Extrapulmonary chronic respiratory failure (ECRF) excluding obesity hypoventilation syndrome is an heterogenous category composed of central neuromuscular disease and chest wall disease in which hypercapnic chronic respiratory failure is frequent. Its diagnosis in patients with neurological impairment is based on respiratory follow-up mainly focussed on reported symptoms, blood gases and pulmonary function test [1]. Indeed, initiation of long-term home respiratory support with non-invasive ventilation (NIV) [2,3] and mechanical insufflation–exsufflation techniques [4] have been reported to improve survival and quality of life.
However, obstructive sleep apnea (OSA) is a common disorder in this population [5,6]. Thus, focusing almost exclusively on hypercapnia and NIV initiation may lead to underestimate the possible benefit of OSA treatment on symptoms and hypoventilation [7].
Overall, there is currently a lack in scientific knowledge on the effect of continuous positive airway pressure (CPAP) therapy for patients with an overlap between OSA and ECRF failure albeit this treatment is reported to be used sometimes [8].
We hypothesized that CPAP treatment for OSA in a context of ECRF could improve emerging nocturnal hypoventilation and might be beneficial on nocturnal hematosis – i.e. both hypoxia and hypercapnia – for those patients.
Thus, we conducted a retrospective study, with the primary objective of describing patients with neurological disorders and OSA for whom CPAP was first offered despite other NIV initiation criteria. The secondary objectives were to describe changes in nocturnal gas exchange under CPAP therapy in these patients and to explore potential factors associated with lack of improvement.
We conducted a retrospective, single-center study in a tertiary care center for patients with neurological disorders identified using a systematic screening tool derived from the institutional “Cohort360” system. Cohort360 is the AP-HP's (Assistance Publique – Hopitaux de Paris) Health Data Hub (HDH). It is a data center coding the reasons for patients’ consultation diagnosis and treatment. The AP-HP's Health Data Hub (HDH) is committed to absolute respect for the confidentiality of the personal data included in cohort360, and has been authorized by the CNIL (no. 1980120). Patients are informed (verbal consent approved by our IRB) of their inclusion in this database and may withdraw their approval at any time. Patients who refused access to their data were not included in the study.
The keywords “CPAP” and “TcCO2” were used to identify all patients referred to our sleep unit (between January 2018 and December 2024) which have been treated by CPAP with any TcCO2 measurement at baseline and/or during follow-up.
Finally, inclusion criteria were: patients with ECRF, presenting at least one criteria for NIV initiation (Supplementary Figure 1) [9] and an apnea hypopnea index (AHI) ≥10 events per hour. Patients needed to have performed blood gases, pulmonary function test (PFT) and sleep study (polygraphy or polysomnography) at baseline. Only patients with TcCO2 measurement at baseline and under CPAP have been included.
To describe patients to whom CPAP was first offered despite other NIV initiation criteria, we collected demographic data (age, sex, BMI, neurological condition), PFT results (FVC, MIP, MEP, SNIP), blood gazes (pH, PCO2, HCO3−, base excess) and AHI from the sleep study. Qualitative data were reported as percentage, quantitative variables were reported as mean and standard deviation or median and IQR [first quartile−third quartile] for non-normal variables.
To compare nocturnal TcCO2 and SpO2 results before and after CPAP initiation, unpaired Student t tests and Mann–Whitney U tests were used according to the normality of variable distribution assessed by Shapiro–Wilk test.
To identify factors associated with CPAP failure, characteristics of subpopulations with and without improvement in mean TcCO2 defined as a decrease of 5mmHg or a value <45mmHg on CPAP were compared with Chi2 tests. Significance was set at p<0.05.
Among 179 patients screened, 30 patients met inclusion criteria (Supplementary Figure 2). The main reasons for exclusion were missing baseline or follow-up data (53.1%) or lack of any NIV initiation criteria (25.1%).
