Bronchiectasis is a chronic lung disease, in which multimorbidity is common. The existence of comorbidities is of importance, as some of them have been associated with increased morbidity and mortality.1,2 In addition, gender-related differences in the clinical features of bronchiectasis have been reported previously.2–5 However, there is scarce data about gender differences in the impact of these comorbidities and their prognosis in this population. We aimed to analyze the impact of comorbidities and gender differences in survival in patients with bronchiectasis.
Patients admitted for a first episode of exacerbation of bronchiectasis according to the International Classification of Diseases version 9 (ICD-9) 494.1 or J47.1 (ICD-10) “Bronchiectasis with acute exacerbation”, between 2014 and 2021 were initially included in the analysis. This was an observational ambispective study, and the main endpoint was survival. Patients were grouped into the most clinically relevant patterns of disease, (a) metabolic diseases; (b) cardiovascular diseases; (c) respiratory diseases; (d) neurologic-psychiatric diseases; (e) osteoarticular; (f) cancer and (g) miscellanea. Comparison of proportions was made with the chi square test. Survival curves were constructed according to the Kaplan–Meier method. Hazard ratios for survival time were calculated with Cox regression models. The analysis for the potential differences in mortality according to gender was conducted with the stcox command of STATA. All calculations were performed using the version 18 of STATA.
Finally, 137 patients were included in the analysis. The mean age for the whole cohort was 73.4 years; 99 (69.2%) were female with a median predicted FEV1 of 60.7%. Mean BSI, E-FACED, BACI and Charlson comorbidity index were, respectively, 7.7, 2.3, 6.2 and 2.7.
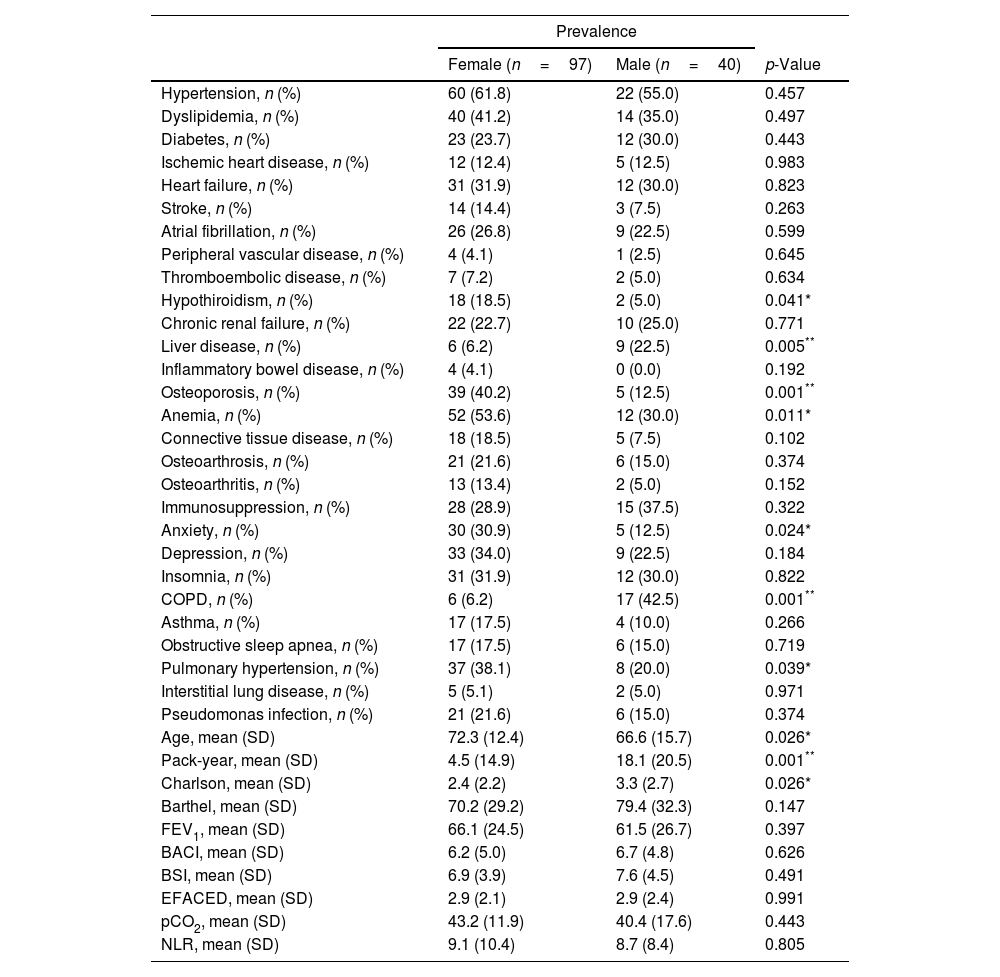
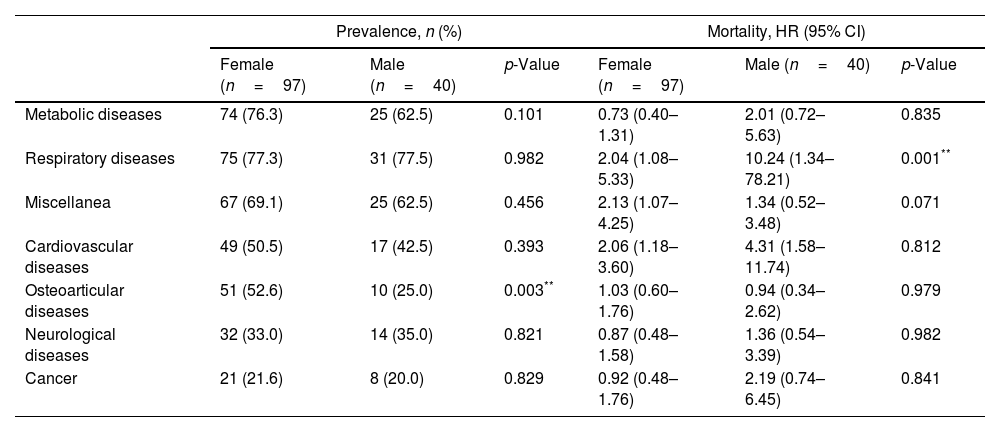
