The search for effective treatment for all people with cystic fibrosis (PwCF) has been one of the main objectives of research into the disease. With the advent of CFTR (cystic fibrosis transmembrane conductance regulator) modulators, considerable benefits have been achieved, especially with the use of elexacaftor/tezacaftor/ivacaftor (ETI), which has led to significant improvement in lung function, body mass index (BMI) and quality of life (QoL) in individuals with at least one F508del variant.1–4 ETI has also been approved in the United States for another 177 variants considered responsive on in vitro studies.5
Nevertheless, a considerable portion of PwCF is still not eligible for this modulator. Although in Europe and the United States more than 80% of cystic fibrosis (CF) patients carry the F508del allele,6,7 in Brazil, currently the country with the sixth largest absolute number of patients in the world,8 only 52.8% of patients have this variant.9
Among non-F508del variants, N1303K (p.Asn1303Lys) is the third most frequent.8 Similarly to F508del, N1303K leads to alterations in processing and traffic of CFTR protein, as well as defects in its conductance.10 To date, the use of CFTR modulators has not been approved for individuals carrying N1303K. In vitro studies with “Fisher Rat Thyroid Cells” (FRT) demonstrated a functional improvement of 9.4% in wild-type CFTR protein after treatment with ETI, which does not exceed the 10% threshold considered positive for responsiveness established by the FDA (U.S. Food and Drug Administration).11 However, real-life studies have shown considerable clinical benefit with ETI in these patients.10,12,13
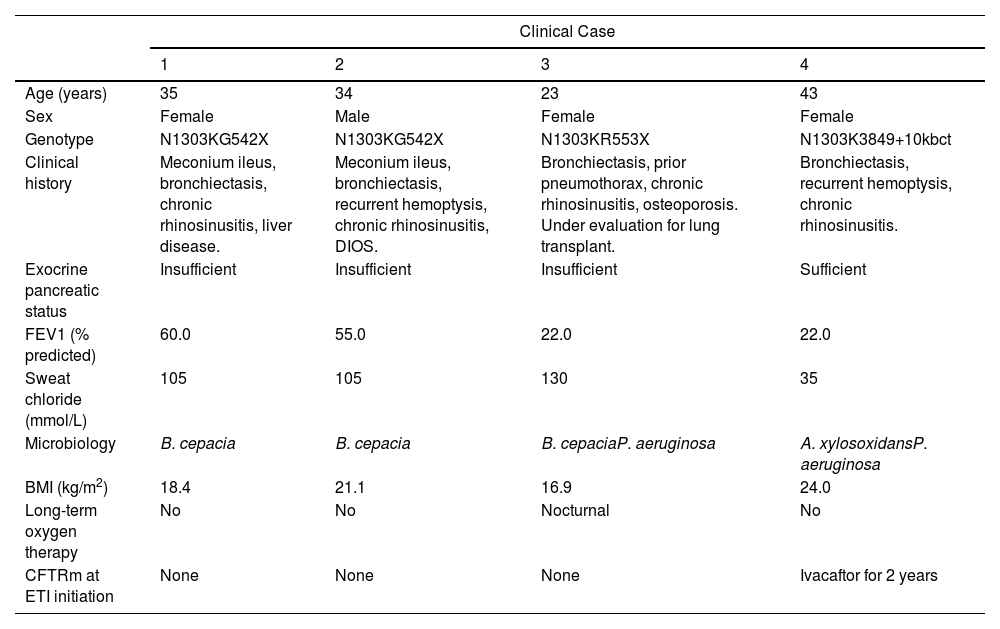
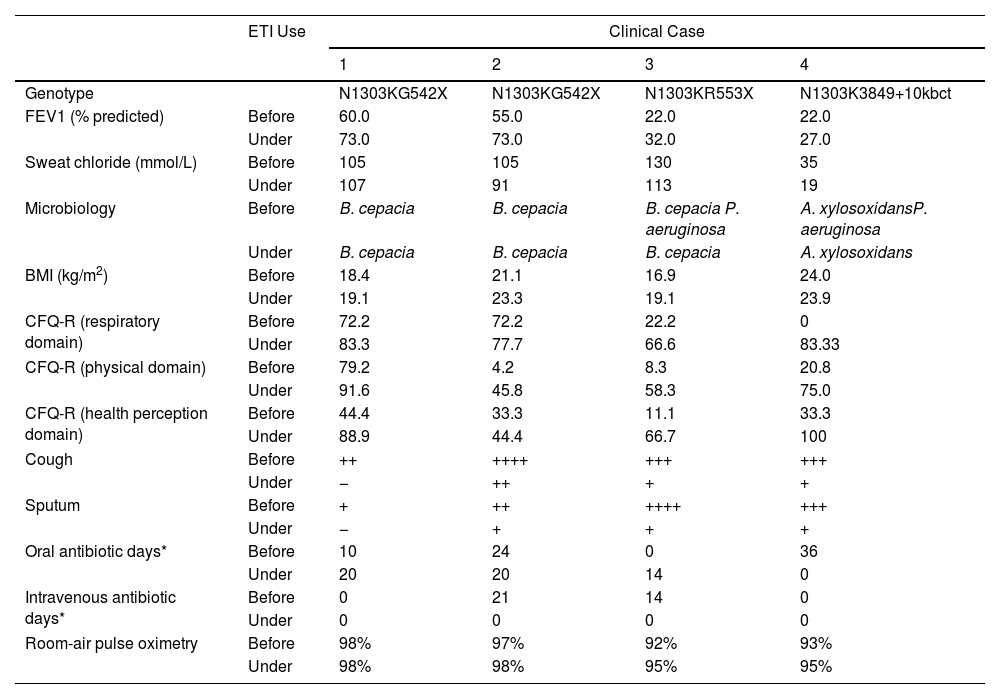