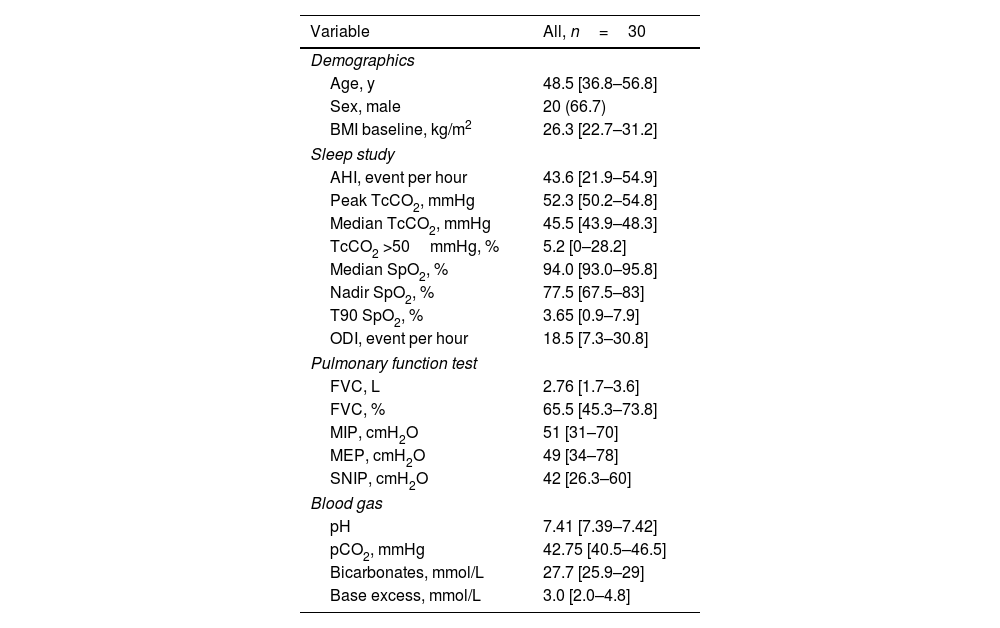
Demographics and baseline data were shown in Table 1. ECRF etiologies were mainly spinal cord injury (n=16) slowly progressive neuromuscular disease (n=8) or other disease categories (multiple sclerosis, restrictive thoracic disorders). Patients were mostly men (66.7%) slightly overweight (median BMI 26.3).
Baseline characteristics.
| Variable | All, n=30 |
|---|---|
| Demographics | |
| Age, y | 48.5 [36.8–56.8] |
| Sex, male | 20 (66.7) |
| BMI baseline, kg/m2 | 26.3 [22.7–31.2] |
| Sleep study | |
| AHI, event per hour | 43.6 [21.9–54.9] |
| Peak TcCO2, mmHg | 52.3 [50.2–54.8] |
| Median TcCO2, mmHg | 45.5 [43.9–48.3] |
| TcCO2 >50mmHg, % | 5.2 [0–28.2] |
| Median SpO2, % | 94.0 [93.0–95.8] |
| Nadir SpO2, % | 77.5 [67.5–83] |
| T90 SpO2, % | 3.65 [0.9–7.9] |
| ODI, event per hour | 18.5 [7.3–30.8] |
| Pulmonary function test | |
| FVC, L | 2.76 [1.7–3.6] |
| FVC, % | 65.5 [45.3–73.8] |
| MIP, cmH2O | 51 [31–70] |
| MEP, cmH2O | 49 [34–78] |
| SNIP, cmH2O | 42 [26.3–60] |
| Blood gas | |
| pH | 7.41 [7.39–7.42] |
| pCO2, mmHg | 42.75 [40.5–46.5] |
| Bicarbonates, mmol/L | 27.7 [25.9–29] |
| Base excess, mmol/L | 3.0 [2.0–4.8] |
Data are provided as number (%) or median and IQR [first quartile−third quartile] as appropriate.
