). Therefore, consensus was reached for 43 (42 agreement and 1 disagreement) of the total 48 statements.
Results of the Delphi Survey.
| Statement | Experts in Range of Agreement, n (%) | Experts in Range of Disagreement, n (%) | Result | |
|---|---|---|---|---|
| Block 1. Definition of exacerbators | ||||
| P1 | For a patient to be considered an exacerbator, they must have had at least 2 moderate exacerbations in the previous year, defined as requiring at least outpatient treatment with systemic corticosteroids and/or antibiotics | 51 (87.9) | 2 (3.4) | Consensus/agreement(1st round) |
| P2 | To be considered an exacerbator, the patient must have had 1 severe exacerbation requiring hospital admission in the previous year | 44 (75.9) | 7 (12.1) | Consensus/agreement(1st round) |
| P3 | A patient who has at least 3 mild exacerbations per year (change in the intensity of symptoms, change of treatment without the need for systemic corticosteroids and/or antibiotics, without being admitted to hospital or treated in the emergency department) should be considered an exacerbator. | 10 (16.7) | 34 (56.7) | No consensus/disagreement(2nd round) |
| P4 | A severe exacerbator is defined as one that has many symptoms (mMRC≥2) | 2 (3.3) | 51 (85.0) | No consensus/disagreement(2nd round) |
| P5 | A patient with 2 or more severe exacerbations in the previous year is defined as a “severe exacerbator” | 53 (91.4) | 1 (1.7) | Consensus/agreement(1st round) |
| P6 | A patient with potentially pathogenic microorganisms isolated in the airway, bronchiectasis, or cardiovascular comorbidities should be considered a potential exacerbator | 41 (70.7) | 4 (6.9) | Consensus/agreement(1st round) |
| P7 | There are 4 types of exacerbators: infectious, inflammatory, comorbid, and mechanical | 50 (83.3) | 1 (1.7) | Consensus/agreement(2nd round) |
| P8 | Patients with COPD-asthma phenotype (ACO) have mostly eosinophilic exacerbations and respond better to steroids than other phenotypes | 51 (87.9) | 1 (1.7) | Consensus/agreement(1st round) |
| P9 | In patients with emphysema, exacerbations have, above all, a mechanical or pauci-inflammatory component | 44 (75.9) | 5 (8.6) | Consensus/agreement(1st round) |
| P10 | The same agent (viruses, bacteria, etc.) can trigger different types of exacerbations depending on the patient's exacerbator phenotype | 75 (75.0) | 0 (0) | Consensus/agreement(2nd round) |
| P11 | A study of cellularity (eosinophilia) and microorganisms in respiratory samples is needed to characterize some exacerbations | 53 (88.3) | 1 (1.7) | Consensus/agreement(2nd round) |
| P12 | Airway and pulmonary parenchymal lesions detected in imaging tests (mainly CT) are useful for identifying exacerbation patterns in COPD and for the differential diagnosis of other entities. | 41 (70.7) | 4 (6.9) | Consensus/agreement(1st round) |
| P13 | The risk of death increases in exacerbators due to a persistent systemic inflammatory response that causes an increase in cardiovascular events during and after the exacerbation | 49 (84.5) | 3 (5.2) | Consensus/agreement(1st round) |
| P14 | Mucus hypersecretion aggravates lung function decline in exacerbator patients | 44 (75.9) | 3 (5.2) | Consensus/agreement(1st round) |
| P15 | Airway inflammation aggravates lung function decline in exacerbator patients | 52 (89.7) | 1 (1.7) | Consensus/agreement(1st round) |
| P16 | Chronic bronchial infection aggravates lung function decline in exacerbator patients | 54 (93.1) | 1 (1.7) | Consensus/agreement(1st round) |
| P17 | The severity of obstruction is a predictive factor in an exacerbator | 48 (80.0) | 3 (5.0) | Consensus/agreement(2nd round) |
| P18 | Symptoms are a predictive factor in an exacerbator | 41 (70.7) | 4 (6.9) | Consensus/agreement(1st round) |
| P19 | Comorbidities are a predictive factor for an exacerbator | 47 (81.0) | 2 (3.4) | Consensus/agreement(1st round) |
| P20a | Peripheral blood eosinophilia>2% is a marker of non-infectious inflammatory exacerbations. | 2 (3.3)13 (21.7) | 19 (31.7)10 (6.7) | No consensus (limit)/indeterminate 2nd round |
| P21 | Worsening of clinical symptoms of COPD is diagnosed when exacerbation is ruled out and there are no data suggesting decompensation of any of the patient's comorbidities | 48 (82.8) | 2 (3.4) | Consensus/agreement(1st round) |
| P22 | Intervention must be made in an exacerbator when a high risk of exacerbations or hospital admission is identified, and during periods of increased environmental pollution, in winter, or if there is occupational exposure or a persistent smoking habit | 50 (86.2) | 2 (3.4) | Consensus/agreement(1st round) |
| P23 | HRCT must be performed during the follow-up of exacerbators | 49 (84.5) | 3 (5.2) | Consensus/agreement(1st round) |