This study reports the clinical evolution of 4 adult PwCF with N1303K and a second non-F508del allele, after treatment with ETI for 12 weeks. The detailed characteristics of the patients are described in Table 1. After 3 months using ETI, all of them reported subjective reduction in cough frequency and sputum production. In addition, there was significant improvement in QoL, assessed by Cystic Fibrosis Questionnaire-Revised (CFQ-R). Objective parameters showed gain in lung function and increase in BMI. Clinical response was more expressive in PwCF carrying N1303K/minimal function (MF) genotype, with a gain of 10–17% in FEV1 and 0.65–2.19kg/m2 in BMI. Regarding pulmonary exacerbations, there was a reduction in antibiotic use in 3 of the 4 PwCF evaluated. There was only a small variation in sweat chloride and no safety-related events. Clinical evolution after ETI is presented in Table 2.
Demographic and Clinical Characteristics Prior to ETI Initiation.
| Clinical Case | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age (years) | 35 | 34 | 23 | 43 |
| Sex | Female | Male | Female | Female |
| Genotype | N1303KG542X | N1303KG542X | N1303KR553X | N1303K3849+10kbct |
| Clinical history | Meconium ileus, bronchiectasis, chronic rhinosinusitis, liver disease. | Meconium ileus, bronchiectasis, recurrent hemoptysis, chronic rhinosinusitis, DIOS. | Bronchiectasis, prior pneumothorax, chronic rhinosinusitis, osteoporosis. Under evaluation for lung transplant. | Bronchiectasis, recurrent hemoptysis, chronic rhinosinusitis. |
| Exocrine pancreatic status | Insufficient | Insufficient | Insufficient | Sufficient |
| FEV1 (% predicted) | 60.0 | 55.0 | 22.0 | 22.0 |
| Sweat chloride (mmol/L) | 105 | 105 | 130 | 35 |
| Microbiology | B. cepacia | B. cepacia | B. cepaciaP. aeruginosa | A. xylosoxidansP. aeruginosa |
| BMI (kg/m2) | 18.4 | 21.1 | 16.9 | 24.0 |
| Long-term oxygen therapy | No | No | Nocturnal | No |
| CFTRm at ETI initiation | None | None | None | Ivacaftor for 2 years |
BMI: body mass index; CFTRm: cystic fibrosis transmembrane conductance regulator modulator; ETI: elexacaftor/tezacaftor/ivacaftor; FEV1: forced expiratory volume in first second.