Survival at one, two and five years after discharge was 0.843 (95% CI 0.769–0.894), 0.749 (95% CI 0.665–0.814) and 0.535 (95% CI 0.442–0.619), respectively. Survival after 8 years was 0.398 (95% CI 0.303–0.495). There were no gender differences in survival (p=0.856). Regarding the impact of comorbidities on survival, we found that patients with COPD and heart failure showed an increased risk of dying, with a HR of 2.33 (95% CI 1.26–4.32) and 2.33 (95% CI 1.32–4.10), respectively. Table 1 shows the prevalence of comorbidities according to gender. Briefly, we found men had a higher comorbidity burden than women, measured by a higher Charlson index score. When we analyzed the prevalence of groups of comorbidities (Table 2), we only found differences in the case of osteoarticular conditions, which were more frequent in women. In the same table, we compared the mortality in men and women for each group of diseases. We only found differences in the case of respiratory diseases, with a HR of 10.24 in men vs a HR of 2.04 in women (p value=0.001).
Prevalence of comorbidities according to gender.
| Prevalence | |||
|---|---|---|---|
| Female (n=97) | Male (n=40) | p-Value | |
| Hypertension, n (%) | 60 (61.8) | 22 (55.0) | 0.457 |
| Dyslipidemia, n (%) | 40 (41.2) | 14 (35.0) | 0.497 |
| Diabetes, n (%) | 23 (23.7) | 12 (30.0) | 0.443 |
| Ischemic heart disease, n (%) | 12 (12.4) | 5 (12.5) | 0.983 |
| Heart failure, n (%) | 31 (31.9) | 12 (30.0) | 0.823 |
| Stroke, n (%) | 14 (14.4) | 3 (7.5) | 0.263 |
| Atrial fibrillation, n (%) | 26 (26.8) | 9 (22.5) | 0.599 |
| Peripheral vascular disease, n (%) | 4 (4.1) | 1 (2.5) | 0.645 |
| Thromboembolic disease, n (%) | 7 (7.2) | 2 (5.0) | 0.634 |
| Hypothiroidism, n (%) | 18 (18.5) | 2 (5.0) | 0.041* |
| Chronic renal failure, n (%) | 22 (22.7) | 10 (25.0) | 0.771 |
| Liver disease, n (%) | 6 (6.2) | 9 (22.5) | 0.005** |
| Inflammatory bowel disease, n (%) | 4 (4.1) | 0 (0.0) | 0.192 |
| Osteoporosis, n (%) | 39 (40.2) | 5 (12.5) | 0.001** |
| Anemia, n (%) | 52 (53.6) | 12 (30.0) | 0.011* |
| Connective tissue disease, n (%) | 18 (18.5) | 5 (7.5) | 0.102 |
| Osteoarthrosis, n (%) | 21 (21.6) | 6 (15.0) | 0.374 |
| Osteoarthritis, n (%) | 13 (13.4) | 2 (5.0) | 0.152 |
| Immunosuppression, n (%) | 28 (28.9) | 15 (37.5) | 0.322 |
| Anxiety, n (%) | 30 (30.9) | 5 (12.5) | 0.024* |
| Depression, n (%) | 33 (34.0) | 9 (22.5) | 0.184 |