| Block 2. Treatment of exacerbators | ||||
| P1 | Long-acting bronchodilators are the main pharmacological treatment to prevent exacerbations in patients with no criteria for ACO | 50 (86.2) | 1 (1.7) | Consensus/agreement(1st round) |
| P2 | Dual bronchodilation must be considered the treatment of choice in COPD exacerbator, except in those with ACO | 47 (81.0) | 3 (5.2) | Consensus/agreement(1st round) |
| P3 | Long-acting bronchodilators must not be withdrawn in COPD exacerbators | 54 (93.1) | 2 (3.4) | Consensus/agreement(1st round) |
| P4 | Patients who continue to have exacerbations despite optimal bronchodilator therapy should be evaluated for other treatable traits, in order to offer a more personalized management | 56 (96.6) | 1 (1.7) | Consensus/agreement(1st round) |
| P5 | Bronchodilator therapy impacts on exacerbations, acting on lung function by reducing hyperinsufflation | 53 (91.4) | 1 (1.7) | Consensus/agreement(1st round) |
| P6 | Bronchodilator therapy impacts on exacerbations by acting on mucus production and mucociliary clearance | 45 (77.6) | 1 (1.7) | Consensus/agreement(1st round) |
| P7 | Bronchodilator therapy is sufficient in low-risk symptomatic patients and in former exacerbators whose exacerbations are well controlled. | 52 (89.7) | 2 (3.4) | Consensus/agreement(1st round) |
| P8 | The use of single-agent bronchodilators is indicated in COPD patients with a maximum of 2 outpatient exacerbations and mild or moderate obstruction. | 42 (70.0) | 8 (13.3) | Consensus/agreement(2nd round) |
| P9 | The use of inhaled corticosteroids (ICS) is recommended to prevent exacerbations in patients who continue to suffer exacerbations despite dual bronchodilation | 52 (89.7) | 1 (1.7) | Consensus/agreement(1st round) |
| P10 | ICS can be withdrawn in patients who are stable for at least 1 year, with no severe obstruction and eosinophilia <300c/mm3, provided dual bronchodilation is maintained. | 46 (79.3) | 3 (5.2) | Consensus/agreement(1st round) |
| P11 | De-escalation is a form of treatment optimization | 48 (82.8) | 3 (5.2) | Consensus/agreement(1st round) |
| P12 | The core treatment of exacerbators should include antimuscarinics. Non-steroidal anti-inflammatory drugs and/or mucolytics can be combined depending on phenotype, tolerance, and clinical response | 51 (87.9) | 2 (3.4) | Consensus/agreement(1st round) |
| P13 | Oral drugs in COPD exacerbators should only be used after inhaled triple therapy. | 40 (66.7) | 11 (18.3) | Consensus (limit)/agreement(2nd round) |
| Block 3. Care process | ||||
| P1 | The implementation of specific care programs will depend on their availability and the extent to which they can be adapted to different healthcare systems. | 51 (87.9) | 1 (1.7) | Consensus/agreement(1st round) |
| P2 | Integrated healthcare programs for exacerbators are efficient, in that they improve the health-related quality of life, and reduce exacerbations, hospitalizations, and costs | 49 (84.5) | 1 (1.7) | Consensus/agreement(1st round) |
| P3 | Despite limited evidence, healthcare programs have been shown to improve health outcomes in severe exacerbators | 49 (84.5) | 3 (5.2) | Consensus/agreement(1st round) |
| P4 | The development of multidimensional personalized self-management programs optimizes the home care of exacerbators | 44 (75.9) | 1 (1.7) | Consensus/agreement(1st round) |
| P5 | The inclusion of mobile technology and telemedicine in self-management programs optimizes the treatment of patients receptive to the use of technology | 41 (70.7) | 5 (8.6) | Consensus/agreement(1st round) |
| P6 | As a general rule, exacerbators must be promptly included in a respiratory rehabilitation program | 50 (86.2) | 2 (3.4) | Consensus/agreement(1st round) |
| P7 | Exacerbation in a severe exacerbator is a criterion for admission, as it is an indirect marker of disease severity | 34 (56.7) | 14 (23.3) | No consensus/agreement(2nd round) |
| P8 | The care and management of the exacerbator must be coordinated within the framework of an integrated primary care/pulmonology care program | 54 (93.1) | 2 (2.4) | Consensus/agreement(1st round) |
| P9 | Exacerbators should always be monitored by the respiratory medicine department in specific integrated multicomponent programs | 55 (94.8) | 1 (1.7) | Consensus/agreement(1st round) |
| P10 | Home care with the support of other available resources (dedicated clinics, day hospital, telemedicine, etc.) is essential for exacerbators | 51 (87.9) | 3 (5.2) | Consensus/agreement(1st round) |
| P11 | As a general rule, exacerbators should be followed up in specialized outpatient clinics | 53 (91.5) | 0 | Consensus/agreement(1st round) |
Divided into 2 questions in the second round.