AHI, apnea hypopnea index; BMI, body mass index; FVC, forced vital capacity; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; ODI, oxygen desaturation index; pCO2, diurnal CO2 level in blood gases; SNIP, sniff nasal inspiratory pressure; SpO2, oxygen saturation; TcCO2, transcutaneous carbon dioxide; TcCO2 >50%, time with TcCO2 above 50mmHg in percentage of recording time; T90 SpO2, percentage time spent with SpO2 below 90%.
Seventy percent had an AHI >30/h, including 47% with an AHI >45/h. Also, 63% met at least three NIV initiation criteria (85% ≥2 criteria). The most frequent NIV initiation criteria were symptoms with VC <80% (66.7%), MIP ≤−60cmH2O (53.3%) followed by Nasal SNIP ≤−40cmH2O and SpO2 ≤90% for ≥5% of the night (43.3%). One third presented with a daytime PaCO2 ≥45mmHg and one third with nocturnal TcCO2 ≥50mmHg (Supplementary Figure 1).
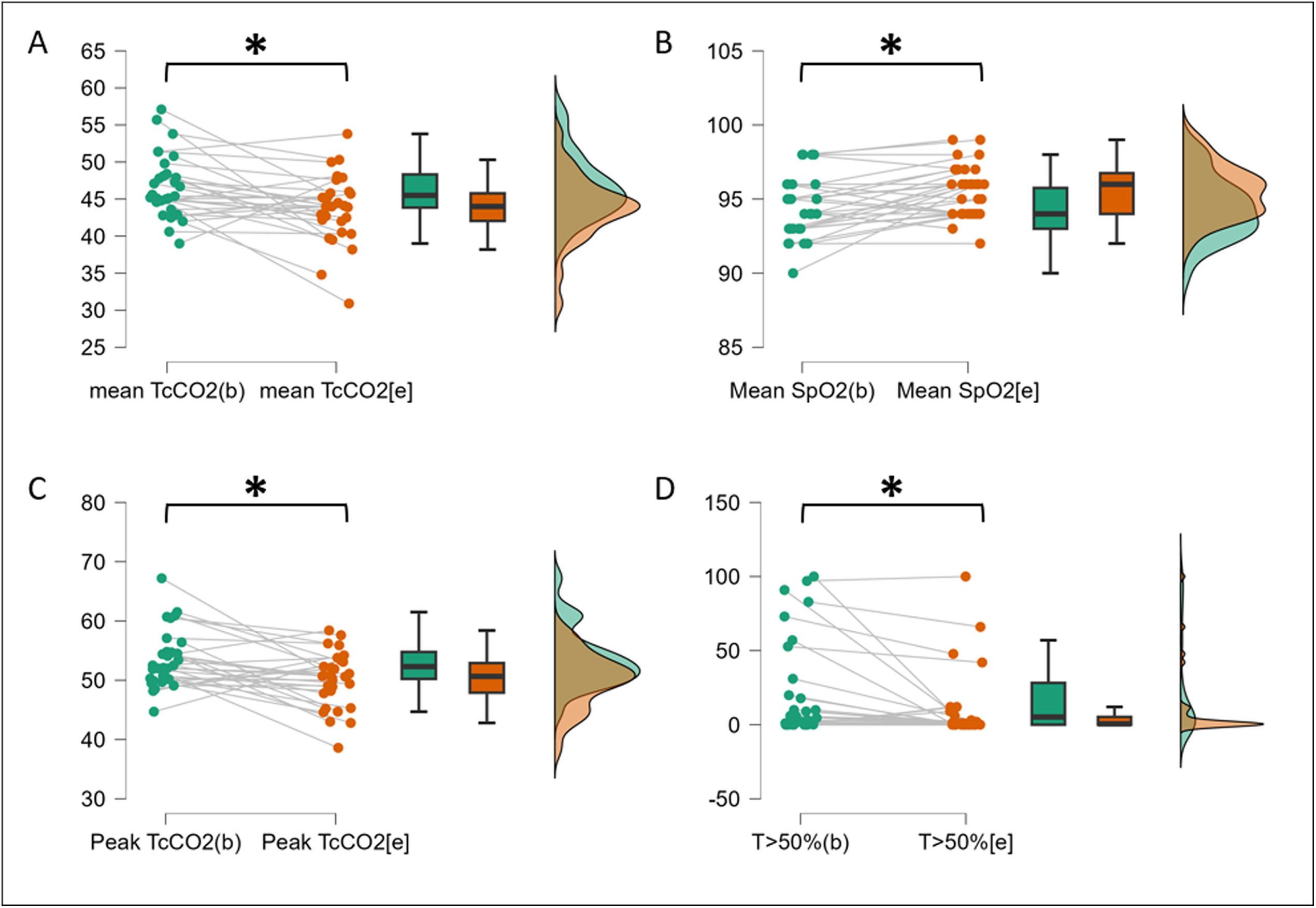
After CPAP initiation, nocturnal TcCO2 decreased significantly from 46.7±4.3 to 43.7±4.5mmHg (p=0.005) (Fig. 1 and Supplementary Table 1). Peak TcCO2 and time with TcCO2 >50mmHg also decreased significantly from 53.5±4.8mmHg to 50.0±4.6mmHg and from 22.5±33.6 to 10.2±23.3% (p=0.003 and 0.013 respectively). Mean SpO2, nadir SpO2 and oxygen desaturation index significantly improved with CPAP (Supplementary Table 1). Supplementary Figure 3 shows the example of nocturnal TcCO2 evolution in a patient removing CPAP during the night.
TcCO2 and SpO2 measurement before and after CPAP initiation. (A) Mean TcCO2 as mmHg before and after CPAP initiation. (B) Mean SpO2 as % before and after CPAP initiation. (C) Peak TcCO2 as mmHg before and after CPAP initiation. (D) Time TcCO2 >50mmHg as % before and after CPAP initiation. Green: baseline assessment in ambient air; orange: endpoint assessment with CPAP. CPAP, continuous positive airway pressure; SpO2, oxygen saturation; TcCO2, transcutaneous carbon dioxide; T>50%, time with TcCO2 above 50mmHg in percentage of recording time.