Clinical Evolution After ETI Initiation.
| ETI Use | Clinical Case | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Genotype | N1303KG542X | N1303KG542X | N1303KR553X | N1303K3849+10kbct | |
| FEV1 (% predicted) | Before | 60.0 | 55.0 | 22.0 | 22.0 |
| Under | 73.0 | 73.0 | 32.0 | 27.0 | |
| Sweat chloride (mmol/L) | Before | 105 | 105 | 130 | 35 |
| Under | 107 | 91 | 113 | 19 | |
| Microbiology | Before | B. cepacia | B. cepacia | B. cepacia P. aeruginosa | A. xylosoxidansP. aeruginosa |
| Under | B. cepacia | B. cepacia | B. cepacia | A. xylosoxidans | |
| BMI (kg/m2) | Before | 18.4 | 21.1 | 16.9 | 24.0 |
| Under | 19.1 | 23.3 | 19.1 | 23.9 | |
| CFQ-R (respiratory domain) | Before | 72.2 | 72.2 | 22.2 | 0 |
| Under | 83.3 | 77.7 | 66.6 | 83.33 | |
| CFQ-R (physical domain) | Before | 79.2 | 4.2 | 8.3 | 20.8 |
| Under | 91.6 | 45.8 | 58.3 | 75.0 | |
| CFQ-R (health perception domain) | Before | 44.4 | 33.3 | 11.1 | 33.3 |
| Under | 88.9 | 44.4 | 66.7 | 100 | |
| Cough | Before | ++ | ++++ | +++ | +++ |
| Under | − | ++ | + | + | |
| Sputum | Before | + | ++ | ++++ | +++ |
| Under | − | + | + | + | |
| Oral antibiotic days* | Before | 10 | 24 | 0 | 36 |
| Under | 20 | 20 | 14 | 0 | |
| Intravenous antibiotic days* | Before | 0 | 21 | 14 | 0 |
| Under | 0 | 0 | 0 | 0 | |
| Room-air pulse oximetry | Before | 98% | 97% | 92% | 93% |
| Under | 98% | 98% | 95% | 95% | |
BMI: body mass index; CFQ-R: Cystic Fibrosis Questionnaire-Revised; ETI: elexacaftor/tezacaftor/ivacaftor; FEV1: forced expiratory volume in first second.
Our results highlight the benefit resulting from ETI in PwFC carrying N1303K/non-F508del genotypes. The first three patients carried the N1303K and a nonsense variant, intrinsically considered non-responsive to CFTR modulators.12 Thus, the clinical response in these patients demonstrates the direct effect of ETI on N1303K. The absolute improvement in FEV1 was +10% to 17%, which is comparable to values found after ETI in PwCF with F508del/MF genotypes [+13.9% (95% CI 12.8–15)].4 There was significant gain in FEV1 (+10%) even in the patient requiring oxygen, whose initial FEV1 was below 30%. Similar results were seen in patients with F508del and advanced lung disease treated with ETI, in which 64% of oxygen-using patients gained 6–11% in FEV1.14 Nutritional improvement was also significant, with an increase of 0.65–2.19kg/m2 in BMI, comparable to studies with ETI in PwCF carrying F508del/MF genotype (median 1.13kg/m2).4
The fourth case differs from the others because it was a patient with a N1303K/residual function genotype (3849+10kbct), who had been on ivacaftor for 2 years prior to ETI. This possibly explains the lower increase in FEV1 (+5%), as ivacaftor itself appears to be responsible for part of the rescue of the native N1303K expressed in very small quantities on the cell membrane of these patients.15–17 There was a reduction in symptoms and improvement in QoL after treatment. However, there was no change in BMI, probably because the patient had pancreatic sufficiency and preserved nutritional status before treatment.