| Insomnia, n (%) | 31 (31.9) | 12 (30.0) | 0.822 |
| COPD, n (%) | 6 (6.2) | 17 (42.5) | 0.001** |
| Asthma, n (%) | 17 (17.5) | 4 (10.0) | 0.266 |
| Obstructive sleep apnea, n (%) | 17 (17.5) | 6 (15.0) | 0.719 |
| Pulmonary hypertension, n (%) | 37 (38.1) | 8 (20.0) | 0.039* |
| Interstitial lung disease, n (%) | 5 (5.1) | 2 (5.0) | 0.971 |
| Pseudomonas infection, n (%) | 21 (21.6) | 6 (15.0) | 0.374 |
| Age, mean (SD) | 72.3 (12.4) | 66.6 (15.7) | 0.026* |
| Pack-year, mean (SD) | 4.5 (14.9) | 18.1 (20.5) | 0.001** |
| Charlson, mean (SD) | 2.4 (2.2) | 3.3 (2.7) | 0.026* |
| Barthel, mean (SD) | 70.2 (29.2) | 79.4 (32.3) | 0.147 |
| FEV1, mean (SD) | 66.1 (24.5) | 61.5 (26.7) | 0.397 |
| BACI, mean (SD) | 6.2 (5.0) | 6.7 (4.8) | 0.626 |
| BSI, mean (SD) | 6.9 (3.9) | 7.6 (4.5) | 0.491 |
| EFACED, mean (SD) | 2.9 (2.1) | 2.9 (2.4) | 0.991 |
| pCO2, mean (SD) | 43.2 (11.9) | 40.4 (17.6) | 0.443 |
| NLR, mean (SD) | 9.1 (10.4) | 8.7 (8.4) | 0.805 |
COPD: chronic obstructive pulmonary disease; SD: standard deviation; FEV1: forced expiratory volume in 1 second; BACI: Bronchiectasis Aetiology and Comorbidities Index; BSI: bronchiectasis severity index; NLR: neutrophil lymphocyte ratio.
Prevalence of groups of comorbidities and mortality according to gender.
| Prevalence, n (%) | Mortality, HR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Female (n=97) | Male (n=40) | p-Value | Female (n=97) | Male (n=40) | p-Value | |
| Metabolic diseases | 74 (76.3) | 25 (62.5) | 0.101 | 0.73 (0.40–1.31) | 2.01 (0.72–5.63) | 0.835 |
| Respiratory diseases | 75 (77.3) | 31 (77.5) | 0.982 | 2.04 (1.08–5.33) | 10.24 (1.34–78.21) | 0.001** |
| Miscellanea | 67 (69.1) | 25 (62.5) | 0.456 | 2.13 (1.07–4.25) | 1.34 (0.52–3.48) | 0.071 |
| Cardiovascular diseases | 49 (50.5) | 17 (42.5) | 0.393 | 2.06 (1.18–3.60) | 4.31 (1.58–11.74) | 0.812 |
| Osteoarticular diseases | 51 (52.6) | 10 (25.0) | 0.003** | 1.03 (0.60–1.76) | 0.94 (0.34–2.62) | 0.979 |
| Neurological diseases | 32 (33.0) | 14 (35.0) | 0.821 | 0.87 (0.48–1.58) | 1.36 (0.54–3.39) | 0.982 |
| Cancer | 21 (21.6) | 8 (20.0) | 0.829 | 0.92 (0.48–1.76) | 2.19 (0.74–6.45) | 0.841 |
HR: Hazard ratio; CI: confidence interval.