The number and percentage of experts in the range of agreement/disagreement are shown. Agreement on the question was consensual when the score of the responses was 7–9, and disagreement was consensual when it was 1–3. The remaining votes were classified as “indeterminate”. Consensus was considered when at least two thirds of the responses were in the median range.
The statements that achieved more than 85% consensus among experts within the median range of agreement/disagreement and those that the Committee considered relevant are highlighted below, by topic block.
Diagnosis of Exacerbators- •
For a patient to be considered an exacerbator, they must have had at least 2 moderate exacerbations in the previous year, defined as requiring at least outpatient treatment with systemic corticosteroids and/or antibiotics (87.9%, agreement).
- •
A patient with 2 or more severe exacerbations in the last year is defined as a “severe exacerbator” (91.4%, agreement).
- •
Patients with COPD-asthma phenotype (asthma-COPD overlap [ACO]) have mostly eosinophilic exacerbations and respond better to steroids than other phenotypes (87.9%, agreement).
- •
A study of cellularity (eosinophilia) and microorganisms in respiratory samples is needed to characterize some exacerbations (88.3%, agreement).
- •
Chronic bronchial infection aggravates lung function decline in exacerbator patients (93.1%, agreement).
- •
Long-acting bronchodilators must not be withdrawn in COPD exacerbators (93.1%, agreement).
- •
Patients who continue to have exacerbations despite optimal bronchodilator therapy should be evaluated for other treatable traits, in order to offer more personalized management (96.6%, agreement).
- •
Bronchodilator therapy impacts on exacerbations, acting on lung function by reducing hyperinsufflation (91.4%, agreement).
- •
The care and management of the exacerbator patient must be coordinated within the framework of an integrated primary care/pulmonology care program (93.1%, agreement).
- •
Exacerbators should always be monitored by the respiratory medicine department in specific integrated multicomponent programs (94.8%, agreement).
- •
As a general rule, exacerbators should be followed up in specialized outpatient clinics (91.5%, agreement).
In contrast, the panel of experts failed to reach consensus on the following statements:
- •
A patient who has at least 3 mild exacerbations per year (change in the intensity of symptoms, change of treatment without the need for systemic corticosteroids and/or antibiotics, without being admitted to hospital or treated in the emergency department) should be considered an exacerbator.
- •
Peripheral blood eosinophilia>2% is a marker of non-infectious inflammatory exacerbations.
- •
Oral drugs in COPD exacerbators should only be used after inhaled triple therapy.
- •
Exacerbation in a severe exacerbator is a criterion for admission, as it is an indirect marker of disease severity.
Consensus documents aim to offer expert opinion in areas of uncertainty, where there is insufficient evidence or controversy. This document focuses on the issue of exacerbators, where there are multiple areas of uncertainty. The most important of the recommendations include the novel proposal for defining severe exacerbators and the recognition of 4 subtypes of exacerbators (infectious, inflammatory, comorbid, and mechanical). The document also introduces for the first time the concept of worsening as a differential factor in exacerbations, and makes specific recommendations for the diagnosis and management of these patients, and the systematic use of chest high-resolution computed tomography (HRCT) or treatment guided by treatable traits in patients who continue to present exacerbations despite dual bronchodilation. An integrated healthcare process is also proposed, with closer specialized monitoring, especially for severe exacerbators.