Among the 21 patients (70%) with a baseline mean TcCO2 ≥45mmHg, those with baseline MIP ≥60cmH2O (p=0.027) and SNIP ≥40cmH2O (p=0.020) are more likely to improve mean nocturnal TcCO2 under CPAP (Supplementary Table 2). Baseline mean nocturnal TcCO2 >50mmHg (p=0.604), SpO2 <90% for more than 5% of recording time (p=0.466) and SpO2 <88% for mor than 5min (p=0.195) were not associated with CPAP failure. Patients with VC ≥50% and diurnal PaCO2 ≤45mmHg tend to improve mean nocturnal TcCO2 under CPAP, but statistics fail to reach significance (p=0.088 and p=0.094 respectively).
This retrospective study identified patients with ECRF meeting both OSA criteria and NIV initiation criteria for whom CPAP has been initiated.
The primary analysis restricted on patients with TcCO2 evaluated before and after CPAP initiation as well as PFT and blood gases at baseline showed significant improvement in TcCO2 measured with each peak TcCO2, mean TcCO2 and time TcCO2 >50mmHg. Also, there were significant improvements in SpO2 as measured with each mean SpO2, nadir SpO2 and time SpO2 <90%. The secondary analysis of patients with initial increased nocturnal TcCO2 showed more univocal improvement in TcCO2 in patients with less initial inspiratory muscles impairment but no difference in patients with more severe nocturnal hematosis impairments.
Thus, this work illustrates that in the context of moderate to severe OSA and ECRF, CPAP initiation can improve nocturnal hematosis both on TcCO2 and SpO2. These results are in line with previous literature which mainly assessed symptoms [10,11].