Several findings arose from the present study. Firstly, there were differences in the pattern of comorbidities according to sex, with COPD and liver disease being more frequent in men and osteoporosis in women. Secondly, respiratory and cardiovascular comorbidity patterns were associated with higher mortality in our population. And last, there were no gender differences in overall survival. However, we found that men have a higher risk of death from respiratory causes than women, due to a higher prevalence of COPD in this group.
The impact of comorbidities on survival in patients with bronchiectasis has been previously described,2,6,7 being a cause of increased mortality in this population.8 Metabolic, respiratory, and miscellaneous cluster pattern comorbidities were the main comorbidities identified in our study. In addition, respiratory and cardiovascular comorbidity patterns were associated with higher mortality in our cohort. Consistently, in the multivariate analysis adjusted by BSI, the comorbidities associated with higher mortality were COPD and heart failure, similar to previous studies.2,9
According to Cole's vicious cycle, structural abnormalities, chronic inflammation, and recurrent infections can cause airflow limitation, which induces premature death associated with respiratory causes.
Bronchiectasis are considered to be an independent risk factor for cardiovascular disease.10 Although the mechanisms involved in this association are not well described and likely multifactorial, chronic inflammation and the “spill-over” of inflammatory factors from the bronchial tree into the bloodstream could be a possible explanation.11 Because the risk of coronary heart disease is higher in patients with bronchiectasis than in the general population, such comorbidities require close attention in patients with bronchiectasis.
Increasing evidence suggests the role of gender on airway disease.3–5 Gender differences in disease prevalence, severity, progression, and outcome have been well described in individuals with COPD, asthma and cystic fibrosis.4,5 Although there are only few studies that have investigated the association between gender and clinical features in bronchiectasis, gender differences in this airway disease are also clinically evident and should be considered.
In our study, we found men had a higher comorbidity burden than women, measured by a higher Charlson index score. Consistent with previous research,3,12 COPD-related bronchiectasis patients were significatively more frequent among males and had worse lung function measured by FEV1. This probably is in line with the evidence that the proportion of male smokers was much higher than that of female smokers. In addition, Zhou et al.3 found that there was still a gender difference in lung function of patients with bronchiectasis after excluding confounders (smoking and COPD), having males a poorer lung function than females. On the contrary, in other studies,4,13,14 women had a poorer lung function than men. This is probably related to the fact that women had a higher frequency of Pseudomonas aeruginosa infection.3,5,13 Recent studies reported sex-related differences in anatomical structure, pulmonary function, and microbiome composition that might affect early pulmonary infection in females. As female sex hormones affect airway cilia function, microbial colonization may be influenced by gender.15,16 In this study, although differences in microbiological characteristics were detected between men and women, these differences were not statistically significant.
Furthermore, several studies have found different comorbidities to be more frequent in females than males with bronchiectasis such as depression, anxiety and rheumatologic or connective tissue diseases.3,5,13–16 In our study we found women were more often affected by osteoporosis and when we analyzed the prevalence of groups of comorbidities, we only found differences in the case of osteoarticular conditions, which were more frequent in females. On the other hand, liver disease was more prevalent in men.
Regarding survival, we compared the mortality in male and female for each group of diseases. We only found differences in the case of respiratory diseases which caused greater mortality in male probably because COPD and smoking were more common in men than in women.
In terms of disease severity, in most studies4,13,15,17 females are reported to have more severe disease, poorer clinical outcomes and a survival disadvantage compared to males across all age groups. The proposed factors that may contribute to this fact are the role of hormones (specially estrogens), the greater presence of P. aeruginosa, a lower therapeutic adherence and poorer attendance at follow-up appointments for treatment and pulmonary rehabilitation compared to men.13 In our study we did not find differences by gender in terms of survival.
In conclusion, respiratory and cardiovascular diseases were associated with increased mortality in patients with bronchiectasis. We found a different pattern of comorbidities between male and female patients, but these differences were not associated with a difference in survival after a first admission for exacerbation of bronchiectasis.
Authors’ contributionsStudy design: IGO, AR, JAC.
Data collecting: BUR, MC, CF.
Statistical analysis: IGO, JR.
Wrote the manuscript: IGO, BUR.
Read critically the manuscript: IGO, BUR, MC, CF, AR, JAC.
Data availability statementAll data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.
Not applicable.