Definition of ExacerbatorsThe concept and definition of what we understand by exacerbator is clearly forms the foundation of subsequent recommendations. Exacerbators or exacerbator phenotypes have previously been defined in the literature,14,17 and have even have been included in CPGs.12,17 However, these definitions are rather controversial with regard to the frequency of events required, the minimum intensity of exacerbations, if a single hospitalization automatically defines a patient with differential features, and the stability of the concept over time. An exacerbator was defined as a patient who had at least 2 moderate (or more severe) exacerbations in the previous year, defined as episodes that require at least ambulatory treatment with systemic corticoids and/or antibiotics. This definition reached a consensus of 87.9% in the first round. Similarly, the experts also consider that a patient who has needed hospital admission in the previous year is an exacerbator (75.9% consensus). This consensus definition is, then, virtually identical to the GesEPOC proposal, and thus reinforces the definition made in those guidelines.17 The proposal refers to the number of exacerbations in the previous year and not to the patient's accumulated history. This factor is important, since it introduces a certain dynamic component. A patient may be an exacerbator at a certain moment, but with time and perhaps with treatment may cease to be defined as such. In the Eclipse study, only 12% of patients classified as exacerbators in the first year of follow-up continued to be exacerbators over the entire 3-year follow-up period.14
One of the most innovative aspects of this document is that it differentiates the “severe exacerbator” as one who has had at least 2 severe exacerbations in the last year. To the best of our knowledge, this definition had not been published previously and could potentially have implications in clinical practice. Repeated severe exacerbations have proven to be an independent adverse prognostic factor,9 but this is not the case with less severe exacerbations. The clinical profile of these cases also differs, and their greater use of resources20,21 means that preventive and therapeutic strategies need to be intensified and personalized (case management) in this subgroup of patients. In general, exacerbations become more frequent and severe as COPD progresses. However, the rate at which they occur seems to reflect an independent susceptibility phenotype14 that needs to be identified.
Another innovative aspect of this consensus document is the proposal to differentiate “worsening of symptoms without exacerbation” from a proper exacerbation. This concept, which achieved 82.8% consensus, was defined as “the absence of exacerbation or signs of decompensation of any of the patient's comorbidities”. Some authors have proposed the term “unstable COPD” to define a worsening of symptoms that is not accompanied by other biological changes, such as tachycardia, tachypnea, desaturation and/or increase in biomarkers.22
Exacerbator SubtypesThe working group reached consensus in identifying exacerbator 4 subtypes (infectious, inflammatory, comorbid, and mechanical). This approach has considerable clinical implications, in that 4 different mechanisms are suggested, with important connotations regarding prevention. Patients with repeated infectious exacerbations usually present neutrophilic inflammation with a significant bacterial burden during stable phases.23,24 In these patients, treatment with inhaled antibiotics can be very useful. There is also evidence that patients with this profile are up to 6 times more likely to have a bacterial-type exacerbation.23 Another proposal is to include patients with predominantly Th2 inflammation in stable phase in the group called “inflammatory exacerbators”. We suggest that these patients, identified in some guidelines as patients with asthma and COPD overlap (ACO)17 or by the presence of increased peripheral eosinophilia (>300eosinophils/mm3),16 may have more eosinophilic exacerbations and a better response to inhaled steroids.23 An important proposal of the consensus document is to define a comorbid exacerbator group and a mechanical exacerbator group (characterized by a worsening of symptoms due to hyperinsufflation with no increase in inflammation). Some studies suggest that the presence of comorbidity, particularly cardiovascular, is associated with an increase in systemic inflammation and risk of exacerbations,25,26 so it follows that the optimization of treatment of these concomitant diseases can be extremely useful. With regard to the mechanical exacerbator, the experts propose the existence of a group of exacerbators characterized by emphysema and signs of air trapping/hyperinsufflation. Functional alterations with a low level of inflammation would be predominant in these patients. Patients with this profile, labelled by some CPGs as exacerbator phenotype with emphysema, have a lighter infective burden and poorer response to inhaled steroids.17
In the opinion of the experts, several considerations must be taken into account to understand these subtypes and predisposing factors. In the first place, eosinophilia must be determined in respiratory samples and microorganisms must be identified. Eosinophilia is easy to analyze in a standard blood test, a procedure that is recommended in all exacerbators, regardless of their severity. Ideally, eosinophilia in sputum should also be analyzed, but as very few centers are equipped for this, this recommendation cannot be generalized. The microbiological study of sputum, however, is not systematically recommended, but it is especially indicated in cases with suspicion of chronic bronchial infection. Similarly, airway and pulmonary parenchymal lesions detected in imaging tests (mainly HRCT) are useful for identifying exacerbation patterns and determining the differential diagnosis of COPD. It is interesting to note that these procedures are considered helpful for recognizing the exacerbation subtype (emphysema, wall thickening, bronchiectasis, cardiovascular, etc.). Moreover, mucus hypersecretion is identified as a factor that favors lung function decline in exacerbators, perhaps due to inflammation.