In terms of pulmonary exacerbations, a decrease in oral and/or intravenous antibiotic use was noted in three evaluated PwCF, which is similar to what was described in patients with the F508del variant during ETI use.4 Regarding microbiology, recent studies have reported that, although ETI improves mucus rheology and microbiome sputum diversity, most PwCF persist with chronic airway infection.18,19 In our case series, we observed that Burkholderia cepacia and Achromobacter xylosoxidans persisted in respiratory cultures after ETI whereas Pseudomonas aeruginosa was not detected in two PwCF who previously had positive cultures. However, this finding should be taken with caution, due to the exclusive use of culture-based methods for bacterial identification and the limited duration of evaluation.
Our findings are in line with previous studies that evaluated the use of ETI in PwCF carrying N1303K variant. These patients were considered consistent responders to ETI by Burgel et al., with a median FEV1% variation of +27.5 (IQR +20.7 to +41.2).12 Recently, Sadras et al. described a series of 8 cases with a mean gain of 18.4% in FEV1 and 0.79±0.33kg/m2 in BMI after treatment.17 In this study, clinical evaluation was accompanied by in vitro analysis using intestinal organoids derived from six patients, and a significant increase in CFTR function was demonstrated in five. Rescue of the CFTR protein in vitro in patients with N1303K has also been described using rectal organoids and in cultures of nasal epithelium cells.15,16 Clinical and in vitro response to ETI were also demonstrated in an 11-year-old patient with N1303K/E193X genotype. After 10 months of treatment, the patient had an absolute increase of 21% in FEV1, 1.1kg/m2 in BMI and significant improvement in respiratory symptoms.20
The PwCF in our study also showed sustained improvement in QoL after 12 weeks. In respiratory domain, there was a gain of 5.55–83.34 points in CFQ-R, with two cases achieving values higher than those found in patients with one F508del variant after 4 weeks (median of +17.4 points), and equal or higher than described in the first 22 patients using ETI in Brazil (mean of +46.7 points).4,21 Also, there was considerable gain in physical and health perception domains. The greatest benefits in QoL were seen in patients with more severe disease who, on the other hand, showed smaller variations in lung function. This reinforces the importance of assessing multiple outcomes when analyzing clinical response to treatment.
Regarding sweat chloride values, we observed a drop of less than 20mmol/L in all PwCF evaluated, despite the clinical response. Furthermore, in one of the patients, who showed a 13% improvement in FEV1 after treatment, the sweat chloride increased by 2mmol/L. The small variation in sweat chloride in patients with N1303K using ETI has already been described.17,20 The possible reasons for this dissociation between clinical and physiological response are not yet fully understood. They may be related to differences in processing, regulation, and responsiveness of mutant CFTR in different cell types, or in availability and activity of ETI in different tissues.12,17,20 This could also explain the lack of response observed in studies of N1303K with FRT.11
Finally, our positive results, along with previous published data, demonstrate the possibility of effective use of ETI for variants not yet eligible for treatment with modulators, such as N1303K. Thus, the proof of ETI effectiveness cannot be restricted to large clinical trials, as it would be impossible to conduct such studies in the context of rarer mutations. There is growing real-life evidence demonstrating significant clinical improvement in the presence of non-F508del variants, in addition to in vitro analyzes using different cell lines. Individualised assessment of response through compassionate use programmes has even been suggested as a way to reduce the number of PwCF who are not yet eligible for modulator treatment,12 given the well-established safety profile of ETI.22
In conclusion, our study demonstrates that patients with a N1303K/non-F508del genotype can significantly benefit from ETI treatment in several aspects, including lung function, weight, and QoL after 12 weeks of treatment. Further studies should be carried out in this population.
Ethical ApprovalThe study was previously approved by the local Research Ethics Committee and written informed consent obtained from all participants.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of InterestsAll authors received lecture and travel fees from Vertex Pharmaceuticals.