The coronavirus disease 2019 (COVID-19) pandemic has created a public health emergency challenging the health care system capabilities. The shortage of medical resources, in particular of mechanical ventilators represents a major concern, leading to some centers considering the use of a single mechanical ventilator for two patients (co-venting). Protocols designed to co-ventilate are based on the use of a single setting delivering pressure-controlled ventilation (PCV) for two patients with similar mechanical support needs and under neuromuscular blockade. Despite these precautions, the sharing of mechanical ventilators has raised numerous concerns among scientific societies.1 Uneven distribution of tidal volume (VT) between the two patients is a major risk, which could theoretically be circumvented by matching patients by size and respiratory mechanics at initiation mechanical ventilation. Nevertheless, the dynamic characteristics of patients in respiratory failure cause fluctuations of lung compliance (C) and airway resistance (R). Recently, Gattinoni et al. proposed two primary phenotypes of COVID-19 pneumonia: “type L” (low elastance) and “type H” (high elastance).2 Patients could transition through both phenotypes during the course of the disease depending on various factors. Therefore, a dynamic and (probably) unpredictable pattern of respiratory mechanics should be expected in COVID-19 patients undergoing mechanical ventilation.
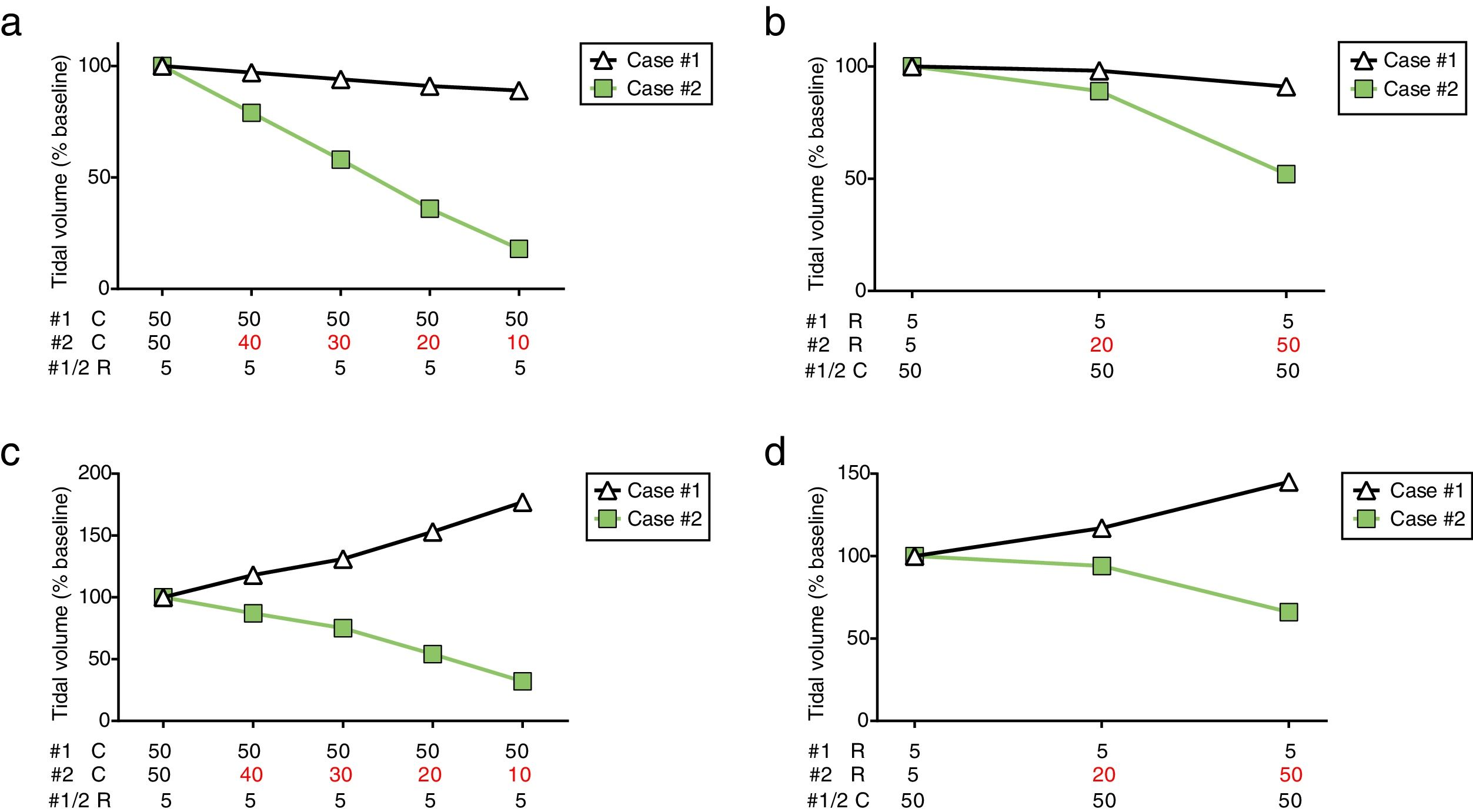
To describe the impact that different C and R would have on VT during co-ventilation, a mechanical ventilator (Puritan Bennett 840, Medtronic, Minneapolis, MN) was connected to a dual-chamber lung simulator (Training and Test Lung, Michigan Instruments, Grand Rapids, MI) using two tubing sets connected through T-tubes, as previously described.3 Each of the simulator chambers represented a different patient (simulated case #1 and #2, respectively). Stable, relatively normal C (50mL/cmH2O) and R (5cmH2O/s) were maintained for case #1 throughout the experiment, while different abnormal conditions were simulated for case #2. Pressure, flow and VT were registered for each chamber individually (SAMAY MV16, Uruguay).