Exacerbation and Persistent Systemic InflammationExacerbators, especially severe exacerbators, have a clearly increased risk of death.9 There is no clear evidence to indicate which mechanisms induce this increase in mortality. In the opinion of the experts, exacerbators are at increased risk of death due to a persistent systemic inflammatory response that causes an increase in cardiovascular events during and after the exacerbation (with an agreement of 84.5%). This statement may clearly have important therapeutic connotations, and is formulated on the basis of indirect data. Persistent systemic inflammation in COPD has been linked to increases in all-cause death and more frequent exacerbations.27 However, it is not clear whether the phenomenon is a cause or a consequence of repeated exacerbations. An increase in systemic inflammation and oxidative stress is known to occur during exacerbations, and these mechanisms could influence any of the extrapulmonary manifestations of COPD (nutritional, metabolic or cardiovascular changes).28 The persistence of residual systemic inflammation has also been seen to increase with time, especially in the case of repeated exacerbations.29,30 In this setting, reducing systemic inflammation should be beneficial; however, this has not been fully demonstrated. Recent data from the IMPACT STUDY31 suggest a reduction in mortality in the group of severe patients with frequent exacerbations treated with triple therapy. However, these data should be interpreted with caution, as they were generated in a secondary analysis. In patients with a cardiovascular profile, the use of combined LABA/ICS therapy showed no benefits in terms of survival.32
Treatment of ExacerbatorsThe working group identifies long-acting bronchodilators as the main preventive therapy for exacerbations in patients with no criteria for ACO. It is clear that the effect of this treatment on exacerbations affects lung function, mucus production, and mucociliary clearance. In the opinion of the experts, this treatment is sufficient in low-risk symptomatic patients and in well-controlled exacerbators. The document proposes the use of single-agent bronchodilators in COPD patients with a maximum of 2 outpatient exacerbations (non-exacerbators) and mild or moderate obstruction. Dual bronchodilation is the treatment of choice in exacerbators, with the exception of patients with ACO. If exacerbations persist despite optimal bronchodilator treatment, other treatable traits must be evaluated, and patient management must be personalized (inhaled corticosteroids [ICS] in cases of peripheral blood eosinophilia, nebulized antibiotics in cases of persistent bronchial infection, low-dose macrolides in patients with bronchiectasis, etc.).
Almost 90% of participants agreed that the use of ICS should be recommended to prevent exacerbations that persist despite dual bronchodilation. The strongest evidence to date comes from the TRIBUTE33 and IMPACT31 studies, which demonstrated the superiority of triple therapy compared to dual bronchodilation in the prevention of exacerbations. This benefit is more pronounced in the presence of significant peripheral eosinophilia and less marked in cases with eosinopenia.31,33 The same biomarker has also been proposed to guide ICS withdrawal. Experts recommend evaluating the discontinuation of ICS in patients who are stable for at least a year, with no severe obstruction, and eosinophilia<300c/mm3, provided dual bronchodilation is maintained. Finally, dose tapering is seen as a way to optimize treatment with ICSs.