The physiological rational of our result needs to be debated. Indeed, CPAP has no beneficial effect on inspiratory muscle effort during wake [12]. However, an increase in the work of breathing (WOB) has been demonstrated in eucapnic as well as hypercapnic patients with OSA [13]. The latter WOB was calculated as the computed area enclosed within the inspiratory oesophageal pressure tidal volume curve which reflects inspiratory muscles work. Therefore, added to the respiratory challenge of sleep physiologically inducing a 10% decrease in minute ventilation, the increase in WOB induced by OSA is likely to hasten chronic respiratory failure [14]. Also, to reinforce our results a parallel can be drawn with obesity hypoventilation syndrome (OHS) for which CPAP has demonstrated to be as efficient as NIV for stable patients [15]. In OHS with OSA, mechanisms of hypercapnia include imbalance between CO2 load during prolonged apnoeic events and limited elimination during resumption of ventilation [16]. Although OSA is less likely to contribute in central chronic respiratory failure than in OHS, our results suggest that it could nevertheless play a role in hypercapnia.
We acknowledge the retrospective and unicentric nature of the study and lack of symptoms measurements may introduce selection bias. Yet in our center, CPAP is prescribed according to French guidelines: AHI >30/h, or >15/h with symptoms and/or cardiovascular comorbidities. Also, the modest hypercapnia reflects that CPAP, not being standard care here, was initiated in patients with mild respiratory failure. Moreover, extra pulmonary cause of respiratory failure is an heterogenous group of patients which limits the internal validity of this work and we cannot exclude a lack of power related to the small sample size. Finally AHI >10/h is an unusual threshold corresponding to our experience of PAP introduction in this specific population for whom usual thresholds may not fully apply.
However, to our knowledge, this work is the first to assess nocturnal hematosis with both TcCO2 and SpO2 benefits of CPAP initiation in patients with central chronic respiratory failure and comorbid OSA. These encouraging results support the need for randomized control study to identify the phenotypes of patients which benefit the most of CPAP initiation. Indeed, given the costs of CPAP as compared to NIV treatment and the discomfort associated with NIV drawbacks [17–19] CPAP could be considered as the first line option for selected patients. Moreover, the determination of CPAP impact may also help in the appropriate expiratory pressure settings of NIV if it is subsequently required. Insufficient pressure may lead to persistent OSA which can compromise NIV efficiency. This advocates for both sleep assessment and CPAP as a treatment option in this population.
CRediT authorship contribution statementAL, PT, MP and HP contributed to the conception and design of the study. All the authors have written or edited the manuscript. Each co-author made substantial contributions to the manuscript; drafted sections of the manuscript and revised it critically for important intellectual content; provided final approval of the version to be published; agreed to be accountable for all aspects of the manuscript and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Guarantor: AL.
Ethics approvalThe AP-HP's Health Data Hub (HDH) is committed to absolute respect for the confidentiality of the personal data included in cohort360 and has been authorized by the CNIL (no. 1980120).
Declaration of generative AI and AI-assisted technologies in the writing processNone declared.
FundingThe authors do not declare a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interestAL reports consulting fees from Air Liquide Medical Systems and lecturing fees from SOS Oxygène, Oxylis and Lowenstein Medical, outside the submitted work.
HP reports personal fees from ASV Santé, SOS Oxygène, ISIS Medical, Breas Medical, ResMed, Sanofi – Genzyme, and Sanofi – Biogen, outside the submitted work.
PT reports no support for the present manuscript. For other works he reports travel and congress grant as well as honoraria from Asdia, Resmed, Linde, Bastide and ALLP; grant support through her institution from “Agir pour les maladies chroniques” foundation.
MP reports no support for the present manuscript. For other works he reports grants contracts consulting fees, honoraria for lectures, travel grants, participation on advisory board, stock and receipt of equipment from Resmed, Philips Respironics, Asten Santé, Kernel Biomedical, SOS Oxygen, Chiesi, Lowenstein, Bastide, Elivie, Antadir, Jazz Pharmaceutical, Fisher & Paykel, Orkyn Sanofi, GSK and Air Liquide Medical.
The other authors have no conflicts of interest to disclose.