During PCV the ventilator was set at peak pressure of 18cmH2O, positive end-expiratory pressure (PEEP) of 10cmH2O, respiratory rate of 15breaths/min, inspiratory–expiratory ratio of 1:2. Mechanical ventilation was initiated with identical C (50mL/cmH2O) and R (5cmH2O/s) for both simulated patients and baseline measurements were obtained. Afterwards, different pathological scenarios were simulated to occur to case #2. Progressive reduction of lung C (maintaining R=5 cmH2O/s) resulted in a substantial contraction of VT for case #2, leading to a decrease of up to 18% from baseline when C was 10mL/cmH2O. Case #1 presented a gradual but modest reduction of VT as C of case #2 declined (Fig. 1a). Later, airway R of case #2 was increased while maintaining C at 50mL/cmH2O (Fig. 1b). Tidal volume was relatively preserved for case #1 and case #2 at R=20 cmH2O/s (98% and 89% from baseline, respectively). However, a severe increase in R (50cmH2O/s) resulted in a drastic reduction of VT for case #2, while a minor decrease was observed for case #1 (52% and 91% from baseline, respectively).
Tidal volume distribution during pressure-controlled ventilation (a, b) or volume-controlled ventilation (c, d) while modifying compliance or resistance of case #2. PCV: pressure-controlled ventilation; VCV: volume-controlled ventilation; #1: simulated case #1; #2: simulated case #2; C: lung compliance (in mL/cmH2O); R: airway resistance (in cmH2O/s).
The same experimental protocol was repeated in volume-controlled ventilation (VCV) with VT set at 800mL while maintaining the other settings unchanged. As observed in PCV, the decrease in lung C or increase in airway R determined a progressive reduction of VT for case #2. More importantly, this reduction was paralleled by an increase in VT for case #1 (Fig. 1c and d). Therefore, case #1 and case #2 could potentially receive highly unequal VT such as 177% and 32% from baseline, respectively (C=10mL/cmH2O).
Ventilating two patients with a single mechanical ventilator has been proposed as a last resort in a crisis standard of care, as could occur during COVID-19 pandemic. This strategy obviously presents significant limitations that could expose both patients to an excessive risk of adverse events. Changes in respiratory mechanics may occur unexpectedly as a result of diverse situations (bronchospasm, secretions, hyperinflation, lung edema, pneumothorax, etc.). Branson et al. have already shown the disparity of VT distribution among four simulated patients connected to a single ventilator, as C and R were modified.4 Here, we aimed to reproduce a scenario that we believe is more likely to occur during the COVID-19 outbreak, co-ventilating two simulated patients that might present relatively preserved or extremely abnormal respiratory mechanics.2,5,6 As we demonstrated in this simulation-based analysis, variation of a single characteristic (C or R) on one patient can drastically affect the way gas is distributed. Of note, we observed similar results when simulation was performed at different PEEP levels (5, 10 and 15cmH2O). Our study design represents an oversimplification of what might occur on a clinical setting, in which both patients could present changes in C and R, in similar or opposite directions. In this scenario, both patients (in different ways) could be exposed to a significant risk of hypo or hyperventilation, with hypercapnia and volutrauma among the most feared consequences. Despite a thoughtful setting of alarm parameters, without individual respiratory monitoring the entailed risk of late detection of these phenomena is too high. As expected, VCV was associated to a more uneven distribution of VT, significantly increasing the risks.
In summary, ventilator sharing could result in deleterious effects related to inadequate VT distribution. This study addressed a single aspect of the issues related to patient co-venting, using a simulation experimental setting, while many other concerns remain to be studied. Notwithstanding, ventilating two patients with a single mechanical ventilator appears to be an unsafe practice. Further research and safety measurements are required before it could be recommended in exceptional circumstances.
FundingNone (This research has not received specific aid from public sector agencies, commercial sector or non-profit entities).
Conflicts of interestNone.