Integrated Care ProcessThe working group reached consensus regarding the usefulness of integrated care programs. In general, these services improve quality of life and exercise capacity and reduce hospital admissions and length of stay. These benefits were described in a Cochrane review published in 201334 (26 trials with 2997 patients) and in various publications.35,36 However, a Dutch study conducted in primary care with a 24-month follow-up found no benefit over standard care, with the exception of a greater level of integration and an improvement in activities of daily living.37 Despite this controversy, the experts support the use of specific integrated multicomponent programs, in which pharmacological treatment, respiratory rehabilitation, health education, and case management are optimized, and the social aspects of the disease are addressed. This approach must be coordinated between primary care, which is much closer to the family environment of the patient, and respiratory care. However, given the complexity, heterogeneity, and high risk of these patients, we believe that exacerbators, especially severe cases, must undergo protocolized follow-up led by the pulmonology department. In less severe cases, monitoring should be primarily performed at the primary care level. However, these patients are still considered to be at risk, and therefore must receive optimized multidimensional assessment, treatment and follow-up.
LimitationsIt was expected that some of the statements would not reach consensus. COPD is a dynamic field, and our knowledge of this pathology is continuously being updated. There is no doubt that new research will help to clarify the controversial issues that did not achieve consensus. Furthermore, the consensus that were reached must always be treated with caution until they are endorsed by scientific evidence.
Another possible limitation of this document is that, due to the characteristics inherent to the method, the results can only be taken as expert opinions. The participants were exclusively specialists in pulmonology. This fact may also have influenced some of the statements, and other specialties, such as primary care, or the viewpoint of the patient, should ideally have been included. We believe, however, that the number of respondents and their distribution across Spain, together with the high degree of participation achieved, add great value to this document.
ConclusionsThis document uses consensus methodology to addresses several ambiguities and controversies that affect the management of exacerbators. Respondents agreed on 89% of the proposed statements. Some of these are of interest for their innovative nature, including the definition of “severe exacerbator”, the identification of 4 exacerbator subtypes (infectious, inflammatory, comorbid, and mechanical) with their proposed biological mechanisms and specific personalized diagnostic and/or therapeutic recommendations, and the recommendation that these high-risk cases be coordinated and managed within specific multicomponent programs. The recommendations put forward by the experts seek to clarify some ambiguities and controversies; however, further research is needed to validate their conclusions.
FundingThis project received unconditional funding from Boehringer Ingelheim, which did not intervene in data collection, analysis, or interpretation of the results, or in drafting the manuscript.
Conflict of InterestsThe ExpertEx project receives sponsorship from Boehringer Ingelheim.
The authors have expressed the following conflicts of interest:
Bernardino Alcazar Navarrete has received honoraria for delivering lectures or payment for participation in advisory committees from GlaxoSmithKline, Novartis AG, Boehringer Ingelheim, Chiesi, Laboratories Menarini, Gebro, and AstraZeneca, not associated with this study.
Julio Ancochea Bermúdez has received honoraria for scientific consulting and/or speaking engagements from Actelion, Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Carburos Médica, Chiesi, Faes Farma, Ferrer, GlaxoSmithKline, InterMude, Linde Healthcare, Menarini, MSD, Mundipharma, Novartis, Pfizer, Roche, Rovi, Sandoz, Takeda, and Teva.
José Luis Izquierdo Alonso has given lectures and participated in meetings funded by AstraZeneca, Bayer, Bial, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GlaxoSmithKline, Gebro Pharma, Menarini, Novartis, Orion, Pfizer, Takeda, and Teva.
Francisco García-River has given lectures funded by Boehringer Ingelheim, Novartis, Chiesi, GlaxoSmithKline, Menarini and Rovi, participated in advisory committees for Boehringer Ingelheim, Esteve, GlaxoSmithKline, Grebo Pharma, AstraZeneca, Menarini and Novartis and has acted as principal investigator in projects funded by GlaxoSmithKline, and Menarini.
Juan José Soler-Cataluña has received honoraria for scientific consultancy and/or for speaking at conferences from AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Laboratorios Esteve, Menarini, Mundipharma, Novartis, and Rovi.
Marc Miravitlles has received honoraria for speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Grifols and Novartis, and for participation in advisory committees from AstraZeneca Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Gebro Pharma, CSL Behring, Novartis, and Grifols.
Please cite this article as: Alcázar Navarrete B, Ancochea Bermúdez J, García-Río F, Izquierdo Alonso JL, Miravitlles M, Rodríguez González-Moro JM, et al. Paciente exacerbador con enfermedad pulmonar obstructiva crónica: recomendaciones en procesos diagnósticos, terapéuticos y asistenciales. Arch Bronconeumol. 2019;55:478–487.
The authors have prepared this article on behalf of the Expert Meeting Expert Panel. The list of the 67 participants in the Consensus, by operational group and alphabetical order of first surname are presented in Annex 1.